Журнал высшей нервной деятельности им. И.П. Павлова, 2022, T. 72, № 3, стр. 343-359
Роль зубчатой извилины в осуществлении функций гиппокампа: эпилептический мозг
В. Ф. Кичигина 1, *, Л. В. Шубина 1, И. Ю. Попова 1
1 ФГБУН Институт теоретической и экспериментальной биофизики РАН
Пущино, Россия
* E-mail: vkitchigina@gmail.com
Поступила в редакцию 28.10.2021
После доработки 03.12.2021
Принята к публикации 20.12.2021
- EDN: HPUNAZ
- DOI: 10.31857/S0044467722030042
Аннотация
Височная эпилепсия (ВЭ) характеризуется потерей клеток гиппокампа, часто приводящей к его склерозу, последующей реорганизацией гиппокампальной сети и дефицитом декларативной памяти. Несмотря на огромное количество экспериментальных, доклинических и клинических исследований, существует все еще ограниченное понимание основных механизмов, лежащих в основе развития ВЭ. Существует предположение, что именно ЗИ играет решающую роль в механизмах развития этого заболевания. Считают, что при ВЭ нарушается защитная функция ЗИ, основанная на низкой возбудимости гранулярных нейронов и предохраняющая пирамидные клетки гиппокампа от гиперактивации при сильных возбуждающих воздействиях. У пациентов с височной эпилепсией в хилусе ЗИ обнаружена потеря мшистых клеток. Уязвимость мшистых клеток рассматривается некоторыми авторами как критический фактор в развитии ВЭ: эти нейроны в норме ведут себя как предохранители, а их гибель разрывает естественную нейронную сеть, приводя к возникновению патологической активности. В предлагаемой работе рассматриваются изменения морфологических и функциональных свойств ЗИ в эпилептическом мозге, роль спрутинга мшистых волокон и нейрогенеза в развитии ВЭ, а также нарушения когнитивных функций гиппокампа при потере зубчатой извилиной ее защитной роли в условиях гиперактивации.
ВВЕДЕНИЕ
Височная эпилепсия (ВЭ) – это разновидность фокальной эпилепсии, при которой судорожная активность (пароксизм) или само органическое поражение мозга, ее вызывающее, локализовано целиком или частично в височной доле. Область возможной локализации эпилептогенного поражения может включать как структуры самой височной доли (гиппокамп, амигдалярный комплекс, неокортекс), так и ряд других образований (поясную извилину, орбитофронтальную кору и др.) (Карлов, 2000). Основным повреждением мозга при височной эпилепсии считается гибель нейронов гиппокампа, часто приводящая к его склерозу, а также последующая реорганизация гиппокампальной сети (Babb, Brown, 1987; de Lanerolle et al., 1992, Lowenstein 2001; см. также обзор Viscomi et al., 2010). Такие события обычно приводят к нарушению эпизодической (автобиографической) памяти (Burgess et al., 2002; Tulving, 2002; Inostrosa et al., 2013).
Приобретенная (ненаследуемая) ВЭ может развиваться как следствие черепно-мозговой травмы, инсульта или токсических воздействий, которые приводят к резкому повышению возбудимости гиппокампальных нейронов (Engel, 2001). Это событие опосредуется дисбалансом тормозных и возбуждающих нейромедиаторов и изменением эффективности функционирования рецепторных комплексов. Ведущая роль в эпилептогенезе принадлежит глутамату и ГАМК. Высокая концентрация глутамата в межклеточном пространстве в гиппокампе, по-видимому, является основной причиной гибели нервных клеток и последующих морфологических перестроек при эпилептогенезе (Haglid et al., 1994; Ueda et al., 2002). Показано, что периодическое кратковременное повышение глутамата в гиппокампе приводит к развитию экспериментальной эпилепсии; при этом во время интериктальной (межсудорожной) фазы накопление глутамата происходит за счет нарушения механизмов его захвата (Ueda et al., 2002). В процессе эпилептогенеза происходит также возрастание количества всех трех типов глутаматных рецепторов (AMPA, NMDA и метаботропных), в результате чего возрастает эффективность возбуждающей синаптической передачи, наблюдаются гиперактивация нейронов и накопление внутриклеточных ионов кальция – события, ведущие к гибели клеток. Обнаружено, что для индукции клеточной гибели важна локализация NMDA-рецепторов: активация экстрасинаптических NMDA-рецепторов обеспечивает клеточную смерть, в то время как активация синаптических, напротив – нейропротекцию. Такое различие определяется активацией различных геномных программ и противоположными влияниями на внутриклеточные сигнальные пути (см. обзор Hardingham, Bading, 2010). В “эксайтотоксической” гипотезе (Olney, 1969) следует различать “острую” гибель клеток, как результат входа в постсинаптическую клетку катионов и воды, которая может быть кальций-независимой, и “отсроченную”, по сценарию апоптоза, которая является кальций-зависимой (см. обзор Freund, Buzsaki, 1996).
При височной эпилепсии (ВЭ) около трети пациентов резистентны к существующим методам лечения. Остаются особенно опасными генерализованные конвульсии, ассоциируемые с тяжелым состоянием и смертностью. В отдельных случаях, чтобы избежать судорожных приступов, единственным методом лечения является хирургическое удаление склеротических структур, прежде всего гиппокампа, что вызывает серьезные когнитивные расстройства. Современные методы лечения эпилепсии в основном основываются на симптоматических стратегиях, фармакологических или хирургических, при этом оба подхода направлены на подавление судорог, но не на эпилептогенез (Loscher, Schmidt, 2004). Эти факты указывают на низкий уровень понимания механизмов нарушений функционирования мозга, приводящих к генерации судорожной активности.
Для изучения механизмов развития ВЭ используются экспериментальные модели ВЭ у животных: каинатная, пилокарпиновая, а также модель киндлинга (раскачки). Каинатная и пилокарпиновая модели создаются введением животным нейротоксинов каиновой кислоты или литий-пилокарпина, соответственно, приводящим к развитию эпилептического статуса (продолжающихся несколько часов судорожных приступов, в которых различают иктальную, т.е. судорожную фазу, и интериктальную, межсудорожную); обычно это приводит к постепенному формированию патологического очага. Для моделирования ВЭ посредством киндлинга используют высокочастотную стимуляцию глутаматергических волокон, чаще всего перфорантного пути (см. обзор Morimoto et al., 2004).
Какую роль играет ЗИ в развитии височной эпилепсии, приводящей к серьезным нарушениям функционирования мозга? Выяснение этой роли может помочь пониманию механизмов развития этой опасной болезни и предотвращению ее возникновения.
Анатомические аномалии в зубчатой извилине при височной эпилепсии
Именно нарушение структуры ЗИ рассматривается некоторыми авторами как возможная причина начальных этапов развития этой патологии, поскольку ЗИ является основным входным звеном в гиппокамп со стороны возбуждающего энторинального входа, обеспечивающего распространение судорожной активности в поля СА3 и СА1 (McNamara, 1994). В нейронной сети ЗИ наиболее уязвимыми являются мшистые клетки. Причина их уязвимости, как предполагается, в основном обусловлена пресинаптическими механизмами: гигантские синапсы многочисленных гранулярных нейронов, заканчивающихся на мшистых клетках, могут выделять глутамат в больших концентрациях, что приводит к эксайтотоксичности (Buckmaster, Schwartzkroin, 1994; Sloviter, 1994; Scharfman, 1999; Scharfman, Myers, 2012). Пептиды, такие как нейротрофический фактор головного мозга (BDNF), которые находятся в плотных пузырьках внутри гигантских бутонов, могут способствовать высвобождению глутамата, усугубляя эту эксайтотоксичность (Scharfman, 2005). Кроме этого, мшистые клетки имеют низкие уровни δ-субъединицы ГАМКА-рецепторов, обычно способствующей ГАМКергическому ингибированию; это может сделать клетку уязвимой к токсическому влиянию глутамата (Tong et al., 2015).
У пациентов с ВЭ в хилусе обнаружена потеря части мшистых клеток (Blümcke et al., 1999). Уязвимость мшистых клеток рассматривается как ключевой фактор: эти нейроны в норме ведут себя как предохранители, и их быстрая гибель разрывает цепь после изменения электрической активности в сети ЗИ (Buckmaster, Schwartzkroin, 1994; Ratzliff et al., 2002). В чем заключается преобразование цепи ЗИ после гибели мшистых клеток? В норме мшистые нейроны хилуса ЗИ проецируются на ее интернейроны, которые, в свою очередь, образуют ГАМКергический вход к гранулярным клеткам; по этой причине гибель возбуждающих хилусных мшистых клеток может вызывать гипервозбудимость гранулярных нейронов (Sloviter, 1991b). Следовательно, утрата части мшистых клеток снижает их ингибирующее влияние на гранулы, опосредованное через тормозные интернейроны; как следствие, на гранулярных клетках образуются “свободные” синаптические локусы (ранее занятые синапсами, образованными ГАМКергическими окончаниями), в результате чего между ними формируются возвратные возбуждающие связи (McNamara, 1994). Позднее эта гипотеза была переоценена с использованием трансгенных мышей с токсин-опосредованной гибелью мшистых клеток. На этих животных было продемонстрировано, что обширное удаление мшистых клеток вызывало повышение возбудимости гранулярных нейронов, хотя само по себе отсутствие мшистых клеток недостаточно, чтобы вызвать клиническую эпилепсию (Jinde et al., 2013). Интересно, что, хотя у пациентов с ВЭ обнаруживается, как отмечалось выше, потеря мшистых клеток в хилусе (Blümcke et al., 1999), часть их остается сохранной (Seress et al., 2009); при этом выжившие мшистые клетки становятся про-эпилептогенными, за счет чрезмерного усиления активности проецирующихся на них гранулярных нейронов (Santhakumar et al., 2000; Ratzliff et al., 2002). Это свойство мшистых клеток легло в основу гипотезы о сверхчувствительных мшистых клетках (“irritable mossy cell”) как причине начала эпилептогенеза (Santhakumar et al., 2000). Кроме этого, накапливающиеся данные свидетельствуют о том, что эктопические гранулярные нейроны ненормально интегрированы и гипервозбудимы и, следовательно, могут способствовать генерации и распространению судорог (Scharfman et al., 2000; Dashtipour et al., 2001; Jung et al., 2004).
Другим морфологическим нарушением в ЗИ при ВЭ является гибель хилусных тормозных нейронов (Buckmaster, Jongen-Rêlo, 1999), проецирующихся к дистальным дендритам гранул, что, как предполагается, приводит к растормаживанию последних (Sloviter, 1987; 1991а, 1991б; Cossart et al., 2001; Sun et al., 2007; Sloviter et al., 2012).
Исследования на материале, взятом у пациентов с ВЭ, прошедших оперативное удаление гиппокампа, показали, что кроме утраты хилусных тормозных нейронов также гибнут интернейроны гранулярного и субгранулярного слоев, в основном корзинчатые клетки (Babb, 1987; de Lanerolle et al., 1992; Mathern et al., 1997). Подобные явления выявлены также в исследованиях на мышах, при этом частота судорожных событий у них коррелировала с гибелью интернейронов гранулярного слоя (но не парвальбумин-содержащих тормозных клеток) (Buckmaster et al., 2017). Следует отметить, что в интерпретации этих исследований проблемой является то, что клетки в основном идентифицируют путем окрашивания на клеточные маркеры, и о потере клеток судят чаще всего по изменению экспрессии этих маркеров, что не всегда соответствует действительной гибели нейронов. Это было постулировано, когда потеря парвальбумин-экспрессирующих клеток, к которым относятся корзинчатые и аксо-аксональные интернейроны, не сопровождалась потерей ГАМКергических контактов в этих локусах у пациентов с ВЭ, а в пилокарпиновой модели ВЭ у крыс предполагаемые контакты корзиночных клеток в ЗИ сохранялись (Obenaus et al., 1993; Wittner et al., 2001). Дальнейшее исследование иммунореактивности на парвальбумин в образцах ткани у пациентов с ВЭ, проведенное на световом и электронно-микроскопическом уровнях, показало, что парвальбумин-содержащие интернейроны в CA1 сохранялись до тех пор, пока пирамидные клетки CA1 (их мишени) не дегенерируют. Это исследование показало, что интернейроны, которые изначально экспрессируют парвальбумин, могут выживать, даже если иммунореактивность на парвальбумин исчезает. Кроме того, несмотря на многочисленные сообщения о потере парвальбумин-содержащих клеток, в пилокарпиновой модели у мышей гибли корзинчатые клетки, экспрессирующие холецистокинин, а парвальбумин-иммунореактивные корзинчатые клетки оставались сохранными (Wyeth et al., 2010). Таким образом, вопрос о том, какие интернейроны гибнут, а какие просто изменяются, и к каким функциональным последствиям это приводит, является пока не вполне решенным; на этот вопрос еще предстоит ответить в будущем.
В целом у пациентов с ВЭ и у животных на моделях эпилепсии в ЗИ обнаружено несколько анатомических аномалий. Во-первых, происходит спрутирование мшистых волокон, причем выявлено спрутирование двух типов: во внутреннем молекулярном слое (рис. 1) и в хилусе ЗИ; при этом в среднем один гранулярный нейрон у эпилептических крыс образует на 75% проекций больше по сравнению с контрольной группой животных (за счет формирования коллатералей) (Tauck, Nadler, 1985; Buckmaster, Dudek, 1999; Longo et al., 2003; Nadler, 2003; Buckmaster, 2012). Во-вторых, образуются входы возвратных коллатералей аксонов клеток CA3 на гранулах (Nadler et al., 1980; Sutula et al., 1989; Nadler, 2003; Sutula, Dudek, 2007; Scharfman, Myers, 2012). Выявлена также значительная потеря соматостатин-содержащих интернейронов в хилусе (Sloviter, 1987, 1991а, 1991б; Cossart et al., 2001; Sun et al., 2007; Sloviter et al., 2012).
Рис. 1.
Гиппокампальная формация в нормальном и эпилептическом мозге. (а) Гранулярный слой зубчатой извилины плотно упакован телами нейронов малого диаметра (гранулярными клетками). Прямо над слоем гранулярных клеток находится молекулярный слой (ml), который считается бесклеточным, поскольку он содержит апикальные дендриты гранул. Внешний молекулярный слой получает информацию от энторинальной коры через перфорантный путь (ПП). Аксоны гранулярных клеток (мшистые волокна) достигают хилуса и проецируются на мшистые клетки и пирамидные нейроны CA3. Аксоны мшистых клеток проецируются на дендриты гранулярных клеток во внутреннем молекулярном слое контралатерального гиппокампа. (б) В эпилептическом гиппокампе, где гибнут мишени аксонов гранулярных нейронов (мшистые клетки) в хилусе, аксоны гранулярных клеток прорастают и интенсивно иннервируют внутренний молекулярный слой зубчатой извилины – явление, называемое спрутингом мшистых волокон (показано пунктиром). Адаптировано из Cavarsan et al., 2018 (Open access).
Fig. 1. Hippocampal formation in the normal and epileptic brain. (а) The granular layer of the dentate gyrus is densely packed with small-diameter neuronal bodies (granular cells). Directly above the granular cell layer is the molecular layer (ml), which is considered acellular because it contains the apical dendrites of the granules. The outer molecular layer receives information from the entorhinal cortex via the perforant pathway (ПП). Granular cell axons (mossy fibers) reach the hilus and project onto mossy cells and CA3 pyramidal neurons. Mossy cell axons are projected onto granular cell dendrites in the inner molecular layer of the contralateral hippocampus. (б) In the epileptic hippocampus, where hilar mossy cells die, the axons of the granular neurons sprout and intensively innervate the inner molecular layer of the dentate gyrus, a phenomenon called sprouting of mossy fibers (shown by the dotted line). Adapted from Cavarsan et al., 2018 (Open access).
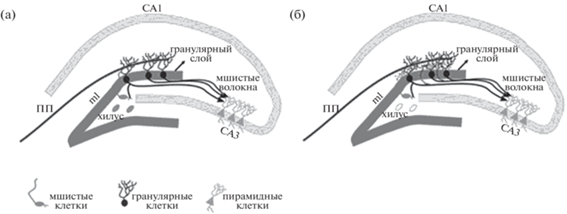
Кроме гибели нейронов при ВЭ обнаружены дисперсия гранулярного слоя и генерация эктопических гранулярных нейронов в хилусе (Houser et al., 1990; Parent et al., 2006). Обнаружены также синаптическая реорганизация гранулярных клеток (Houser et al., 1990; Babb et al., 1991) и астроглиоз (Van Paesschen et al., 1997; Das et al., 2012; Johnson et al., 2016).
Молекулярные внутрисинаптические нарушения в ЗИ при эпилептогенезе
Относительно качественных и количественных внутрисинаптических нарушений необходимо отметить, что в химических синапсах выявлены изменения субъединиц как ионотропных, так и метаботропных глутаматных рецепторов, а также ГАМКА- и ГАМКБ-рецепторов. Кроме этого, обнаружены изменения в электрических синапсах (щелевых контактах, gap junctions), как в нейрональных, так и в астроцитарных (см. обзор Ren, Curia, 2021). В этом отношении авторы отмечают некоторые разногласия в данных, полученных на пациентах, с одной стороны, и на моделях ВЭ у животных, с другой. Например, обнаружено возрастание мРНК и уровня белка АМПА-рецепторов у больных ВЭ, что редко выявляется на моделях у животных; такие же разногласия отмечаются в отношении ГАМКБ-рецепторов, которые возрастают в ЗИ у пациентов, однако этого не выявлено на моделях ВЭ у животных (см. обзор Ren, Curia, 2021). Причины такого несоответствия клинических и экспериментальных результатов пока неясны. В указанной обзорной работе отмечается, что в целом в ЗИ, как и в гиппокампе, при ВЭ наблюдается возрастание глутаматергических рецепторов наряду с общим снижением ГАМКергических ионотропных рецепторов и увеличением субъединиц щелевых контактов, что вызывает дисбаланс между возбуждением и торможением. В отдельных областях гиппокампа при эпилептогенезе было показано возрастание субъединиц ГАМК-рецепторов по сравнению со здоровым гиппокампом, в чем можно увидеть “безуспешную” попытку противодействия гипервозбудимости нейронной сети во время эпилептогенеза (Ren, Curia, 2021).
Нарушения электрической активности
Изменение структуры ЗИ приводит к изменению возбудимости гранулярных нейронов: если в нормальных условиях гранулы демонстрируют низкий уровень возбудимости, что эффективно ограничивает возбуждение пирамидных клеток и сдерживает поток активности в пределах гиппокампа (Coulter, Carlson, 2007; Hsu, 2007), то после ЭС гранулярные нейроны становятся гипервозбудимыми (Sloviter, 1987, 1991b; Harvey, Sloviter, 2005), что снижает их способность контролировать распространение возбуждающих сигналов из неокортекса. Синаптическая реорганизация в ЗИ и образование входов возвратных коллатералей аксонов клеток CA3 на гранулах также существенно изменяют возбудимость гранулярных нейронов (Nadler, 2003; Sutula, Dudek, 2007; Scharfman, Myers, 2012). Кроме этого, образование в хилусе ЗИ эктопических гранул (Houser, 1990; Parent, et al., 2006) вызывает формирование из них сверх-связанных возбуждающих локусов (Cameron et al., 2011; Scharfman, Pierce, 2012). Учитывая эти факты, было предложено рассматривать ЗИ как структуру, выполняющую в норме фильтрующую (“gate”) функцию, – явление, получившее название “dentate gating” (Lothman et al., 1992), ограничивающее возбуждение пирамидных клеток гиппокампа. Нарушение этой функции считается рядом авторов потенциальным механизмом для генерации иктальных и интериктальных событий у людей и животных (Heinemann et al., 1992; Houser, 1992; Hsu, 2007; но см. Ratzliff et al., 2004; Howard et al., 2007). Интересно, что оптогенетическое повышение или подавление активности гранулярных нейронов может спровоцировать или подавить судорожные приступы соответственно (Krook-Magnuson et al., 2015).
В исследовании, проведенном на срезах гиппокампа, взятого после операции у пациентов с лекарственно-устойчивой ВЭ, при регистрации активности в ЗИ было выявлено, что низкочастотная стимуляция хилуса ЗИ склеротического гиппокампа вызывает судорожно-подобные события при незначительном повышении концентрации внеклеточного калия; в то же время в несклеротическом гиппокампе пациентов с диагнозом ВЭ для этого требовалось более значительное повышение концентрации калия (Gabriel et al., 2004); это свидетельствует о том, что при ВЭ возбудимость в ЗИ возрастает.
В срезах склеротического гиппокампа, взятых от пациентов с фармакорезистентной ВЭ, при сравнении активности в полях СА1-СА3 и ЗИ было выявлено, что ЗИ обнаруживает наибольшую склонность к генерации эпилептиформно-подобных эпизодов, вызываемых повышением концентрации внеклеточного калия и стимуляцией хилуса (Reyes-Garcia et al., 2018). На основании этих и более ранних результатов (Beck et al., 1996; Wittner et al., 2001; Gabriel et al., 2004) предполагается, что такие патологические события являются результатом реорганизации нейронной сети в склеротическом гиппокампе человека, а именно спрутинга мшистых волокон (см. рис. 1), а также возникновения калиевого тока (Ik); эти события считаются важными факторами при генерации популяционных ответов, проявляющих признаки гипервозбуждения (Reyes-Garcia et al., 2018).
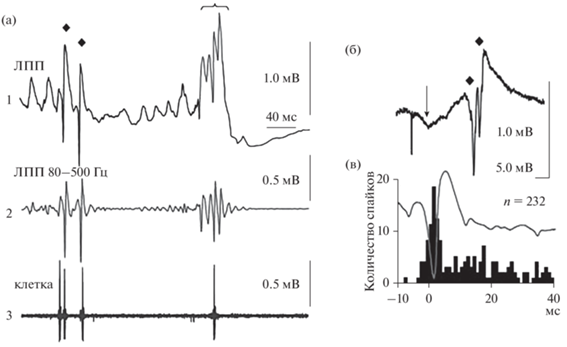
Интересно, что в нормальном гиппокампе человека во время медленноволнового сна регистрируются физиологические относительно высокочастотные (риппл)-осцилляции с частотой 80–200 Гц; обычно они приурочены к определенной фазе медленных волн (от пика до впадины) (Frauscher et al., 2015; von Ellenrieder et al., 2016; Song et al., 2017; Weiss et al., 2020) и важны для консолидации памяти, осуществляемой в покое и во сне (Axmacher et al., 2008). В эпилептическом мозге частота риппл-осцилляций увеличивается (Jacobs et al., 2008, 2016). У пациентов с ВЭ во время предоперационной оценки судорожного очага с помощью внутримозговых записей посредством микроэлектродов показано, что в ЗИ регистрируются патологические высокочастотные (или быстрые) риппл-осцилляции (пВЧО) (200–600 Гц) (Bragin et al., 1999a, 1999b, 2011; Staba et al., 2002). Показано также, что в гиппокампе они связаны с другой фазой медленных волн (от впадины до пика) (Weiss et al., 2020). У грызунов в норме в ЗИ не регистрируются быстрые риппл-осцилляции; поэтому при создании модели ВЭ появление осцилляций на частоте выше 100 Гц рассматривалось как патологические эпилептиформные события, т.е. это являлось маркером эпилептогенеза (Bragin et al., 2011; Csicsvari et al., 2003). Во время быстрых риппл-осцилляций полевой потенциал представлял собой популяционные спайки, состоящие из суммы нейронных спайков ЗИ (Bragin et al., 2011). В этой работе на пилокарпиновой модели ВЭ у эпилептических мышей, анестезированных уретаном и кетамином и находящихся в стереотаксическом аппарате, регистрировали активность идентифицированных гранулярных клеток и интернейронов ЗИ при одновременной регистрации пВЧО. Гранулярные клетки разряжались преимущественно синхронно с пВЧО и одиночными популяционными спайками (рис. 2), в то время как интернейроны уменьшали частоту разрядов (Bragin et al., 2011).
Рис. 2.
Разряды идентифицированных гранулярных клеток зубчатой извилины во время одиночных популяционных спайков и патологических высокочастотных осцилляций (пВЧО). (а) Пример двух одиночных популяционных спайков (ромбы) и пВЧО (фигурная скобка), сопровождаемых разрядами гранулярной клетки. A1 – экспериментальные (сырые) записи в частотном диапазоне 0.1 Гц–5.0 кГц. A2 – те же данные, отфильтрованные в полосе частот 200–500 Гц. A3 – разряды гранулярной клетки, зарегистрированной стеклянным микроэлектродом, расположенным на расстоянии 200 мкм от вольфрамового микроэлектрода для регистрации полевых потенциалов на A1. (б) Вызванный полевой потенциал в ответ на стимуляцию перфорантного пути. Вслед за популяционным ВПСП, обозначенным стрелкой, следуют два популяционных спайка (ромбы). (в) Гистограмма разрядов гранулярной клетки (черный цвет) во время 232 популяционных спайков (серый цвет), где “0” – начало популяционного спайка. Адаптировано из: Bragin et al., 2011 (Full access).
Fig. 2. Discharges of identified granular cells of the dentate gyrus during single population spikes and pathological high-frequency oscillations (pHFO). (а) An example of two single population spikes (rhombuses) and pHPO (curly brace), accompanied by granular cell discharges. A1. Experimental (raw) data recorded with 0.1 Hz–5.0 kHz frequency band. A2. The same data filtered in the 200–500 Hz frequency band. A3. Discharges of a granular cell recorded by the glass microelectrode located 200 μm from the tungsten microelectrode which recorded the field potentials in A1. (б) An evoked field potential in response to perforant path stimulation. The beginning of a population EPSP indicated by the arrow is followed by two population spikes (diamonds). (в) Histogram of granular cell discharges (black) during 232 population spikes (gray), where “0” is the beginning of the population spike. Adapted from: Bragin et al., 2011 (Full access).
Кроме введения пилокарпина для создания моделей ВЭ животным вводят другие нейротоксины или применяют высокочастотную стимуляцию возбуждающих волокон, что инициирует эпилептический статус (ЭС). В течение нескольких часов или дней после ЭС в ЗИ наблюдались активация нейрогенеза (Parent et al., 1997; Scott et al., 2000; Covolan et al., 2000; Shapiro et al., 2007; Hester, Danzer, 2013; Bielefeld et al., 2014), продукция эктопических гранулярных клеток (Dudek, 2004; Pierce et al., 2005, 2007; Parent et al., 2006; Scharfman et al., 2007) и базальных дендритов (Spigelman et al., 1998; Avanzi et al., 2010; Sanchez et al., 2012; Kelly, Beck, 2017). Поздними изменениями в ЗИ, которые могут по времени совпадать с началом спонтанных припадков, являются синаптическая реорганизация (Sloviter, 1999; Kienzler et al., 2009; Zhang et al., 2014) и дисперсия гранулярных клеток (Houser, 1990; Mello et al., 1992; Lowenstein, 2001; Jessberger et al., 2005; El Bahh et al., 2008).
Роль спрутинга мшистых волокон в развитии височной эпилепсии
Одно из самых заметных явлений, наблюдающихся при эпилептогенезе, – прорастание, или спрутинг, мшистых волокон (СМВ) во внутренний молекулярный слой ЗИ (Cavarsan et al., 2018) (рис. 1). СМВ происходит в две фазы: (1) повреждения клеток вызывают повышение нейрональной активности и высвобождение ростовых факторов (Ikegaya et al., 1999; Binder et al., 2001; Koyama et al., 2004); и (2) рост аксонов и образование аксонных коллатералей у гранулярных клеток (Bekirov et al., 2008; Shibata et al., 2013; Song et al., 2015). Для выявления аномальных содержащих цинк терминалей во внутреннем молекулярном слое ЗИ окрашивание по методу Тимма считается “золотым стандартом”; однако недавние данные показали, что СМВ можно отслеживать in vivo в МРТ-исследованиях (Nairismägi et al., 2006; Malheiros et al., 2015). Предполагается, что спрутинг происходит, во-первых, из-за образования вакантных синаптических сайтов (Longo et al., 2003) на проксимальных дендритах гранулярных клеток, вызванного гибелью нейронов хилуса после повреждающего воздействия (Sloviter, 1987), и, во-вторых, подавлением хемопеллентов, таких как Sema3A (Holtmaat et al., 2003). Sema3A в норме секретируется в аксонах клеток энторинальной коры, проецирующихся в молекулярный слой ЗИ; как предполагается, этот путь в норме активен в гиппокампальной формации (см. обзор Tamagnone, Comoglio, 2000), но не активен после ЭС. Это говорит о том, что подавление хемопеллентов, таких как Sema3A, может действовать как молекулярный элемент, способствующий формированию возвратных проекций мшистых волокон во внутренний молекулярный слой ЗИ после ЭС.
В настоящее время оспариваются причины (Schmeiser et al., 2017) и роль спрутинга при ВЭ (Elmer et al., 1997; Nissinen et al., 2001). Вопрос о том, является ли СМВ эпилептогенным или адаптивным процессом, остается спорным. Первоначально СМВ описывали как результат образования возвратных возбуждающих проекций гранулярных клеток (Tauck, Nadler, 1985), вызванного гибелью их мишеней, в частности, гибелью клеток хилуса, которая является одной из первых находок экспериментальных моделей ВЭ (Mello, Covolan, 2009). Однако нет четких доказательств того, что потеря именно мшистых клеток, а не других нейронов хилуса запускает СМВ (Gorter et al., 2001), и, хотя реорганизация внутригиппокампальной сети может быть причиной эпилептиформной активности в гиппокампе, некоторые исследования показывают, что СМВ не обязательно связан с возникновением спонтанных судорожных припадков (Heng et al., 2013). Переоцененная в 1990-х годах “гипотеза прорастания мшистых волокон” (McNamara, 1994) утверждает, что увеличение возбудимости гранулярных клеток является следствием патологической перестройки нейронных цепей, в которых возбуждающие гранулярные нейроны иннервируют сами себя, “выстраивая” возвратные возбуждающие сети. Эта гипотеза подтвердилась многими фактами, некоторые из которых описаны ниже. Доказательства проэпилептогенной роли СМВ включают, в частности, его присутствие примерно у 60% пациентов с ВЭ (Sutula et al., 1989; Isokawa et al., 1993) и у животных в моделях эпилепсии (Cronin et al., 1992; Mathern et al., 1993; Wuarin, Dudek, 2001). Кроме этого, электронно-микроскопические исследования показывают, что окончания проросших мшистых волокон образуют асимметричные (возбуждающие) контакты с дендритными шипиками гранул (Represa et al., 1993). Получены также электрофизиологические данные, подтверждающие эту гипотезу. На срезах гиппокампа крыс производилась парная стимуляция ПП (с задержкой 40 мс) и регистрировались ответы в гранулярном слое. В срезах, взятых от нормальных животных, на первый стимул наблюдались популяционные ВПСП и популяционные спайки, в то время как на второй стимул последний компонент отсутствовал, вероятно, в результате включения возвратного торможения. Однако в срезах, взятых от крыс с каинатной моделью ВЭ, в ответах присутствовали оба компонента; это означает, что гранулярные нейроны расторможены, т.е. гипервозбудимы. Эти результаты коррелировали с наличием устойчивого СМВ в гиппокампальных срезах (Tauck, Nadler, 1985). Все это позволяет предположить, что аберрантный СМВ связан с утратой фильтрующей (“gate”), т.е. защитной, функции ЗИ. Похожие результаты были получены в срезах, взятых от животных с введением каината, когда антидромная стимуляция гранулярных клеток вызывала судорожно-подобные залпы потенциалов действия (Cronin et al., 1992; Wuarin, Dudek, 2001). Важное свидетельство проэпилептогенной роли СМВ было продемонстрировано при киндлинге – модели ВЭ, в которой степень прорастания мшистых волокон увеличивалась с возрастанием количества судорожных приступов (Cavazos et al., 1991).
Хотя СМВ положительно коррелирует с потерей мшистых клеток у пациентов с ВЭ (Schmeiser et al., 2017b) и на моделях ВЭ у животных (Pierce et al., 2005; Polli et al., 2014), уместен вопрос: необходим и достаточен ли этот процесс для возникновения судорожных приступов? Существуют данные, показывающие, что СМВ может быть вызван экспериментально без судорог, в результате длительной потенциации (Adams, Lee, 1997) или повреждения ПП (Zimmer, 1973, 1974), а также генетической мутацией (Colling et al., 1997). После электростимуляции миндалины у некоторых животных развивались судороги, но отсутствовал СМВ (Nissinen et al., 2001). В пилокарпиновой и каинатной моделях наличие и интенсивность СМВ положительно коррелировали с количеством спонтанных судорожных припадков и степенью гибели гранулярных клеток ЗИ и пирамидных нейронов в полях СА1 и СА3, но не в хилусе ЗИ (Polli et al., 2014). Эти результаты согласуются с предыдущими данными (Liu et al., 2005; Mitsueda-Ono et al., 2013) и указывают, что, несмотря на важность СМВ, он может развиваться независимо от гибели клеточных мишеней мшистых волокон (Ratzliff et al., 2002); он присутствует у животных со спонтанными судорожными приступами, но его наличие не обязательно связано с их возникновением (Nissinen et al., 2001). Соответственно, хотя у животных, проявляющих большее количество судорог, наблюдается большее количество цинксодержащих терминалей, ультраструктурный анализ молекулярного слоя ЗИ не показал повышенного числа возбуждающих синапсов, что поддерживает идею о том, что СМВ связан с заменой или восстановлением утраченных контактов, а не с повышением возбудимости (Buckmaster, 2014; Bittencourt et al., 2015), т.е. что он является адаптивным процессом. Кроме этого, эксперименты с манипулированием СМВ показали, что введение рапамицина успешно подавляет СМВ, но дает противоречивые результаты в отношении развития эпилепсии (Zeng et al., 2009; Buckmaster, Lew, 2011). Некоторые другие данные указывают на то, что СМВ – активное явление, возможно, нормальный репаративный механизм, который может в определенных условиях становиться патогенным (Buckmaster, 2014; Yamawaki et al., 2015).
Таким образом, относительно роли спрутинга мшистых волокон в развитии ВЭ в литературе нет единого мнения; эти противоречия, вероятно, будут разрешены в будущем.
Роль нейрогенеза в развитии височной эпилепсии
Выявлено, что при судорогах в гранулярном слое ЗИ усиливается нейрогенез, сохраняющийся в мозге взрослых млекопитающих (Cameron et al., 1993; Parent et al., 1997; Bengzon et al., 1997). Гранулярные клетки гиппокампа, образовавшиеся за несколько недель до и после эпилептогенного повреждения мозга, могут аномально интегрироваться со сформированной сетью ЗИ, потенциально опосредуя эпилептогенез в височной доле. Вполне вероятно также, что продолжение нейрогенеза может приводить к увеличению СМВ, который дополнительно усиливается последующими спонтанными судорогами и вызывает повышенную возбудимость в ЗИ (но см. Zeng et al., 2009; Buckmaster, Lew, 2011).
Показано, что после индуцированного пилокарпином ЭС как новорожденные, так и зрелые гранулы вносят свой вклад в прорастание и аберрантную реорганизацию мшистых волокон (Parent et al., 1997, 1999). У взрослых грызунов через несколько недель после введения пилокарпина, вызывающего ЭС, предполагаемые предшественники гранулярных нейронов напоминают “эктопические” гранулоподобные клетки, обнаруженные в образцах гиппокампа, взятых от людей с ВЭ (Houser, 1990); они встречаются до 21 месяцев после ЭС, вызванного конвульсантом (Scharfman et al., 2000). Показано, что эпилептические припадки приводят не только к СМВ, но и к гипертрофии нейронов и проецированию базальных дендритов хилусных нейронов ко вновь рожденным гранулярным клеткам (Parent et al., 1997; Scharfman et al., 2002; Pun et al., 2012). С другой стороны, нейроны, рожденные за 1 неделю до эпилептогенного повреждения, могут создавать аберрантные дендритные проекции в хилусе ЗИ (Walter et al., 2007), тогда как клетки, рожденные за 1 месяц до инсульта, способствуют аберрантному разрастанию аксонов во внутренний молекулярной слой (Kron et al., 2010). Таким образом, эти морфологические аномалии создают de novo возвратные возбуждающие петли внутри ЗИ – процесс, гипотетически способствующий эпилептогенезу (Jessberger, Parent, 2015).
Однако относительно роли нейрогенеза в развитии ВЭ в литературных данных имеются противоречия; в одних работах показано, что ингибирование нейрогенеза гиппокампа у взрослых особей после острых судорожных приступов приводило к уменьшению их количества (Jung et al., 2004, 2006; Cho et al., 2015), в то время как другие исследования указывали на то, что блокирование нейрогенеза у взрослых с помощью облучения в малых дозах не меняло ступенчатого прогрессирования киндлинга (Pekcec et al., 2011) или даже немного усиливало его (Raedt et al., 2007). Позднее эти результаты были дополнены посредством удаления после эпилептогенного инсульта новых гранулярных клеток при использовании стратегии экспрессии рецептора дифтерийного токсина у мышей. Экспрессия этого рецептора была индуцирована у гранулярных клеток, рожденных за 5 недель до вызванного пилокарпином эпилептического статуса; затем, через 3 дня после эпилептогенного воздействия, эти клетки удалялись. Это воздействие привело к сокращению частоты судорог на 50%. Но когда животных обследовали через 2 месяца после этой процедуры, то было обнаружено не только сокращение частоты приступов, но также увеличение их продолжительности на 20%. Авторы предполагают, что этот парадоксальный эффект может отражать нарушение гомеостатических механизмов, которые при частых припадках обычно сокращают их продолжительность. В целом, эти данные, подтверждая давнюю гипотезу о том, что вновь образованные гранулярные клетки являются проэпилептогенными и имеют значение в возникновении судорог, вносят дополнение в представление о том, что процедура устранения вновь рожденных гранулярных клеток, применяемая в клинически значимый момент времени после эпилептогенного инсульта, может иметь модифицирующие болезнь эффекты в развитии эпилепсии (Hosford et al., 2016).
Сообщалось также, что гранулярные нейроны, рождающиеся после эпилептогенных воздействий, демонстрируют различные уровни возбудимости (Cameron et al., 1993; Scharfman et al., 2000; Jakubs et al., 2006; Thind et al., 2008; Zhan et al., 2010; Ribak et al., 2012; Myers et al., 2013). Хилусные эктопические гранулы получают больше возбуждающих сигналов и повышают возбудимость гиппокампа (Cameron et al., 1993; Scharfman et al., 2000; Zhan et al., 2010; Myers et al., 2013), тогда как нейроны в гранулярном слое либо демонстрируют пониженную возбудимость (Jakubs et al., 2006), либо получают чрезмерное возбуждение (Thind et al., 2008; Ribak et al., 2012). Более того, на трансгенных мышах с использованием оптогенетических методов недавно было продемонстрировано, что, несмотря на наличие СМВ гранулярных клеток, рожденных после ЭС, образованные ими синапсы не были функционально активны и не могли вызывать возвратное возбуждение (Hendricks et al., 2017). Таким образом, эти эксперименты противоречат выводам, сделанным в более ранней работе (Hosford et al., 2016).
Нарушение когнитивных функций при ВЭ
ЗИ имеет фундаментальное значение для когнитивных функций, осуществляемых гиппокампом. Одним из нарушений, сопутствующих ВЭ, является когнитивный дефицит, который в повседневной жизни не менее опасен, чем сами судороги (Holmes, 2013). Тем не менее нейронные механизмы такого дефицита не совсем понятны. Многочисленные работы на грызунах показали, что при ВЭ нарушается, в частности, пространственная дискриминация (Gilbert et al., 2001; Clelland et al., 2009; Nakashiba et al., 2012; Kheirbek et al., 2013), что влечет за собой дефицит эпизодической памяти при ВЭ (Burgess et al., 2002; Tulving, 2002; Inostrosa et al., 2013). Как отмечалось выше, в ЗИ при эпилептогенезе наблюдается резкое повышение возбудимости (Dengler et al., 2016); при этом повышение или подавление активности гранулярных нейронов с помощью оптогенетических методов может спровоцировать или подавить судорожные приступы соответственно (Krook-Magnuson et al., 2015). В недавней работе на мышах (Kahn et al., 2019) на пилокарпиновой модели ВЭ было обнаружено, что гранулярные клетки ЗИ проявляют чрезмерную возбудимость и мыши не справляются с ЗИ-зависимой задачей пространственной дискриминации. В этой работе использовали хемогенетический метод (DREADDs, designer receptors exclusively activated by designer drugs) (Roth, 2016), дающий возможность снизить гиперактивность гранулярных клеток, что позволяло восстановить поведенческие характеристики; в этом случае эпилептические животные осуществляли задачу так же, как контрольные мыши дикого типа. Кроме того, создание гипервозбудимости гранулярных клеток у контрольных мышей посредством возбуждения хемогенетических рецепторов также приводило к дефициту пространственной памяти, наблюдаемой у мышей с эпилепсией. Однако при чрезмерно сниженной возбудимости гранулярных клеток животные как с эпилепсией, так и контрольные снова обнаруживали нарушенные поведенческие характеристики. Эти двунаправленные манипуляции показывают, что для гранулярных нейронов существует оптимальное окно возбудимости, которое необходимо для успешного осуществления когнитивных функций (Kahn et al., 2019).
ЗАКЛЮЧЕНИЕ
Факты, указывающие на решающую роль ЗИ в развитии височной эпилепсии и нарушениях функционирования мозга при ВЭ, достаточно убедительны. Являясь основным входным звеном в гиппокамп со стороны глутаматергического неокортикального входа, ЗИ регулирует возбудимость пирамидных нейронов гиппокампа и предохраняет их от генерации патологической активности. Спрутинг мшистых волокон, возможно, вносит вклад в развитие гипервозбудимости гранулярных клеток, при этом повышенная скорость нейрогенеза после судорог может приводить к увеличению СМВ. Однако вопрос о том, является ли СМВ эпилептогенным или адаптивным процессом, остается спорным, так же как и вопрос о роли самого нейрогенеза в развитии ВЭ. Таким образом, вопрос о том, какие изменения в ЗИ играют решающую роль в эпилептогенезе: гибель тормозных интернейронов (и каких именно), потеря мшистых клеток, образование на гранулах синапсов коллатералями возвратных аксонов клеток CA3, спрутинг мшистых волокон, аномальное встраивание вновь рожденных и “молодых” нейронов в гиппокампальную сеть, – этот вопрос пока не решен. Наиболее вероятно, что все эти изменения в комплексе, а не какое-то одно из них, приводят к развитию эпилепсии. Более того, существующая гипотеза о нарушении фильтрующей (“gate”) функции ЗИ, рассматриваемая долгое время в качестве основной причины эпилептогенеза, еще не получила окончательной экспериментальной поддержки; в этом отношении также существуют сомнения. Относительно качественных и количественных внутрисинаптических нарушений в ЗИ при височной эпилепсии существуют разногласия в данных, полученных на пациентах и на моделях ВЭ у животных, причины которых пока неясны.
Прогресс новых технологий (оптогенетических, визуализационных подходов, магнитоэнцефалографии) поможет в будущем решить эти вопросы.
Список литературы
Карлов В.А. Эпилепсия как клиническая и нейрофизиологическая проблема. Журнал неврологии и психиатрии. 2000. 100 (9): 7–15.
Adams B., Lee M., Fahnestock M., Racine R. Long-term potentiation trains induce mossy fiber sprouting. Brain Res. 1997. 775: 193–7.
Axmacher N., Elger C.E., Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain J Neurol. 2008. 131: 1806–17.
Avanzi R.D., Cavarsan C.F., Santos J.G. Jr., Hamani C., Mello L.E., Covolan L. Basal dendrites are present in newly born dentate granule cells of young but not aged pilocarpine-treated chronic epileptic rats. Neuroscience. 2010. 170: 687–91.
Babb T.L., Brown W.J. Pathological findings in epilepsy. Surgical treatment of the epilepsies. Ed. Engel. J.Jr. New York: Raven Press, 1987. 511–540 pp.
Babb T.L., Kupfer W.R., Pretorius J.K., Crandall P.H., Levesque M.F. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991. 42: 351–363.
Beck H., Blümcke I., Kral T., Clusmann H., Schramm J., Wiestler O.D., Heinemann U., Elger C.E. Properties of a delayed rectifier potassium current in dentate granule cells isolated from the hippocampus of patients with chronic temporal lobe epilepsy. Epilepsia. 1996. 37: 892–901.
Bekirov I.H., Nagy V., Svoronos A., Huntley G.W., Benson D.L. Cadherin-8 and N-cadherin differentially regulate pre- and postsynaptic development of the hippocampal mossy fiber pathway. Hippocampus. 2008. 18: 349–63.
Bengzon J., Kokaia Z., Elmer E., Nanobashvili A., Kokaia M., Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc. Natl. Acad. Sci. U S A 1997. 94: 10432–10437.
Bielefeld P., van Vliet E.A., Gorter J.A., Lucassen P.J., Fitzsimons C.P. Different subsets of newborn granule cells: a possible role in epileptogenesis? Eur. J. Neurosci. 2014. 39(1): 1–11.
Binder D.K., Croll S.D., Gall C.M., Scharfman H.E. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001. 24: 47–53.
Bittencourt S., Covolan L. C.H., Hamani C., Longo B., Faria F., Freymuller E., Freymuller E., Ottersen O.P., Mello L.E. Replacement of asymmetric synaptic profiles in the molecular layer of dentate gyrus following cycloheximide in the pilocarpine model in rats. Front Psychiatry. 2015. 6: 157.
Blümcke I., Zuschratter W., Schewe J.C., Suter B., Lie A.A., Riederer B.M., Meyer B., Schramm J., Elger C.E., Wiestler O.D. Cellular pathology of hilar neurons in Ammon’s horn sclerosis. The Journal of Comparative Neurology. 1999. 414: 437–453.
Bragin A., Benassi S.K., Kheiri F., Engel J. Jr. Further evidence that pathological high frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia. 2011. 52(1): 45–52.
Bragin A., Engel J.Jr., Wilson C.L., Fried I., Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999a. 9: 137–142.
Bragin A., Engel Jr.J., Wilson C.L., Fried I., Mathern G.W. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999b. 40: 127–137.
Bragin A., Jandó G., Nádasdy Z., van Landeghem M., Buzsáki G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J. Neurophysiol. 1995. 73: 1691–1705.
Buckmaster P.S. Mossy cell dendritic structure quantified and compared with other hippocampal neurons labeled in rats in vivo: Epilepsia. 2012. 53 (Suppl 1): 9–17.
Buckmaster P. Does mossy fiber sprouting give rise to the epileptic state? Issues in Clinical Epileptology: A View From the Bench. Advances in Experimental Medicine and Biology. Ed. Scharfman H., Buckmaster P. Dordrecht: Springer, 2014. 161–168 pp.
Buckmaster P.S., Abrams E., Wen X. Seizure frequency correlates with loss of dentate gyrus GABAergic neurons in a mouse model of temporal lobe epilepsy. J Comp Neurol. 2017. 525 (11): 2592–2610.
Buckmaster P.S., Dudek F.E. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J. Neurophysiol. 1997. 77: 2685–2696.
Buckmaster P.S., Lew F.H. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J. Neurosci. 2011. 31: 2337–2347.
Buckmaster P.S., Jongen-Rêlo A.L. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J. Neurosci. 1999. 19(21): 9519–9529.
Buckmaster P.S., Strowbridge B.W., Kunkel D.D., Schmiege D.L., Schwartzkroin P.A. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus. 1992. 2: 349–362.
Buckmaster P.S., Schwartzkroin P.A. Hippocampal mossy cell function: a speculative view. Hippocampus. 1994. 4: 393–402.
Burgess N., Maguire E., O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron 2002. 35: 625–641.
Cameron H.A., Woolley C.S., McEwen B.S., Gould E. Differentiationof newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993. 56: 337–344.
Cameron M.C., Zhan R., Nadler J.V. Morphologic integration of hilar ectopic granule cells into dentate gyrus circuitry in the pilocarpine model of temporal lobe epilepsy. The Journal of Comparative Neurology. 2011. 519: 2175–2192.
Cavarsan C.F., Malheiros J., Hamani C., Najm I., Covolan L. Is Mossy Fiber Sprouting a Potential Therapeutic Target for Epilepsy? Front. Neurol. 2018. 9: 1023.
Cavazos J.E., Golarai G., Sutula T.P. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci. 1991. 11(9): 2795–2803.
Cho K.-O., Lybrand Z.R., Ito N., Kelly R., Tafacory F., Zhang L., Good L., Ure K., Kernie S.G., Birnbaum S.G., Scharfman H.E., Eisch A.J., Hsieh J. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun. 2015. 6: 6606.
Colling S., Khana M., Collinge J., Jefferys J. Mossy fibre reorganization in the hippocampus of prion protein null mice. Brain Res. 1997. 755: 28–35.
Cossart R., Dinocourt C., Hirsch J.C., Merchan-Perez A., De F.J., Ben-Ari Y., Esclapez M., Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001. 4: 52–62.
Coulter D.A., Carlson G.C. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res. 2007. 163: 235–243.
Covolan L., Ribeiro L.T., Longo B.M., Mello L.E. Cell damage and neurogenesis in the dentate granule cell layer of adult rats after pilocarpine- or kainate-induced status epilepticus. Hippocampus. 2000. 10(2): 169–180.
Cronin J., Obenaus A., Houser C.R., Dudek F.E. Electrophysiology of dentate granule cells after kainate-induced synaptic reorganization of the mossy fibers. Brain Res. 1992. 573(2): 305–310.
Das A., Wallace G.C., Holmes C., McDowell M.L., Smith J.A., Marshall J.D., Bonilha L., Edwards J.C., Glazier S.S., Ray S.K., Banik N.L. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience. 2012. 220: 237–246.
Dashtipour K., Tran P.H., Okazaki M.M., Nadler J.V., Ribak C.E. Ultrastructural features and synaptic connections of hilar ectopic granule cells in the rat dentate gyrus are different from those of granule cells in the granule cell layer. Brain Res. 2001. 890: 261–271.
de Lanerolle N.C., Brines M.L., Kim J.H., Williamson A., Philips M.F., Spencer D.D. Neurochemical remodeling of the hippocampus in human temporal lobe epilepsy. Ed. Engel J.Jr., Wasterlain C., Cavalheiro E.A., Heinemann U., Avanzini G. Epilepsy Res. (Suppl. 9). Amsterdam: Elsevier Science, 1992. 205–220 pp.
Dengler C.G., Coulter D.A. Normal and epilepsy-associated pathologic function of the dentate gyrus. Prog Brain Res. 2016. 226: 155–78.
Dudek F. Seizure-induced neurogenesis and epilepsy: involvement of ectopic granule cells? Epilepsy Curr. 2004. 4: 103–104.
El Bahh B., Lespinet V., Lurton D., Coussemacq M., Le Gal La Salle G., RougierA. Correlations between granule cell dispersion, mossy fiber sprouting, and hippocampal cell loss in temporal lobe epilepsy. Epilepsia. 1999. 40: 1393–1401.
Elmer E., Kokaia Z., Kokaia M., Lindvall O., McIntyre D. Mossy fibre sprouting: evidence against a facilitatory role in epileptogenesis. Neuroreport. 1997. 8: 1193–1196.
Engel Jr.J. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001. 42: 796–803.
Gabriel S., Njunting M., Pomper J.K., Merschhemke M., Sanabria E.R.G., Eilers A., Kivi A., Zeller M., Meencke H.-J., Cavalheiro E.A., Heinemann U., Lehmann T.-N. Stimulus and potassium-induced epileptiform activity in the human dentate gyrus from patients with and without hippocampal sclerosis. J. Neurosci. 2004. 24: 10416–10430.
Gilbert P.E., Kesner R.P., Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001. 11: 626–636.
Gorter J., van Vliet E., Aronica E., Lopes da Silva F. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatinimmunoreactive neurons. Eur. J. Neurosci. Biobehav. Rev. 2001. 13: 657–669.
Haglid K.G., Wang S., Qiner Y., Hamberger A. Excitotoxicity. Experimental correlates to human epilepsy. Mol. Neurobiol. 1994. 9: 259–263.
Harvey B.D., Sloviter R.S. Hippocampal granule cell activity and c-Fos expression during spontaneous seizures in awake, chronically epileptic, pilocarpine-treated rats: implications for hippocampal epileptogenesis. J Comp Neurol. 2005. 488: 442–463.
Heinemann U., Beck H., Dreier J.P., Ficker E., Stabel J., Zhang C.L. The dentate gyrus as a regulatedgate for the propagation of epileptiform activity. Epilepsy Res. 1992. Suppl 7: 273–280.
Hendricks L., Chen Y., Bensen A., Westbrook G., Schnell E. Short-term depression of sprouted mossy fiber synapses from adult-born granule cells. J Neurosci. 2017. 37: 5722–5735.
Heng K., Haney M., Buckmaster P. High-dose rapamycin blocks mossy fiber sprouting but not seizures in a mouse model of temporal lobe epilepsy. Epilepsia. 2013. 54: 1535–1541.
Hester M.S., Danzer S.C. Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci. 2013. 33: 8926–8936.
Houser C.R. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Research. 1990. 535: 195–204.
Houser C.R. Morphological changes in the dentate gyrus in human temporal lobe epilepsy. Epilepsy Res. 1992. Suppl 7: 223–234.
Holtmaat A., Gorter J., De Wit J., Tolner E., Spijker S., Giger R., Lopes da Silva F.H., Verhaagen J. Transient downregulation of Sema3A mRNA in a rat model for temporal lobe epilepsy. A novel molecular event potentially contributing to mossy fiber sprouting. Exp Neurol. 2003. 182: 142–150.
Hosford B.E., Liska J.P., Danzer S.C. Ablation of newly generated hippocampal granule cells has disease-modifying effects in epilepsy. J. Neurosci. 2016. 36 (43): 11013–11023.
Hsu D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog Brain Res. 2007. 163: 601–613.
Ikegaya Y. Abnormal targeting of developing hippocampal mossy fibers after epileptiform activities via L-type Ca2+ channel activation in vitro. J. Neurosci. 1999. 19: 802–812.
Inostroza M., Brotons-Mas J.R., Laurent F., Cid E., de la Prida L.M. Specific impairment of ‘‘what-where-when’’ episodic-like memory in experimental models of temporal lobe epilepsy. J. Neurosci. 2013. 33: 17749–17762.
Isokawa M., Levesque M.F., Babb T.L., Engel J.Jr. Single mossy fiber axonal systems of human dentate granule cells studied in hippocampal slices from patients with temporal lobe epilepsy. J. Neurosci. 1993. 13(4): 1511–1522.
Jacobs J., LeVan P., Chander R., Hall J., Dubeau F., Gotman J. Interictal highfrequency oscillations (80–500Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008. 49: 1893–1907.
Jacobs J., Banks S., Zelmann R., Zijlmans M., Jones-Gotman M., Gotman J. Spontaneous ripples in the hippocampus correlate with epileptogenicity and not memory function in patients with refractory epilepsy. Epilepsy Behav. 2016. 62: 258–266.
Jakubs K., Nanobashvili A., Bonde S., Ekdahl C.T., Kokaia Z., Kokaia M., Lindvall O. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006. 52: 1047–1059.
Jessberger S., Parent J.M. Epilepsy and adult neurogenesis. Cold Spring Harb Perspect. Biol. 2015. 7: a020677.
Jessberger S., Römer B., Babu H., Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol 2005. 196: 342–351.
Johnson A.M., Sugo E., Barreto D., Hiew C., Lawson J.A., Connolly A.M., Somerville E., Hasic E., Bye A.M., Cunningham A.M. The severity of gliosis in hippocampal sclerosis correlates with pre-operative seizure burden and outcome after temporal lobectomy. Molecular Neurobiology. 2016. 53: 5446–5456.
Jung K.H., Chu K., Kim M., Jeong S.-W., Song Y.-M., Lee S.-T., Kim J.-Y., Lee S.K., Roh J.-K. Continuous cytosine-b-Darabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci. 2004. 19: 3219–3226.
Jung K.H., Chu K., Lee S.T., Kim J., Sinn D.I., Kim J.M., Park D.K., Lee J.J., Kim S.U., Kim M., Lee S.K., Roh J.K. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol. Dis. 2006. 23: 237–246.
Frauscher B., von Ellenrieder N., Ferrari-Marinho T., Avoli M., Dubeau F., Gotman J. Facilitation of epileptic activity during sleep is mediated by high amplitude slow waves. Brain J. Neurol. 2015. 138: 1629–1641.
Freund T.F., Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996. 4: 347–470.
Kahn J.B., Port R.G., Yue C., Takano H., Coulter D.A. Circuit-based interventions in the dentate gyrus rescue epilepsy-associated cognitive dysfunction. Brain. 2019. 142 (9): 2705–2721.
Kelly T., Beck H. Functional properties of granule cells with hilar basal dendrites in the epileptic dentate gyrus. Epilepsia. 2017. 58: 160–171.
Kheirbek M.A., Drew L.J., Burghardt N.S., Costantini D.O., Tannenholz L., Ahmari S.E., Fenton A.A., Hen R. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013. 77: 955–968.
Kienzler F., Norwood B.A., Sloviter R.S. Hippocampal injury, atrophy, synaptic reorganization, and epileptogenesis after perforant pathway stimulation-induced status epilepticus in the mouse. J Comp Neurol. 2009. 515(2): 181–196.
Koyama R., Yamada M.K., Fujisawa S., Katoh-Semba R., Matsuki N., Ikegaya Y. Brain-derived neurotrophic factor induces hyperexcitable reentrant circuits in the dentate gyrus. J Neurosci. 2004. 24: 7215–7224.
Kron M.M., Zhang H., Parent J.M. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J. Neurosci. 2010. 30: 2051–2059.
Krook-Magnuson E., Armstrong C., Bui A., Lew S., Oijala M., Soltesz I. In vivo evaluation of the dentate gate theory in epilepsy. J Physiol. 2015. 593(10): 2379–2388.
Liu R., Lemieux L., Bell G., Sisodiya S., Bartlett P., Shorvon S., Sander J.W.A.S., Duncan J.S. Cerebral damage in epilepsy: a population-based longitudinal quantitative MRI study. Epilepsia. 2005. 46: 1482–1494.
Longo B., Covolan L., Chadi G., Mello L. Sprouting of mossy fibers and the vacating of postsynaptic targets in the inner molecular layer of the dentate gyrus. Exp Neurol. 2003. 181: 57–67.
Loscher W., Schmidt D. New horizons in the development of antiepileptic drugs: the search for new targets. Epilepsy Res. 2004. 60: 77–159.
Lothman E., Bertram E. Epileptogenic effects of status epilepticus. Epilepsia. 1993. 34: 59–70.
Lowenstein D.H. Structural reorganization of hippocampal networks caused by seizure activity. International Review of Neurobiology. 2001. 45: 209–236.
Malheiros J., Paiva F., Longo B., Hamani C., Covolan L. Manganese-enhanced MRI: biological applications in neuroscience. Front Neurol. 2015. 6: 161.
Mathern G.W., Babb T.L., Armstrong D.L. Hippocampal sclerosis. Epilepsy: a comprehensive textbook. Ed. Engel J.Jr., Pedley T.A. Philadelphia: Lippincott-Raven Publishers, 1997. 133–155 pp.
Mathern G., Cifuentes F., Leite J., Pretorius J., Babb T. Hippocampal EEG excitability and chronic spontaneous seizures are associated with aberrant synaptic reorganization in the rat intrahippocampal kainate model. Electroencephalogr Clin Neurophysiol. 1993. 87: 326–339.
Mathern G.W., Pretorius J.K., Leite J.P., Kornblum H.I., Mendoz D., Lozada A., Bertram E.H. Hippocampal AMPA and NMDA mRNA Levels and Subunit Immunoreactivity in Human Temporal Lobe Epilepsy Patients and a Rodent Model of Chronic Mesial Limbic Epilepsy. Epilepsy Res. 1998. 32: 154–171.
McNamara J. Cellular and molecular basis of epilepsy. J Neurosci. 1994. 14: 3413–3425.
Mello L., Covolan L. Neuronal injury and progressive cell damage. Encyclopedia of Basic Epilepsy Research. Ed. Schwartzkroin P.A. London: Academic Press, 2009. 125–128 pp.
Mello L., Cavalheiro E., Tan A., Pretorius J., Babb T., Finch D. Granule cell dispersion in relation to mossy fiber sprouting, hippocampal cell loss, silent period and seizure frequency in the pilocarpine model of epilepsy. Epilepsy Res. 1992. 9: 51–59.
Mitsueda-Ono T., Ikeda A., Sawamoto N., Aso T., Hanakawa T., Kinoshita M., Matsumoto R., Mikuni N., Amano S., Fukuyama H., Takahashi R. Internal structural changes in the hippocampus observed on 3-tesla MRI in patients with mesial temporal lobe epilepsy. Intern Med. 2013. 52: 877–885.
Morimoto K., Fahnestock M., Racine R.J. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog. Neurobiol. 2004. 73: 1–60.
Myers C.E., Bermudez-Hernandez K., Scharfman H.E. The influence of ectopic migration of granule cells into the hilus on dentate gyrus-CA3 function. PLoS ONE. 2013. 8: e68208.
Nadler J.V., Perry B.W., Cotman C.W. Selective reinnervation of hippocampal area CA1 and the fascia dentata after destruction of CA3-CA4 afferents with kainic acid. Brain Res. 1980. 182: 1–9.
Nadler J.V. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003. 28: 1649–1658.
Nairismägi J., Pitkänen A., Narkilahti S., Huttunen J., Kauppinen R., Gröhn O. Manganese-enhanced magnetic resonance imaging of mossy fiber plasticity in vivo. Neuroimage. 2006. 30: 130–5.
Nakashiba T., Cushman J.D., Pelkey K.A., Renaudineau S., Buhl D.L., McHugh T.J., Rodriguez Barrera V., Chittajallu R., Iwamoto K.S., McBain C.J., Fanselow M.S., Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012.149: 188–201.
Nissinen J., Lukasiuk K., Pitkänen A. Is mossy fiber sprouting present at the time of the first spontaneous seizures in rat experimental temporal lobe epilepsy? Hippocampus. 2001. 11: 299–310.
Obenaus A., Esclapez M., Houser C.R. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J. Neurosci. 1993. 13(10): 4470–4485.
Olney J.W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969. 164: 719–721.
Parent J.M., Elliott R.C., Pleasure S.J., Barbaro N.M., Lowenstein D.H. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Annals of Neurology. 2006. 59: 81–91.
Parent J.M., Tada E., Fike J.R., Lowenstein DH. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J. Neurosci. 1999. 19: 4508–4519.
Parent J., Yu T., Leibowitz R., Geschwind D., Sloviter R., Lowenstein D. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997. 17: 3727–3738.
Pekcec A., Lupke M., Baumann R., Seifert H., Potschka H. Modulation of neurogenesis by targeted hippocampal irradiation fails to affect kindling progression. Hippocampus. 2011. 21: 866–876.
Pierce J., Melton J., Punsoni M., McCloskey D., Scharfman H. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp Neurol. 2005. 196: 316–331.
Pierce J., Punsoni M., McCloskey D., Scharfman H. Mossy cell axon synaptic contacts on ectopic granule cells that are born following pilocarpine-induced seizures. Neurosci Lett. 2007. 422: 136–140.
Polli R., Malheiros J., Dos Santos R., Hamani C., Longo B., Tannús A., Mello L.E., Covolan L. Changes in hippocampal volume are correlated with cell loss but not with seizure frequency in two chronic models of temporal lobe epilepsy. Front Neurol. 2014. 5: 111.
Pun R.Y., Rolle I.J., Lasarge C.L., Hosford B.E., Rosen J.M., Uhl J.D., Schmeltzer S.N., Faulkner C., Bronson S.L., Murphy B.L., Richards D.A., Holland K.D., Danzer S.C. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. 2012. 75: 1022–1034.
Raedt R., Boon P., Persson A., Alborn A.-M., Boterberg T., Van Dycke A., Linder B., Smedt T.D., Wadman W.J., Ben-Menachem E., Eriksson P.S. Radiation of the rat brain suppresses seizure-induced neurogenesis and transiently enhances excitability during kindling acquisition. Epilepsia. 2007. 48: 1952–1963.
Ratzliff A.H., Howard A.L., Santhakumar V., Osapay I., Soltesz I. Rapid Deletion of Mossy Cells Does Not Result in a Hyperexcitable Dentate Gyrus: Implications for Epileptogenesis. J. Neurosci. 2004. 24(9): 2259–2269.
Ratzliff A.H., Santhakumar V., Howard A., Soltesz I. Mossy cells in epilepsy: Rigor mortis or vigor mortis? Trends. Neurosci. 2002. 25: 140–144.
Ren E., Curia G. Synaptic Reshaping and Neuronal Outcomes in the Temporal Lobe Epilepsy. Int J. Mol. Sci. 2021. 22(8): 3860.
Represa A., Jorquera I., Le Gal La Salle G., Ben-Ari Y. Epilepsy induced collateral sprouting of hippocampal mossy fibers: does it induce the development of ectopic synapses with granule cell dendrites? Hippocampus. 1993. 3(3): 257–268.
Reyes-Garcia S.Z., Scorza C.A, Araújo N.S., Ortiz-Villatoro N.N., Jardim A., Centeno R., Targas Y.E.M., Faber J., Cavalheiro E.A. Different patterns of epileptiform-like activity are generated in the sclerotic hippocampus from patients with drug-resistant temporal lobe epilepsy. Sci Rep. 2018. 8: 7116.
Ribak C.E., Shapiro L.A., Yan X.-X., Dashtipour K., Nadler J.V., Obenaus A., Spigelman I., Buckmaster P.S. Seizure-induced formation of basal dendrites on granule cells of the rodent dentate gyrus. Jasper’s Basic Mechanisms of the Epilepsies. Eds.: Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., Delgado-Escueta A.V. Bethesda (MD): National Center for Biotechnology Information (US), 2012.
Roth B.L. DREADDs for neuroscientists. Neuron. 2016. 89(4): 683–694.
Viscomi M.T., Oddi S., Latini L. et al. The endocannabinoid system: A new entry in remote cell death mechanisms. Exp. Neurol. 2010. 224: 56–65.
Sanchez R., Ribak C., Shapiro L. Synaptic connections of hilar basal dendrites of dentate granule cells in a neonatal hypoxia model of epilepsy. Epilepsia. 2012. 53: 98–108.
Santhakumar V., Bender R., Frotscher M., Ross S.T., Hollrigel G.S., Toth Z., Soltesz I. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the ‘irritable mossy cell’ hypothesis. J. Physiol. 2000. 524(1): 117–134.
Scharfman H.E. The role of nonprincipal cells in dentate gyrus excitability and its relevance to animal models of epilepsy and temporal lobe epilepsy. Adv. Neurol. 1999. 79: 805–820.
Scharfman H.E. Epilepsy as an example of neural plasticity. Neuroscientist. 2002. 8: 154–173.
Scharfman H.E. Synaptic Plasticity and Transsynaptic Signalling. Stanton P.K., Bramham C.R., Scharfman H.E., editors. Springer. 2005. pp. 201–220.
Scharfman H., Goodman J., McCloskey D. Ectopic granule cells of the rat dentate gyrus. Dev Neurosci. 2007. 29: 14–27.
Scharfman H.E., Goodman J.H., Sollas A.L. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J. Neurosci. 2000. 20: 6144–6158.
Scharfman H.E., Myers C.E. Hilar mossy cells of the dentate gyrus: a historical perspective. Front Neural Circuits. 2012. 6: 106.
Scharfman H.E., Pierce J.P. New insights into the role of hilar ectopic granule cells in the dentate gyrus based on quantitative anatomic analysis and three-dimensional reconstruction. Epilepsia. 2012. 53(Suppl 1): 98–108.
Schmeiser B., Li J., Brandt A., Zentner J., Doostkam S., Freiman T. Different mossy fiber sprouting patterns in ILAE hippocampal sclerosis types. Epilepsy Res. 2017. 136: 115–122.
Shibata K., Nakahara S., Shimizu E., Yamashita T., Matsuki N., Koyama R. Repulsive guidance molecule a regulates hippocampal mossy fiber branching in vitro. Neuroreport. 2013. 24: 609–615.
Scott B., Wojtowicz J., Burnham W. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 2000. 165: 231–236.
Shapiro L.A., Figueroa-Aragon S., Ribak C.E. Newly generated granule cells show rapid neuroplastic changes in the adult rat dentate gyrus during the first five days following pilocarpine-induced seizures. Eur J Neurosci. 2007. 26(3): 583–592.
Sloviter R. Decreased hippocampal inhibition and selective loss of interneurons in experimental epilepsy. Science. 1987. 235: 73–76.
Sloviter R.S. Feedforward and feedback inhibition of hippocampal principal cell activity evoked by perforant path stimulation: GABA-mediated mechanisms that regulate excitability in vivo. Hippocampus. 1991a. 1: 31–40.
Sloviter R.S. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991b. 1: 41–66.
Sloviter R.S. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994. 35: 640–654.
Sloviter R.S. Status epilepticus-induced neuronal injury and network reorganization. Epilepsia. 1999. 40. Suppl 1: S34-9, discussion S40-1.
Sloviter R., Bumanglag A., Schwarcz R., Frotscher M. Abnormal dentate gyrus network circuitry in temporal lobe epilepsy. Jasper’s Basic Mechanisms of the Epilepsies. Eds.: Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., Delgado-Escueta A.V. Bethesda (MD): National Center for Biotechnology Information (US), 2012.
Song M.Y., Tian F.F., Wang Y.Z., Huang X., Guo J.L., Ding D.X. Potential roles of the RGMa-FAK-Ras pathway in hippocampal mossy fiber sprouting in the pentylenetetrazole kindling model. Mol Med Rep. 2015. 11: 1738–1744.
Song I., Orosz I., Chervoneva I., Waldman Z.J., Fried I., Wu C., Sharan A., Salamon N., Gorniak R., Dewar S., Bragin A., Engel J., Sperling M.R., Staba R., Weiss S.A. Bimodal coupling of ripples and slower oscillations during sleep in patients with focal epilepsy. Epilepsia. 2017. 58: 1972–1984.
Staba R.J., Wilson C.L., Bragin A., Fried I., Engel Jr.J. Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002. 88: 1743–1752.
Spigelman I., Yan X., Obenaus A., Lee E., Wasterlain C., Ribak C. Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience. 1998. 86: 109–120.
Sun C., Mtchedlishvili Z., Bertram E.H., Erisir A., Kapur J. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J. Comp Neurol. 2007. 500: 876–893.
Sutula T.P., Dudek F.E. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog. Brain Res. 2007. 163: 541–563.
Sutula T., Cascino G., Cavazos J., Parada I., Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Annals of Neurology. 1989. 26: 321–330.
Tamagnone L., Comoglio P. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol. 2000. 10: 377–383.
Tauck D, Nadler J. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. (1985) 5: 1016– 22.
Thind K.K., Ribak C.E, Buckmaster P.S. Synaptic input to dentate granule cell basal dendrites in a rat model of temporal lobe epilepsy. J. Comp. Neurol. 2008. 509: 190–202.
Tong X., Tong X., Peng Z., Zhang N., CetinaY., Huang C.S., Wallner M., Otis, T. S., Houser C.R. Ectopic expression of α6 and δ GABAA receptor subunits in hilar somatostatin neurons increases tonic inhibition and alters network activity in the dentate gyrus. J. Neurosci. 2015. 35: 16142–16158.
Tulving E. Episodic memory: from mind to brain. Annu. Rev. Psychol. 2002. 53: 1–25.
Van Paesschen W., Revesz T., Duncan J.S., King M.D., Connelly A. Quantitative neuropathology and quantitative magnetic resonance imaging of the hippocampus in temporal lobe epilepsy. Annals of Neurology. 1997. 42: 756–766.
von Ellenrieder N., Frauscher B., Dubeau F., Gotman J. Interaction with slow waves during sleep improves discrimination of physiologic and pathologic high-frequency oscillations (80–500Hz). Epilepsia. 2016. 57: 869–878.
Walter C., Murphy B.L., Pun R.Y., Spieles-Engemann A.L., Danzer S.C. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J. Neurosci. 2007. 27: 7541–7552.
Weiss S.A., Song I., Leng M., Pastore T., Slezak D., Waldman Z., Orosz I., Gorniak R., Donmez M., Sharan A., Wu C., Fried I., Sperling M.R., Bragin A., Engel, Jr. J., Nir Y., Staba R. Ripples Have Distinct Spectral Properties and Phase-Amplitude Coupling With Slow Waves, but Indistinct Unit Firing, in Human Epileptogenic Hippocampus. Front Neurol. 2020. 11: 174.
Wittner L., Maglóczky Z., Borhegyi Z., Halász P., Tóth S., Eross L., Szabó Z., Freund T.F. Preservation of perisomatic inhibitory input of granule cells in the epileptic human dentate gyrus. Neuroscience. 2001. 108: 587–600.
Wuarin J.P., Dudek F.E. Excitatory synaptic input to granule cells increases with time after kainate treatment. J. Neurophysiol. 2001. 85(3): 1067–1077.
Ueda Y., Doi T., Tsuru N., Tokumaru J., Mitsuyama Y. Expression of glutamate transporters and ionotropic glutamate receptors in GLAST knockout mice. Brain Res. Mol. Brain Res. 2002. 104(2): 120–126.
Zhan R.Z., Timofeeva O., Nadler J.V. High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats. J. Neurophysiol. 2010. 104: 3293–3304.
Zhang Y., Xiong T., Tan B., Song Y., Li S., Yang L., Li Y.-C. Pilocarpineinduced epilepsy is associated with actin cytoskeleton reorganization in the mossy fiber-CA3 synapses. Epilepsy Res. 2014. 108: 379–389.
Zeng L.H., Rensing N.R., Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J. Neurosci. 2009. 29: 6964–6972.
Zimmer J. Changes in the Timm sulfide silver staining patter of the rat hippocampus and fascia dentata following early postnatal deaferentiation. Brain Res. 1973. 64: 313–326.
Zimmer J. Long-term synaptic reorganization in rat fascia dentate deafferented at adolescent and adult stages: observations with the Timm method. Brain Res. 1974. 76: 336–342.
Yamawaki R., Thind K., Buckmaster P.S. Blockade of excitatory synaptogenesis with proximal dendrites of dentate granule cells following rapamycin treatment in a mouse model of temporal lobe epilepsy. J. Comp Neurol. 2015. 523: 281–297.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


