Журнал высшей нервной деятельности им. И.П. Павлова, 2022, T. 72, № 6, стр. 851-861
Вклад трансглутаминазы в индукцию и поддержание долговременной синаптической потенциации в нейронах виноградной улитки
А. Б. Зюзина 1, *, П. М. Балабан 1
1 Федеральное государственное бюджетное учреждение науки Институт высшей нервной деятельности
и нейрофизиологии РАН
Москва, Россия
* E-mail: lucky-a89@mail.ru
Поступила в редакцию 11.03.2022
После доработки 31.03.2022
Принята к публикации 26.04.2022
- EDN: KXZAJM
- DOI: 10.31857/S0044467722060120
Аннотация
Ранее было показано, что для успешного формирования долговременной потенциации у наземной улитки Helix lucorum необходим нейромедиатор серотонин. В последнее время в литературе накапливается все больше данных о важной роли серотонина не только как агента, действующего через синаптические рецепторы, но также посредством ковалентного присоединения к своим белковым мишеням внутри клетки путем серотонилирования. Ферментом, обеспечивающим данную модификацию, является трансглутаминаза типа II (трансглутаминаза). В целом на сегодняшний день не сообщалось об исследованиях, направленных на выяснение роли трансглутаминаз в серотонин-зависимой пластичности. В текущем исследовании мы впервые изучили влияние блокады трансглутаминазы с помощью ингибитора монодансилкадаверина на формирование долговременной потенциации синаптических ответов в идентифицированных премоторных (командных) нейронах оборонительного поведения виноградной улитки. Мы показали, что применение ингибитора трансглутаминазы монодансилкадаверина нарушает позднюю фазу долговременной потенциации амплитуды синаптического ответа, вызванной пятикратной тетанизацией сенсорного нерва (второго кожного или интестинального), совмещенной с аппликацией серотонина на in vitro-препарате изолированной центральной нервной системы. Мы также обнаружили, что аппликация монодансилкадаверина сама по себе не влияет на синаптическую передачу в премоторных нейронах. Полученные результаты позволяют предположить, что для поддержания индуцированной серотонином поздней фазы долговременной потенциации синаптических ответов в премоторных (командных) нейронах оборонительного поведения виноградной улитки требуется опосредованное трансглутаминазой серотонилирование.
ВВЕДЕНИЕ
Согласно современным представлениям, серотонин играет решающую роль в формировании и поддержании долговременной синаптической пластичности и долговременной памяти у моллюсков (Kandel, Schwartz, 1982; Balaban et al., 1987; Balaban, Bravarenko, 1993; Balaban, 2002; Alberini, Kandel, 2014; Balaban et al., 2016; Deryabina et al., 2018, Zuzina et al., 2019), однако молекулярный механизм его действия остается неясным. В последнее время в литературе накапливается все больше данных о важной роли серотонина не только как агента, действующего через синаптические рецепторы, но также посредством ковалентного присоединения к глутамину в составе своих белковых мишеней внутри клетки путем серотонилирования (Berger et al., 2009; Paulmann et al., 2009; Walther et al., 2011; Muma, Mi, 2015). Ферментом, обеспечивающим серотонилирование, является трансглутаминаза типа II (ТГ). ТГ — широко распространенный эволюционно консервативный фермент, присутствующий как в прокариотических, так и в эукариотических организмах. К настоящему времени ТГ идентифицированы у широкого круга беспозвоночных животных (Singer et al., 1992; Sugino et al., 2002; Fagutao et al., 2012; Shibata, Kawabata, 2018; Junkunlo et al., 2019; Sirikharin et al., 2019; Junkunlo et al., 2020; Zhu et al., 2021). Анализ нервной ткани аплизии in vitro показал наличие ТГ в гигантском холинергическом нейроне R2 абдоминального ганглия (Ambron, Kremzner, 1982; Facchiano et al., 2010). Несмотря на то, что ТГ широко представлена в мире беспозвоночных животных, ее исследования у наземных улиток проведены не были и ее физиологическая роль неясна.
Было показано, что ТГ участвует в различных клеточных процессах, таких как дифференцировка (Ivashkin et al., 2015, 2019; Farrelly et al., 2019), гибель клеток, воспаление, миграция клеток и заживление ран (Piacentini et al., 2014), в агрегации тромбоцитов и секреции инсулина (Walther et al., 2003; Dale, 2005; Paulmann et al., 2009), в реорганизации дендритных шипиков в нейронах (Muma, Mi, 2015), а нарушение функций ТГ ведет к различным заболеваниям (Facchiano et al., 2006), при этом количество исследований роли ТГ в механизмах синаптической пластичности беспозвоночных незначительно.
О том, что серотонин с помощью ТГ способен связываться с белками, сообщалось неоднократно (Lin et al., 2014; Penumatsa et al., 2014; Hummerich et al., 2015; Muma, Mi, 2015; Ivashkin et al., 2015; Wang et al., 2016; Ivashkin et al., 2019). Недавно было показано, что серотонин служит субстратом для опосредованного ТГ трансаминирования ядерных белков гистонов у эмбрионов как позвоночных, так и беспозвоночных животных (Ivashkin et al., 2019). Примерно в то же время, основываясь на предыдущих наблюдениях (Ballestar et al., 1996), предполагающих, что ТГ модифицируют гистоны, Farrelly с соавт. (Farrelly et al., 2019) описали гистоны как мишени серотонилирования, где серотонилирование гистонов выступало в роли нового эпигенетического регуляторного механизма, способствовавшего потенциации транскрипции (Anastas, Shi, 2019; Fu, Zhang, 2019; Zhao et al., 2019; Zlotorynski, 2019).
В целом на сегодняшний день не сообщалось об исследованиях, направленных на выяснение роли ТГ в серотонин-зависимой пластичности. В текущем исследовании мы впервые изучили влияние блокады трансглутаминазы с помощью ингибитора монодансилкадаверина на формирование долговременной потенциации синаптических ответов в премоторных (командных) нейронах оборонительного поведения виноградной улитки. Мы показали, что применение ингибитора трансглутаминазы монодансилкадаверина нарушает позднюю фазу долговременной потенциации, вызванной пятикратной тетанизацией сенсорного нерва (второго кожного или интестинального), совмещенной с аппликацией серотонина. Мы также обнаружили, что аппликация монодансилкадаверина сама по себе не влияет на синаптическую передачу в премоторных нейронах. Полученные результаты позволяют предположить, что для поддержания индуцированной серотонином поздней фазы долговременной потенциации в премоторных (командных) нейронах оборонительного поведения виноградной улитки требуется опосредованное трансглутаминазой серотонилирование.
МЕТОДИКА
Эксперименты были проведены на улитках Helix lucorum taurica L. массой 20–30 г. За 1–2 нед до эксперимента улиток помещали во влажную среду, где они находились в активном состоянии. Протокол экспериментов утвержден Этической комиссией Института высшей нервной деятельности и нейрофизиологии РАН. Электрофизиологические эксперименты проводили на изолированной ЦНС улиток. Препарирование и идентификацию нейронов осуществляли по стандартной методике (Malyshev, Balaban, 2002). Перед началом препарирования производили инъекцию холодного изотонического раствора MgCl2 для обездвиживания и обезболивания животного. Изолированную ЦНС помещали в физиологический раствор Рингера (мМ): 100 NaCl, 4 KCl, 7 CaCl2, 5 MgCl2, 10 Trizma, pH 7.6.
Внутриклеточную регистрацию активности премоторных (командных) интернейронов париетальных ганглиев (Pa3 и Pa2) проводили при помощи острых стеклянных микроэлектродов, заполненных ацетатом калия (2 M), с сопротивлением 20–30 МОм. Регистрировали возбуждающие постсинаптические потенциалы (ВПСП), вызванные электрической стимуляцией второго педального кожного или интестинального нерва. В каждом эксперименте амплитуду стимула подбирали таким образом, чтобы стимуляция вызывала не потенциалы действия, а ВПСП амплитудой 6–15 мВ.
В начале записи проводили 5 тестовых стимуляций второго кожного или интестинального нерва с интервалом между стимулами 10 мин, затем осуществляли тетанизацию второго кожного или интестинального нерва (пачка стимулов частотой 10 Гц, длительность пачки 10 с, 10-кратное увеличение амплитуды тестового стимула). Всего тетанизацию осуществляли 5 раз с интервалом 5 мин. Перед каждой тетанизацией в экспериментальную ванночку добавляли серотонин (финальная концентрация 10−5 M), который отмывали через 2 мин после тетанизации. После пятой тетанизации продолжали тестирующую стимуляцию второго кожного или интестинального нерва с исходной амплитудой стимула каждые 10 мин в течение, по крайней мере, 4 часов.
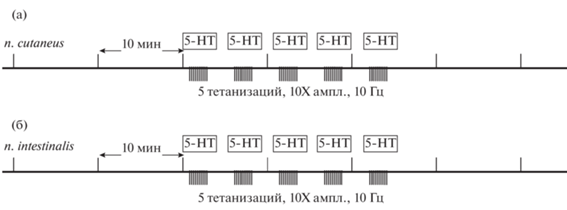
Первые 40 мин записи перфузионная система находилась в замкнутом режиме вплоть до момента первой тетанизации. В сериях экспериментов с ингибитором ТГ в этот временной период в экспериментальной ванночке находился монодансилкадаверин (МДК, является конкурентным субстратом первичных аминов) в концентрации 25 мкМ (низкая концентрация вещества была выбрана исходя из литературных данных, чтобы избежать побочных эффектов ингибитора на возбудимость мембраны, при этом данной концентрации достаточно, чтобы обеспечить ингибирование активности трансглутаминазы на 50–70% (Facchiano et al., 2010)). Мы применяли протокол гомосинаптической потенциации с тестовой стимуляцией по второму кожному нерву и тетанизацией того же второго кожного нерва или тестовой стимуляцией по интестинальному нерву и тетанизацией интестинального нерва совместно с аппликацией серотонина (рис. 1). После первой тетанизации (и первой аппликации серотонина) производился интенсивный “отмыв” препарата, который полностью удалял МДК из ванночки. После последней тетанизации система перфузии переводилась в разомкнутое состояние (отмыв), при этом скорость протока составляла 0.2 мл/мин при объеме ванночки 3 мл. В контрольных экспериментах без тетанизации и аппликации серотонина проток переводили в разомкнутое состояние в тот же момент времени.
Рис. 1.
Протокол эксперимента. Потенциация вызывалась тетанизацией того же нерва ((а) – второго кожного, (б) – интестинального) с 10-кратным увеличением амплитуды. Совместно с тетанизацией применяли 10–5 М-серотонин (расчетная концентрация в омывающем ЦНС растворе). Fig. 1. Experiment protocol. Potentiation was caused by tetanization of the same nerve ((a) – n. cutaneus, (б) – n. intestinalis) with a 10-fold increase in amplitude. Together with tetanization, 10–5 M serotonin was used (calculated concentration in the solution washing the CNS).
Достоверность изменений амплитуды синаптических потенциалов оценивали по статистическому критерию Манна–Уитни.
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЙ
Для изучения влияния ингибирования ТГ на потенциацию были проведены эксперименты на идентифицированных нейронах виноградной улитки, обработанных мембранопроницаемым ингибитором МДК. В первой серии экспериментов тестовая стимуляция и тетанизация проводились по второму кожному нерву. Тестовая стимуляция без тетанизации вызывала постепенное уменьшение амплитуды ВПСП в командных нейронах (привыкание) (рис. 2 (а), незаполненные квадратики, группа Контроль, n = 9). Мы проверили возможные эффекты МДК на амплитуду ВПСП без тетанизации (группа МДК + + Контроль, n = 10) (рис. 2 (а), незаполненные треугольники). Достоверных отличий между амплитудой ответов в группах Контроль и МДК + Контроль обнаружено не было. Пятикратная тетанизирующая стимуляция, совмещенная с аппликацией серотонина, вызывала выраженный рост амплитуд ВПСП (рис. 2 (б), заполненные квадратики, группа 5 × (5-НТ + тет), n = 10). Так, через 2 ч после последней тетанизации и аппликации серотонина амплитуда ВПСП в группе 5 × (5-НТ + + тет) составляла 138.1 ± 17.1% от исходной (рис. 2 (б)), в то время как в группе Контроль (рис. 2 (а)) эффект ослабления ответа приводил к тому, что в той же временной точке амплитуда ВПСП составляла 63.1 ± 5.4%, (p < < 0.0001). Через 4 ч после тетанизации амплитуда ВПСП тетанизированных входов также достоверно превышала амплитуды ответов в группе Контроль (группа 5 × (5-НТ + тет) – 150.0 ± 23.6% (рис. 2 (б)); группа Контроль 31.6 ± ± 5.8% (рис. 2 (а)), p < 0.0001). Аппликация МДК в течение 40 минут до тетанизации + + аппликации серотонина не влияла на амплитуду ВПСП в первые два часа после выработки долговременной потенциации (наблюдаемые различия между группами 5 × (5-НТ + тет) и МДК + 5 × (5-НТ + тет) в первый час эксперимента носили недостоверный характер). Так, через 2 ч после последней тетанизации + + серотонин амплитуда ВПСП в группе МДК + + 5 × (5-НТ + тет) составляла 119.1 ± 8.8% (n = 9) и достоверно не отличалась (р > 0.05) от амплитуд ВПСП в группе 5 × (5-НТ + тет) (138.1 ± 17.1%) (рис. 2 (б), заполненные треугольники). Однако, начиная с временной отметки 2 часа после последней тетанизации + + аппликации серотонина в группе МДК + 5 × × (5-НТ + тет) происходило постепенное снижение амплитуды ВПСП – амплитуды ВПСП данной группы достоверно отличались от амплитуды ответов контрольной группы 5 × (5-НТ + тет), при этом через 4 ч после последней тетанизации + серотонин амплитуда ВПСП в группе МДК + 5 × (5-НТ + + тет) составляла 74.5 ± 4.5%, тогда как в 5 × × (5-НТ + тет)-группе 150.0 ± 23.6% (p < < 0.0005) (рис. 2 (б)).
Рис. 2.
Влияние блокатора трансглутаминазы 2 монодансилкадаверина (МДК) на формирование и поддержание потенциации амплитуды комплексного ВПСП в премоторных (командных) гигантских нейронах париетальных ганглиев виноградной улитки, вызванной тетанизацией различных синаптических входов: второго кожного нерва ((а), (б)) или интестинального нерва ((в), (г)). Заштрихованными прямоугольниками обозначено время, когда монодансилкадаверин присутствовал в экспериментальной ванночке. По оси ординат представлена усредненная амплитуда ВПСП в % от исходного: исходная амплитуда ВПСП при первой стимуляции во всех экспериментах принята за единицу; по оси абсцисс – время в минутах. Данные представлены как среднее ± SEM. * – достоверность была установлена на уровне p < 0.05. Fig. 2. The effect of the transglutaminase 2 blocker monodansylcadaverine (MDK) on the formation and maintenance of complex EPSP amplitude potentiation in premotor (command) giant neurons of the parietal ganglia of the terrestrial snail, caused by tetanization of various synaptic inputs: the second cutaneus nerve ((a), (б)) or the intestinal nerve ((в), (г)). The shaded boxes indicate the time when monodansylcadaverine was present in the experimental bath. The ordinate shows the average EPSP amplitude in % of the initial one: the initial EPSP amplitude during the first stimulation was taken as one in all experiments; the abscissa shows time in minutes. Data are presented as means ± SEM. * – the significance was set at p < 0.05.
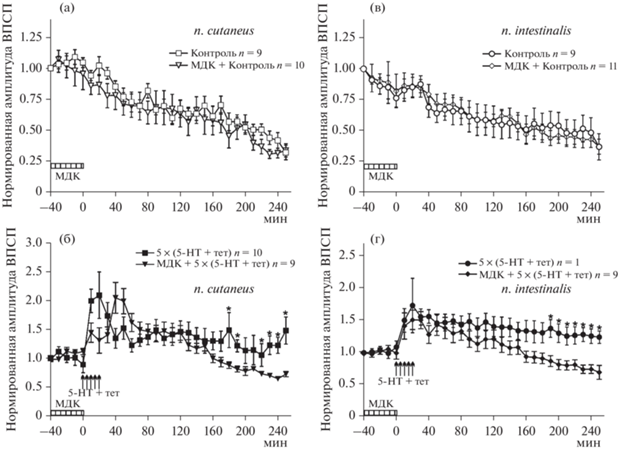
Во второй серии экспериментов использовался гомосинаптический протокол фасилитации, в котором тестовая стимуляция и тетанизация осуществлялись по интестинальному нерву. Результаты оказались сходными с эффектом МДК на амплитуду ВПСП в премоторных (командных) гигантских нейронах париетальных ганглиев виноградной улитки, вызванных стимуляцией по второму кожному нерву. В экспериментах без тетанизации амплитуда ВПСП постепенно снижалась (рис. 2 (в), незаполненные кружки, группа Контроль, n = 9), МДК не оказывал влияние на базовую синаптическую передачу (рис. 2 (в), незаполненные ромбики, группа МДК + + Контроль, n = 11). Достоверных отличий между амплитудой ответов в группах Контроль и МДК + Контроль обнаружено не было. Пятикратная тетанизирующая стимуляция, совмещенная с аппликацией серотонина, вызывала выраженный рост амплитуд ВПСП (рис. 2 (г), заполненные кружки, группа 5 × (5-НТ + тет), n = 11). Так, через 2 ч после последней тетанизации и аппликации серотонина амплитуда ВПСП в группе 5 × × (5-НТ + тет) (рис. 2 (г)) составляла 139.7 ± 14.4% от исходной, в то время как в группе Контроль (рис. 2 (в)) эффект ослабления ответа приводил к тому, что в той же временной точке амплитуда ВПСП составляла 56.1 ± 12.1%, (p < 0.0001). Через 4 ч после тетанизации амплитуда ВПСП тетанизированных входов также достоверно превышала амплитуды ответов в группе Контроль (группа 5 × (5-НТ + тет) – 124.5 ± 9.8% (рис. 2 (г)); группа Контроль 36.8 ± ± 10.6% (рис. 2 (в)), p < 0.0001). Аппликация МДК в течение 40 минут до тетанизации + серотонин не блокировала начальную фазу долговременной потенциации, однако через 2 часа после последней тетанизации + аппликации серотонина в группе МДК + 5 × (5-НТ + + тет) (рис. 2 (г)), заполненные ромбики) амплитуда ВПСП постепенно уменьшалась и при этом через 4 ч после последней тетанизации + серотонин между данными группами наблюдалось достоверное отличие (амплитуда ВПСП в группе МДК + 5 × (5-НТ + тет) составляла 69.7 ± 10.8%, тогда как в 5 × (5-НТ + + тет)-группе 124.5 ± 9.8%; p < 0.01) (рис. 2 (г)).
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
Прежде всего необходимо отметить, что долговременная потенциация синаптических ответов идентифицированных нейронов у моллюсков представляет собой ценную модель для исследования молекулярных механизмов, лежащих в основе формирования и поддержания синаптической пластичности. В данной работе было исследовано влияние ингибирования трансглутаминазы типа II (ТГ) с помощью блокатора монодансилкадаверина (МДК) на пластичность синаптических входов от сенсорных нейронов на гигантские премоторные интернейроны, чья активность связана с запуском оборонительной реакции у виноградной улитки, а потенциация данных входов лежит в основе долговременной оборонительной памяти. Для выяснения возможного влияния МДК на базовую синаптическую передачу в командных нейронах виноградной улитки была поставлена специальная контрольная серия экспериментов без тетанизации. Выяснилось, что само по себе добавление МДК не вызывало изменения амплитуды ВПСП при стимуляции нервов (второго кожного или интестинального) на протяжении всего периода записи в сравнении с контрольными экспериментами. Наблюдаемое нами в экспериментах без тетанизации (как контрольных, так и в экспериментах с МДК) (рис. 2 (а), (в)) постепенное уменьшение амплитуды ВПСП согласуется с литературными данными, полученными на данной модели (Malyshev, Balaban, 2002).
Далее в двух сериях опытов вырабатывали долговременную потенциацию синаптических входов (предположительно глутаматергический вход при стимуляции волокон второго кожного нерва (Bravarenko et al., 2003); предположительно холинергический вход при стимуляции волокон интестинального нерва (Ter-Markarian et al., 1990)) и анализировали изменение амплитуды ВПСП в ответ на добавление МДК. Вне зависимости от эргичности нерва высокочастотная стимуляция нерва с одновременной аппликацией серотонина вызывала долговременное увеличение амплитуды комплексного ВПСП в ответ на тестовую стимуляцию нерва. На фоне МДК также наблюдалось кратковременное увеличение амплитуды ВПСП с последующим нарушением величины долговременной потенциации (рис. 2 (б), (г)). Фармакологическое ингибирование ТГ приводило к достоверному ослаблению поздней фазы долговременной потенциации в премоторных (командных) нейронах оборонительного поведения виноградной улитки. В экспериментах на нервной ткани аплизии сообщалось о наличии белков, иммунореактивных к антителу против ТГ (Facchiano et al., 2010), что, вероятно, отражает присутствие в нервных окончаниях более чем одной ТГ. Опираясь на эти данные, можно объяснить только частичное ослабление потенциации при ингибировании активности ТГ с помощью МДК в наших экспериментах, поскольку другие ТГ могли так или иначе вносить свой вклад, как это продемонстрировано в работе Johnson и соавт. (Johnson et al., 2012). Полученные результаты подтверждают необходимость опосредованных ТГ процессов при формировании долговременных синаптических изменений в нервной системе виноградной улитки. С другой стороны, необходимо отметить, что мы наблюдали лишь частичное, но достоверное ослабление поздних этапов долговременной потенциации при блокаде ТГ, что говорит о наличии других путей действия серотонина при формировании долговременных пластических перестроек, а именно посредством активации серотонинергических рецепторов, как это было показано в ряде более ранних работ (Abramova et al., 2006; Solntseva, Nikitin, 2008). В настоящей работе существенным является также тот факт, что введение ингибитора ТГ без процедуры инициации потенциации не приводило к выраженным эффектам при тестировании любого из нервов. Данное наблюдение предполагает регулирующее влияние ТГ-опосредованных процессов именно на молекулярную систему формирования и поддержания долговременных синаптических перестроек.
Следует отметить, что долговременная потенциация – это сложный многоступенчатый процесс, регулируемый множеством механизмов и требующий нескольких сигнальных каскадов. На основании имеющихся литературных данных и полученных нами результатов мы попробуем предложить вероятный механизм синаптической потенциации, опосредованной серотонином у наземных моллюсков. В литературе принято разделять долговременную потенциацию на раннюю (длительностью 1–3 часа) и позднюю фазы (длящуюся много часов), при этом поздняя фаза долговременной потенциации требует экспрессии генов (Silva et al., 1998; Kandel, 2001; Dudai, 2004; Klann, Sweat, 2008; Alberini, 2009; Allen et al., 2014). Поскольку ингибирование ТГ влияло на позднюю фазу долговременной потенциации (рис. 2 (б), (г)), можно предположить, что ТГ-опосредованные процессы являются частью молекулярного механизма включения экспрессии генов на поздних этапах долговременной потенциации. В пользу данного предположения свидетельствуют следующие литературные данные. Известно, что посттрансляционные модификации гистонов (ацетилирование, метилирование, фосфорилирование, убиквитинилирование, пропионилирование, бутирилирование) могут выступать как транскрипционные контролеры (Huang et al., 2014; Borodinova, Balaban, 2020; Cavalieri, 2021). При этом гистоны представляют собой хороший глутамин-содержащий субстрат для фермента ТГ (Ballestar et al., 1996; Ballestar et al., 2001; Sato et al., 2003; Sileno et al., 2014). Кроме того, совсем недавно было обнаружено, что серотонилирование гистонов может являться эпигенетическим регуляторным механизмом (Anastas, Shi, 2019; Fu, Zhang, 2019; Zhao et al., 2019; Zlotorynski, 2019): серотонилирование гистона H3 модулировало связывание транскрипционного фактора II D с H3K4me3, тем самым влияя на экспрессию генов (Bader, 2019; Farrelly et al., 2019). Примерно в то же время было показано, что серотонин служит субстратом для опосредованного ТГ трансаминирования ядерных белков гистонов у эмбрионов как позвоночных, так и беспозвоночных животных: активность ТГ определяла ядерную локализацию иммунореактивности к серотонину – уровень иммунореактивности к серотонину в ядрах клеток увеличивался при повышении концентрации серотонина, а фармакологическое ингибирование активности ТГ приводило к снижению как яркости, так и ядерной локализации окрашивания (Ivashkin et al., 2019). Способность как ТГ (Lesort et al., 2000), так и серотонина проникать в ядро также не вызывает сомнений (Emanuelsson, 1974; Korneliussen, 1976; Csaba, Sudar, 1978; Сalas et al., 1981; Bosler, Calas, 1982; Csaba et al., 1983; Csaba, Kovacs, 2006; Csaba et al., 2006; Czaker, 2006; Farelly et al., 2019; Ivashkin et al., 2019). Таким образом, можно предположить, что наблюдаемое нами ослабление поздней фазы долговременной потенциации является следствием нарушения такого ТГ-опосредованного процесса, как серотонилирование, где ТГ играет роль в модификации хроматина и регуляции экспрессии генов за счет присоединения молекулы серотонина к гистонам.
Обнаруженный нами эффект ухудшения долговременной потенциации при ингибировании ТГ может также объясняться тем, что ТГ модулирует экспрессию генов (Kuo et al., 2011; Fagutao et al., 2012), регулируя функционирование многочисленных факторов транскрипции (Mann et al., 2006; Tatsukawa et al., 2009; Gundemir et al., 2012; Agnihotri et al., 2013; Eckert et al., 2014). Кроме того, ТГ может выступать в роли модулятора сигнальных путей, увеличивая активацию CREB в нервных и ненейрональных клетках (Satpathy et al., 2009; Obara et al., 2013).
Важным обстоятельством является то, что проявление ТГ своей каталитической активности возможно только в условиях повышенной внутриклеточной концентрации кальция (Bader et al., 2019), при этом повышенные уровни внутриклеточного кальция увеличивают транслокацию ТГ в ядро (Gundemir et al., 2012), где она может опосредовать регуляцию транскрипции. В экспериментах на виноградной улитке было показано, что повышение внутриклеточной концентрации кальция в премоторных интернейронах наблюдается при действии серотонина (Balaban et al., 2004) в результате активации рецепторов клеточной поверхности. Учитывая вышеприведенные факты, можно предположить, что действие серотонина включает ранний этап: опосредованный через внеклеточные взаимодействия лиганд-рецептор, сопровождающийся повышением внутриклеточной концентрации кальция, связыванием кальция ТГ, с переходом последней в открытую активную конформацию, – и поздний этап, который, вероятно, сопровождается вовлечением серотонина во внутриклеточную регуляцию посредством посттрансляционных модификаций белков в результате опосредованного ТГ связывания с остатками глутамина в пептидах-мишенях.
ЗАКЛЮЧЕНИЕ
Данные, полученные в настоящем исследовании на моллюсках, предполагают, что активация трансглутаминазы является важным событием, которое обеспечивает стабилизацию индуцированной серотонином долговременной потенциации в премоторных (командных) нейронах оборонительного поведения виноградной улитки. Основываясь также на предыдущих исследованиях, мы описали вероятную роль трансглутаминазы в формировании опосредованной серотонином долговременной потенциации у наземных моллюсков за счет модификации хроматина и регуляции экспрессии генов за счет присоединения молекулы серотонина к гистонам. Будущие исследования, безусловно, необходимы для выявления белков-мишеней и сигнальных путей, характерных для трансглутаминазы.
Список литературы
Abramova M.S., Nistratova V.L., Moskvitin A.A., Pivovarov A.S. Methiothepin-sensitive serotonin receptors are involved in the postsynaptic mechanism of sensitization of the defensive response in the common snail. Neurosci. Behav. Physiol. 2006. 36(6): 589–596.
Agnihotri N., Kumar S., Mehta K. Tissue transglutaminase as a central mediator in inflammation-induced progression of breast cancer. Breast Cancer Res. 2013. 15(1): 202.
Alberini C.M. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009. 89(1): 121–145.
Alberini C.M., Kandel E.R. The regulation of transcription in memory consolidation. Cold Spring Harb. Perspect. Biol. 2014. 7(1): a021741.
Allen K.D., Gourov A.V., Harte C., Gao P., Lee C., Sylvain D., Splett J.M., Oxberry W.C., van de Nes P.S., Troy-Regier M.J., Wolk J., Alarcon J.M., Hernández A.I. Nucleolar integrity is required for the maintenance of long-term synaptic plasticity. PLoS One. 2014. 9(8): e104364.
Ambron R.T., Kremzner L.T. Post-translational modification of neuronal proteins: evidence for transglutaminase activity in R2, the giant cholinergic neuron of Aplysia. Proc Natl Acad Sci U S A. 1982. 79(11): 3442–3446.
Anastas J.N., Shi Y. Histone Serotonylation: can the brain have “Happy”. Chromatin. Mol. Cell. 2019. 74(3): 418–420.
Bader M. Serotonylation: Serotonin Signaling and Epigenetics. Front. Mol. Neurosci. 2019. 12: 288.
Balaban P.M. Cellular mechanisms of behavioral plasticity in terrestrial snail. Neurosci. Biobehav. Rev. 2002. 26(5): 597–630.
Balaban P.M., Vehovszky A., Maksimova O.A., Zakharov I.S. Effect of 5,7-dihydroxytryptamine on the food-aversive conditioning in the snail Helix lucorum L. Brain Research. 1987. 404(1–2): 201–210.
Balaban P., Bravarenko N. Long-term sensitization and environmental conditioning in terrestrial snails. Exp. Brain Research. 1993. 96(3): 487–493.
Balaban P.M., Korshunova T.A., Bravarenko N.I. Postsynaptic calcium contributes to reinforcement in a three-neuron network exhibiting associative plasticity. Eur. J. Neurosci. 2004. 19(2): 227–233.
Balaban P.M., Vinarskaya A.K., Zuzina A.B., Ierusalimsky V.N., Malyshev A.Y. Impairment of the serotonergic neurons underlying reinforcement elicits extinction of the repeatedly reactivated context memory. Sci. Rep. 2016. 6: 36933.
Ballestar E., Abad C., Franco L. Core histones are glutaminyl substrates for tissue transglutaminase. J. Biol. Chem. 1996. 271(31): 18817–18824.
Ballestar E., Boix-Chornet M., Franco L. Conformational changes in the nucleosome followed by the selective accessibility of histone glutamines in the transglutaminase reaction: effects of ionic strength. Biochemistry. 2001. 40(7): 1922–1929.
Berger M., Gray J.A., Roth B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009. 60: 355−366.
Borodinova A.A., Balaban P.M. Epigenetic regulation as a basis for long-term changes in the nervous system: in search of specificity mechanisms. Biochemistry (Mosc.). 2020. 85(9): 994–1010.
Bosler O., Calas A. Radioautographic investigation of monoaminergic neurons: An evaluation. Brain Res. Bull. 1982. 9(1–6): 151−169.
Bravarenko N.I., Korshunova T.A., Malyshev A.Y., Balaban P.M. Synaptic contact between mechanosensory neuron and withdrawal interneuron in terrestrial snail is mediated by l-glutamate-like transmitter. Neurosci. Let. 2003. 341(3): 237–240.
Calas A., Dupuy J.J., Gamrani H., Gonella J., Mourre C., Condamin M., Pellissier J.F., Van den Bosch P. Radioautographic investigation of serotonin cells. Adv. Exp. Med. Biol. 1981. 133: 51−66.
Cavalieri V. The expanding constellation of histone post-translational modifications in the epigenetic landscape. Genes (Basel). 2021. 12(10): 1596.
Csaba G., Sudar F. Localization of radioactively ́ labelled serotonin in the nucleus of adrenal medulla cells. Acta Anat. (Basel). 1978. 100(2): 237−240.
Csaba G., Sudar F., Ubornyak L. Comparative ́ study of the internalization and nuclear localization of amino acid type hormones in Tetrahymena and rat lymphocytes. Exp. Clin. Endocrinol. Diabetes. 1982. 82(1): 61−67.
Csaba G., Kovacs P. Perinuclear localization of ́ biogenic amines (serotonin and histamine) in rat immune cells. Cell Biol. Int. 2006. 30(11): 861−865.
Csaba G., Kovacs P., Pallinger E. Hormones in ́ the nucleus. Immunologically demonstrable biogenic amines (serotonin, histamine) in the nucleus of rat peritoneal mast cells. Life Sci. 2006. 78(16): 1871−1877.
Czaker R. Serotonin immunoreactivity in a highly enigmatic metazoan phylum, the pre-nervous Dicyemida. Cell Tissue Res. 2006. 326(3): 843−850.
Dale G.L. Coated-platelets: an emerging component of the procoagulant response. J. Thromb. Haemost. 2005. 3(10): 2185–2192.
Deryabina I.B., Muranova L.N., Andrianov V.V., Gainutdinov K.L. Impairing of serotonin synthesis by p-chlorphenylanine prevents the forgetting of contextual memory after reminder and the protein synthesis inhibition. Front. Pharmacol. 2018. 9: 607.
Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 2004. 55: 51–86.
Eckert R.L., Kaartinen M.T., Nurminskaya M., Belkin A.M., Colak G., Johnson G.V., Mehta K. Transglutaminase regulation of cell function. Physiol. Rev. 2014. 94(2): 383–417.
Emanuelsson H. Localization of serotonin in cleavage embryos of Ophryotrocha labronica La Greca and Bacci. Dev. Genes Evol. 1974. 175(4): 253−271.
Facchiano F., Facchiano A., Facchiano A.M. The role of transglutaminase-2 and its substrates in human diseases. Front. Biosci. 2006. 11: 1758–1773.
Facchiano F., Deloye F., Doussau F., Innamorati G., Ashton A.C., Dolly J.O., Beninati S., Facchiano A., Luini A., Poulain B., Benfenati F. Transglutaminase participates in the blockade of neurotransmitter release by tetanus toxin: evidence for a novel biological function. Amino Acids. 2010. 39(1): 257–269.
Fagutao F.F., Maningas M.B., Kondo H., Aoki T., Hirono I. Transglutaminase regulates immune-related genes in shrimp. Fish Shellfish Immunol. 2012. 32(5): 711–715.
Farrelly L.A., Thompson R.E., Zhao S., Lepack A.E., Lyu Y., Bhanu N.V., Zhang B., Loh Y.-H.E., Ramakrishnan A., Vadodaria K.C., Heard K.J., Erikson G., Nakadai T., Bastle R.M., Lukasak B.J., Zebroski H. 3rd, Alenina N., Bader M., Berton O., Roeder R.G., Molina H., Gage F.H., Shen L., Garcia B.A., Li H., Muir T.W., Maze I. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me. Nature. 2019. 567(7749): 535–539.
Fu L., Zhang L. Serotonylation: a novel histone H3 marker. Signal Trans. Target Ther. 2019. 4: 15.
Gundemir S., Colak G., Tucholski J., Johnson G.V. Transglutaminase 2: a molecular Swiss army knife. Biochim. Biophys. Acta. 2012. 1823(2): 406–419.
Huang H., Sabari B.R., Garcia B.A., Allis C.D., Zhao Y. SnapShot: histone modifications. Cell. 2014. 159(2): 458.e1.
Hummerich R., Costina V., Findeisen P., Schloss P. Monoaminylation of fibrinogen and glia-derived proteins: indication for similar mechanisms in posttranslational protein modification in blood and brain. ACS Chem. Neurosci. 2015. 6(7): 1130–1136.
Ivashkin E., Khabarova M.Y., Melnikova V., Nezlin L.P., Kharchenko O., Voronezhskaya E.E., Adameyko I. Serotonin mediates maternal effects and directs developmental and behavioral changes in the progeny of snails. Cell Rep. 2015. 12(7): 1144–1158.
Ivashkin E., Melnikova V., Kurtova A., Brun N.R., Obukhova A., Khabarova M.Y. Transglutaminase activity determines nuclear localization of serotonin immunoreactivity in the early embryos of invertebrates and vertebrates. ACS Chem. Neurosci. 2019. 10(8): 3888–3899.
Johnson K.B., Petersen-Jones H., Thompson J.M., Hitomi K., Itoh M., Bakker E.N. Vena cava and aortic smooth muscle cells express transglutaminases 1 and 4 in addition to transglutaminase 2. Am. J. Physiol. Heart Circ. Physiol. 2012. 302(7): H1355–H1366.
Junkunlo K., Söderhäll K., Söderhäll I. Transglutaminase inhibition stimulates hematopoiesis and reduces aggressive behavior of crayfish, Pacifastacus leniusculus. J. Biol. Chem. 2019. 294(2): 708–715.
Junkunlo K., Söderhäll K., Söderhäll I. Transglutaminase 1 and 2 are localized in different blood cells in the freshwater crayfish Pacifastacus leniusculus. Fish Shellfish Immunol. 2020. 104: 83–91.
Kandel E.R. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001. 294(5544): 1030–1038.
Kandel E.R., Schwartz J.H. Molecular biology of an elementary form of learning: modulation of transmitter release by cuclic AMP. Science. 1982. 218(4571): 433–443.
Klann E., Sweatt J.D. Altered protein synthesis is a trigger for long-term memory formation. Neurobiol. Learn. Mem. 2008. 89(3): 247–259.
Korneliussen H. 5-Hydroxytryptamine: Autoradiographic evidence for uptake into fibroblast cell nuclei. Experientia. 1976. 32(4): 443−445.
Kuo T.-F., Tatsukawa H., Kojima S. New insights into the functions and localization of nuclear transglutaminase 2. FEBS J. 2011. 278(24): 4756−4767.
Lesort M., Tucholski J., Miller M.L., Johnson G.V. Tissue transglutaminase: a possible role in neurodegenerative diseases. Prog. Neurobiol. 2000. 61(5): 439–463.
Lin J.C., Chou C.C., Tu Z., Yeh L.F., Wu S.C., Khoo K.H., Lin C.H. Characterization of protein serotonylation via bioorthogonal labeling and enrichment. J. Proteome Res. 2014. 13(8): 3523–3529.
Malyshev A.Y., Balaban P.M. Identification of mechanoafferent neurons in terrestrial snail: response properties and synaptic connections. J. Neurophysiol. 2002. 87(5): 2364–2371.
Mann A.P., Verma A., Sethi G., Manavathi B., Wang H., Fok J.Y., Kunnumakkara A.B., Kumar R., Aggarwal B.B., Mehta K. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 2006. 66(17): 8788–8795.
Muma N.A., Mi Z. Serotonylation and transamidation of other monoamines. ACS Chem. Neurosci. 2015. 6(7): 961–969.
Obara Y., Yanagihata Y., Abe T., Dafik L., Ishii K., Nakahata N. Gα(h)/transglutaminase-2 activity is required for maximal activation of adenylylcyclase 8 in human and rat glioma cells. Cell Signal. 2013. 25(3): 589–597.
Paulmann N., Grohmann M., Voigt J.P., Bert B., Vowinckel J., Bader M., Skelin M., Jevsek M., Fink H., Rupnik M., Walther D.J. Intracellular serotonin modulates insulin secretion from pancreatic β-cells by protein serotonylation. PLoS Biol. 2009. 7(10): e1000229.
Penumatsa K., Abualkhair S., Wei L., Warburton R., Preston I., Hill N.S., Watts S.W., Fanburg B.L., Toksoz D. Tissue transglutaminase promotes serotonin-induced AKT signaling and mitogenesis in pulmonary vascular smooth muscle cells. Cell. Signal. 2014. 26(12): 2818–2825.
Piacentini M., D’Eletto M., Farrace M.G., Rodolfo C., Del Nonno F., Ippolito G., Falasca L. Characterization of distinct sub-cellular location of transglutaminase type II: changes in intracellular distribution in physiological and pathological states. Cell Tissue Res. 2014. 358(3): 793–805.
Sato N., Ohtake Y., Kato H., Abe S., Kohno H., Ohkubo Y. Effects of polyamines on histone polymerization. J. Protein Chem. 2003. 22(3): 303–307.
Satpathy M., Shao M., Emerson R., Donner D.B., Matei D. Tissue transglutaminase regulates matrix metalloproteinase-2 in ovarian cancer by modulating cAMP-response element-binding protein activity. J. Biol. Chem. 2009. 284(23): 15390–15399.
Shibata T., Kawabata S.I. Pluripotency and a secretion mechanism of Drosophila transglutaminase. J. Biochem. 2018. 163(3): 165–176.
Sileno S., D’Oria V., Stucchi R., Alessio M., Petrini S., Bonetto V., Maechler P., Bertuzzi F., Grasso V., Paolella K., Barbetti F., Massa O. A possible role of transglutaminase 2 in the nucleus of INS-1E and of cells of human pancreatic islets. J. Proteomics. 2014. 96(100): 314–327.
Singer M.A., Hortsch M., Goodman C.S., Bentley D. Annulin, a protein expressed at limb segment boundaries in the grasshopper embryo, is homologous to protein cross-linking transglutaminases. Dev. Biol. 1992. 154(1): 143–159.
Silva A.J., Kogan J.H., Frankland P.W., Kida S. CREB and memory. Annu. Rev. Neurosci. 1998. 21: 127–148.
Sirikharin R., Utairungsee T., Srisala J., Roytrakul S., Thitamadee S., Sritunyalucksana K. Cell surface transglutaminase required for nodavirus entry into freshwater prawn hemocytes. Fish Shellfish Immunol. 2019. 89: 108–116.
Solntseva S.V., Nikitin V.P. Neurochemical mechanisms of food aversion conditioning consolidation in snail Helix lucorum. Ross. Fiziol. Zh. Im. I. M. Sechenova. 2008. 94: 1259–1269.
Sugino H., Terakawa Y., Yamasaki A., Nakamura K., Higuchi Y., Matsubara J., Kuniyoshi H., Ikegami S. Molecular characterization of a novel nuclear transglutaminase that is expressed during starfish embryogenesis. Eur. J. Biochem. 2002. 269(7): 1957–1967.
Tatsukawa H., Fukaya Y., Frampton G., Martinez-Fuentes A., Suzuki K., Kuo T.F., Nagatsuma K., Shimokado K., Okuno M., Wu J., Iismaa S., Matsuura T., Tsukamoto H., Zern M.A., Graham R.M., Kojima S. Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1. Gastroenterology. 2009. 136(5): 1783–95.e10.
Ter-Markarian A.G., Palikhova T.A., Sokolov E.N. The action of atropine and d-tubocurarine on the monosynaptic connections between identified neurons in the central nervous system of the edible snail. Zh. Vyssh. Nerv. Deiat. Im. I.P. Pavlova. 1999. 40: 183–184.
Walther D.J., Peter J.U., Winter S., Holtje M., Paulmann N., Grohmann M., Vowinckel J., Alamo-Bethencourt V., Wilhelm C.S., Ahnert-Hilger G., Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet α-granule release. Cell. 2003. 115(7): 851–862.
Walther D.J., Stahlberg S., Vowinckel J. Novel roles for biogenic monoamines: from monoamines in transglutaminase-mediated post-translational protein wmodification to monoaminylation deregulation diseases. FEBS J. 2011. 278(24): 4740–4755.
Wang Q., Wang D., Yan G., Qiao Y., Sun L., Zhu B., Wang X., Tang C. SERCA2a was serotonylated and may regulate sino-atrial node pacemaker activity. Biochem. Biophys. Res. Commun. 2016. 480(3): 492–497.
Zhao S., Yue Y., Li Y., Li H. Identification and characterization of ‘readers’ for novel histone modifications. Curr. Opin. Chem. Biol. 2019. 51: 57–65.
Zhu J., Shao Y., Chen K., Zhang W., Li C. A transglutaminase 2-like gene from sea cucumber Apostichopus japonicus mediates coelomocytes autophagy. Fish Shellfish Immunol. 2021. 119: 602–612. Zlotorynski E. Histone serotonylation boosts neuronal transcription. Nat. Rev. Mol. Cell Biol. 2019. 20(6): 323.
Zuzina A.B., Vinarskaya A.K., Balaban P.M. Increase in serotonin precursor levels reinstates the context memory during reconsolidation. Invert. Neurosci. 2019. 19(3): 8.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


