Журнал высшей нервной деятельности им. И.П. Павлова, 2022, T. 72, № 3, стр. 317-342
Роль зубчатой извилины в осуществлении функций гиппокампа: здоровый мозг
В. Ф. Кичигина 1, *, Л. В. Шубина 1, И. Ю. Попова 1
1 ФГБУН Институт теоретической и экспериментальной биофизики РАН
Пущино, Россия
* E-mail: vkitchigina@gmail.com
Поступила в редакцию 28.10.2021
После доработки 03.12.2021
Принята к публикации 20.12.2021
- EDN: LXWSUH
- DOI: 10.31857/S0044467722030030
Аннотация
Зубчатая извилина (ЗИ), входящая в состав гиппокамповой формации, является основной мишенью получаемых гиппокампом неокортикальных и субкортикальных афферентов, что составляет анатомическую основу его участия в когнитивных процессах, таких как внимание и память. ЗИ участвует в организации многих когнитивных функций гиппокампа и мозга в целом, включая детекцию новизны, разделение и завершение паттернов (pattern separation and pattern completion), пространственную рабочую память, кодирование информации и консолидацию памяти. Именно в ЗИ впервые была обнаружена долговременная потенциация – пластические изменения в синапсах, схожие с теми, которые происходят при запоминании информации. ЗИ представляет собой уникальную область мозга, одну из немногих, где обнаруживается нейрогенез у взрослых млекопитающих, включая человека. Другой особенностью ЗИ, отличающей ее от гиппокампа, является наличие в ней двух типов глутаматергических нейронов – гранулярных и мшистых клеток. Гранулярные клетки, в норме обладая низкой активностью, ограничивают возбудимость пирамидных нейронов гиппокампа в неблагоприятных условиях. Функции мшистых нейронов ЗИ наименее ясны; эти клетки, иннервируя как глутаматергические, так и ГАМКергические нейроны, вероятно, участвуют в организации сложной сетевой активности как в самой ЗИ, так и в гиппокампе. Несмотря на интенсивное исследование ЗИ, ее роль в деятельности гиппокампа еще во многом неясна. В предлагаемом обзоре рассматриваются анатомические, гистохимические и функциональные особенности ЗИ, активность отдельных клеточных элементов, а также ее роль в гиппокампальных функциях нормального мозга.
1.1. АНАТОМИЧЕСКИЕ И ГИСТОХИМИЧЕСКИЕ ОСОБЕННОСТИ ЗУБЧАТОЙ ИЗВИЛИНЫ
Со времен исследований Рамон-и-Кахала (Ramon y Cajal, 1893) и Лоренте де Но (Lorente de Nо, 1934), описавших типы, характеристики и связи нейронов гиппокампа (сornu ammonis), его принято подразделять на три области собственно гиппокампа (CA1, СА2 и СА3) (Lorente de No, 1934) и зубчатую извилину (ЗИ) (dentate gyrus, DG). Подобно гиппокампу, ЗИ содержит основные глутаматергические нейроны и ГАМКергические интернейроны. Однако, в отличие от гиппокампа, ЗИ содержит два типа основных клеток: гранулярные нейроны и мшистые клетки (Scharfman, 1995; Freund, Buzsaki, 1996).
Анатомическая организация ЗИ сходна у грызунов и приматов. Основная структура ЗИ подробно описана в работе (Amaral et al., 2007) и суммирована на рис. 1. ЗИ, начиная от внешней поверхности, делится на три слоя: молекулярный, гранулярный и полиморфный (хилус). Основными клеточными элементами ЗИ являются гранулярные нейроны; большинство их расположено в гранулярном слое, но небольшое их количество (полулунные гранулярные клетки, см. ниже) находится рядом с этим слоем (Williams et al., 2007; Larimer, Strowbridge, 2010) и в хилусе (эктопические гранулярные клетки) (Scharfman et al., 2007); хилус граничит с другой стороны с дендритным слоем поля CA3c. Дендриты гранулярных клеток находятся в молекулярном слое ЗИ, который лишен клеточных элементов и делится на внешний, средний и внутренний подслои. За исключением самых проксимальных участков, все дендриты густо покрыты шипиками. С противоположной стороны от гранулярных нейронов отходят тонкие (0.1 мкм) немиелинизированные аксоны, так называемые мшистые волокна. Главные аксоны гранулярных нейронов проходят рядом со слоем пирамидных клеток в поле CA3, где они образуют по ходу аксона (en passant) чрезвычайно большие (“гигантские”, до 4–6 мкм в поперечнике) бутоны – характерные окончания на шипиках проксимальных дендритов пирамидных нейронов, имеющие сложное строение (Hamlyn, 1962; Blackstad, 1963; Freund, Buzsáki, 1996). Значительно реже мшистые волокна заканчиваются на других гранулярных клетках (Fricke, Prince, 1984) (рис. 1 (а), рис. 1 (в)).
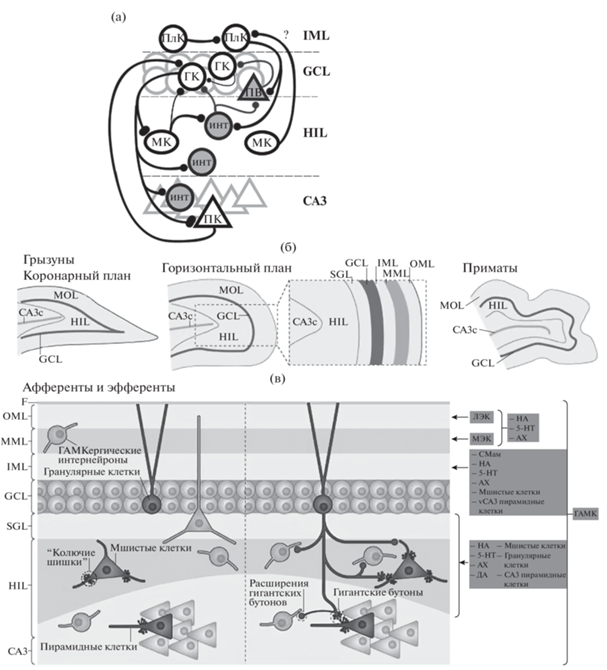
Рис. 1.
Организация зубчатой извилины у грызунов и приматов. (а) Упрощенная схема организации ЗИ. Показаны гранулярные клетки (ГК), полулунные гранулярные клетки (ПлК), мшистые клетки (МК), интернейроны (ИНТ), ПВ – парвальбумин-содержащие интернейроны, пирамидные нейроны поля СА3 гиппокампа (ПК), а также связи между ними: возбуждающие (черные линии) и тормозные (серые линии). IML, внутренний молекулярный слой; GCL, гранулярный клеточный слой; HIL, хилус ЗИ; СА3, поле СА3 гиппокампа. (б) Основная организация зубчатой извилины (ЗИ) в коронарном и горизонтальном планах в мозге грызунов и приматов. Область, выделенная пунктирной линией, представлена при большем увеличении на вставке, где показана ламинарная организация ЗИ, включающей молекулярный слой (MOL), гранулярный слой (GCL) и хилус (HIL). Схематическое изображение структуры ЗИ у приматов показывает сходство с таковой у грызунов, но форма ее более извилистая; кроме того, СА3с у приматов имеет большую протяженность, чем у грызунов, и включает изгиб. (в) Показаны слои ЗИ, куда приходят афферентные волокна. Источники главных афферентных входов обозначены справа (самый правый квадрат означает ГАМКергический вход, иннервирующий все слои, другие квадраты означают волокна из других нейротрансмиттерных систем). Латеральная (LEC) и медиальная энторинальная кора (MEC) иннервируют внешний молекулярный слой (OML) и срединный слой (MML) соответственно. Супрамамиллярный вход (Mamm), холинергический вход, вход от мшистых клеток и от пирамидных нейронов вентрального СА3 (vCA3) иннервируют внутренний молекулярный слой (IML). OML и MML также получают входы от ствола мозга (включая норадренергический (NA), серотонинергический (5-HT) и холинергический (Ach) от передне-базального мозга. Хилус получает разнообразные входы, включая аксоны гранулярных клеток, ГАМКергических нейронов ЗИ, мшистых клеток, пирамидных нейронов СА3, нейромодуляторные входы от ствола мозга (такие как NA и 5-HT, а также дофаминергический (DA)), и холинергический от передне-базального мозга. Эфференты от ЗИ в основном идут от гранулярных нейронов, проецирующихся к HIL и CA3. Аксоны гранулярных клеток образуют, во-первых, гигантские бутоны, иннервируя “колючие шишки” (thorny excrescences) мшистых клеток и пирамидные нейроны СА3, во-вторых, малые бутоны на коллатералях, идущих из хилуса, и, в-третьих, расширения (filamentous extensions) гигантских бутонов. Малые бутоны в основном контактируют с интернейронами, но также с дистальными дендритами мшистых клеток. Подробные пояснения в тексте. ACh, ацетилхолин; F, фиссура; SGZ, субгранулярная зона. (б) и (в) Адаптировано из Scharfman, 2016 (разрешен полный доступ к статье через Институт теоретической и экспериментальной биофизики РАН).
Fig. 1. The organization of the dentate gyrus of rodents and primates. (а) Simplified scheme for organizing the DG. Granular cells (ГК), semilunar granular cells (ПлК), mossy cells (МК), interneurons (ИНТ), parvalbumin-containing interneurons (ПВ), pyramidal neurons of the CA3 field of the hippocampus (ПК), as well as connections between them are shown: excitatory (black lines) and inhibitory (gray lines). IML, inner molecular layer; GCL, granular cell layer; HIL, hilus of the DG; CA3, field CA3 of the hippocampus. (б) The general organization of the (DG) in the coronal and horizontal planes in the rodent and primate brain. The area surrounded by the dashed box is expanded in the inset figure to show the laminar organization of the dentate gyrus, which is composed of a molecular layer (MOL), granule cell layer (GCL) and hilus (HIL). A schematic of the primate dentate gyrus shows that it is similar to that of the rodents; in addition, CA3c is larger in primates than in rodents and includes a reflected blade. (в) The DG layers are shown, where the afferent fibers come. The sources of major afferent inputs are shown on the right (most right box indicates the GABAergic input and other boxes indicate inputs from other neurotransmitters system). GABAergic interneurons innervate all layers. The lateral entorhinal cortex (LEC) and the medial entorhinal cortex (MEC) innervate the outer molecular layer (OML) and the middle molecular layer (MML), respectively. Supramammillary (Mamm), cholinergic, mossy cell and ventral CA3 (vCA3) pyramidal cell axons innervate the inner molecular layer (IML). The OML and MML also receive inputs from the brainstem (including noradrenergic and 5-hydroxytryptamine (5-HT; also known as serotonin) inputs) and from basal forebrain cholinergic neurons. The HIL receives diverse inputs, including the axons of granule cells, dentate gyrus GABAergic neurons, mossy cells, CA3 pyramidal cells, neuromodulatory inputs from the brainstem (such as noradrenaline (NA), 5-HT and dopamine (DA)) and basal forebrain cholinergic neurons. The efferents from the dentate gyrus to other areas arise mainly from granule cells that project to the HIL and CA3. The granule cell axon, called a mossy fibre, is complex. It makes giant boutons that innervate thorny excrescences of mossy cells and pyramidal cells, and small boutons that arise from hilar collaterals and filamentous extensions from the giant boutons. The small boutons primarily contact interneurons but also form contacts on distal dendrites of mossy cells. Detailed explanations in the text. ACh, acetylcholine; F, fissure; SGZ, subgranular zone. (б) and (в) Adapted from Scharfman, 2016 (full access to the article is allowed through the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences).
Гранулярные нейроны обычно считаются однородной популяцией относительно связей и функций. Однако особая субпопуляция гранулярных клеток в виде полулунных (semilunar) гранулярных клеток (Williams et al., 2007; Larimer, Strowbridge, 2010) (рис. 1 (а), рис. 2 (а)), обнаруживает особые свойства и связи. Эти нейроны имеют иную морфологию, чем классические гранулы: в отличие от монополярных клеток, они мультиполярны, и их дендритная ветвь более широко распространяется в молекулярном слое. Полулунные нейроны располагаются преимущественно около супрагранулярного слоя или глубже, во внутреннем молекулярном слое. У этих нейронов есть особая связь с мшистыми клетками хилуса, благодаря которой полулунные нейроны могут активировать мшистые нейроны, вводя их в особое состояние – хилусное возбуждение (hilar up-states) (Williams et al., 2007; Larimer and Strowbridge, 2010). Такое состояние поддерживается за счет особого характера разрядов полулунных клеток (см. ниже) и наличия возвратных связей между этими нейронами. В отличие от типичных гранул, у полулунных нейронов аксон может отдавать коллатерали в пределах гранулярного слоя (Williams et al., 2007). Эти морфологические характеристики позволяют предполагать, что эта популяция гранулярных клеток, хотя и менее многочисленная, находится в “выгодном” положении как источник перисоматических бутонов на парвальбумин-содержащих интернейронах.
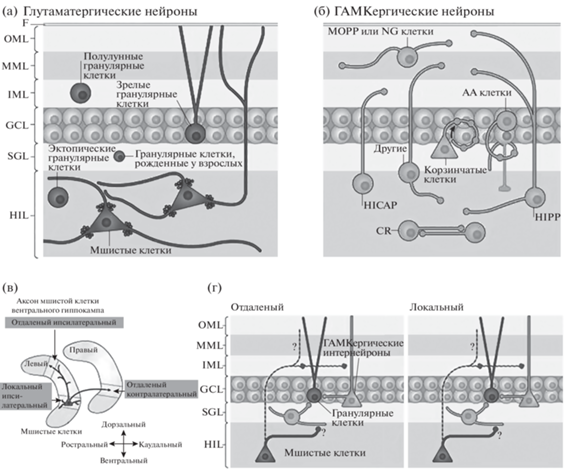
Рис. 2.
Типы клеток и связей в зубчатой извилине. (а) Глутаматергические нейроны зубчатой извилины включают гранулярные и мшистые клетки. Гранулярные клетки расположены не только в гранулярном слое (GCL); есть небольшие группы во внутреннем молекулярном слое (IML) и хилусе (HIL), а предшественники гранулярных нейронов расположены в субгранулярной зоне (SGZ). Мшистые клетки имеют длинные дендриты, некоторые из них простираются в молекулярный слой (MOL; состоящий из внутреннего, среднего и внешнего молекулярного слоев (соответственно IML, MML и OML)). (б) ГАМКергические нейроны зубчатой извилины неоднородны. Их номенклатура основана на расположении тела клетки и терминального поля аксона. Например, клетки MOPP имеют тело клетки в MOL и терминали аксонов в OML и MML, где расположены терминали перфорантного пути. Клетки HICAP (интернейроны с сомой в хилусе) иннервируют IML, где находятся комиссуральные/ассоциативные проекции от мшистых клеток. Нейроны, которые иннервируют сомы гранулярных клеток или начальные сегменты аксонов (перисоматически проецирующиеся нейроны), включают корзинчатые клетки (Basket cells), которые часто имеют сому пирамидальной формы и расположены на границе GCL и HIL, и аксо-аксональные (AA) клетки. AA-клетки часто присутствуют рядом с GCL или в нем самом и иннервируют начальные сегменты аксонов гранулярных клеток. Несколько подтипов ГАМКергических нейронов иннервируют дендриты гранулярных клеток. Наиболее распространенными из них являются клетки в HIL, которые иннервируют OML и MML (клетки HIPP, хилусные клетки, которые проецируются в терминальную зону перфорантного пути). Показан пример нейроглиаформной клетки (NG), которая иннервирует молекулярный слой. Есть некоторые типы ГАМКергических нейронов, у которых есть аксон, иннервирующий более одного слоя (обозначен как “other”), а некоторые интернейроны иннервируют друг друга, например, хилусные клетки, экспрессирующие кальретинин (CR). (в) Организация аксонов мшистых клеток. Схематическое изображение аксона одной мшистой клетки вентрального гиппокампа. Рядом с сомой локальные ипсилатеральные ветви аксона мшистых клеток образуют синапсы в хилусе (HIL) и во внутреннем молекулярном слое (IML). Вдали от сомы отдаленные ипсилатеральные или дальние контралатеральные ветви аксона проецируются в основном в IML. (г) Дальняя (слева) и локальная (справа) ипсилатеральные проекции. На удаленных ипсилатеральных участках аксон в основном образует синапсы на шипиках; поскольку на интернейронах зубчатой извилины мало шипиков, вполне вероятно, что аксон мшистых клеток в IML иннервирует гранулярные клетки. Кроме того, аксон мшистой клетки простирает коллатерали во внутренний, внешний и средний части молекулярного слоя (IML, OML и MML соответственно). Неясно, создают ли синапсы терминали мшистых волокон клеток в OML и MML, или на какие типы нейронов в хилусе проецируются мшистые клетки (обозначено вопросительными знаками). В ипсилатеральной ЗИ аксоны мшистых клеток образуют локальные коллатерали в HIL (особенно вблизи сомы). Там они, вероятно, контактируют с интернейронами, т.к. там находятся многочисленные дендриты интернейронов этой области и отсутствуют дендриты гранулярных клеток. Мшистые клетки также образуют локальные проекции к IML, но их не так много, как на отдаленных участках. GCL, гранулярный слой; SGZ, субгранулярная зона. Подробные пояснения в тексте. Адаптировано из Scharfman, 2016 (разрешен полный доступ к статье через Институт теоретической и экспериментальной биофизики РАН).
Fig. 2. The cell types and connections of the dentate gyrus (а) Glutamatergic cells of the dentate gyrus include granule cells and mossy cells. Granule cells are not only located in the granule cell layer (GCL); there are small subsets in the inner molecular layer (IML) and hilus (HIL), and precursors to granule cells are located in the subgranular zone (SGZ). Mossy cells have long dendrites, some of which extend into the molecular layer (MOL; comprised of the IML, middle molecular layer (MML) and outer molecular layer (OML)). (б) GABAergic neurons of the dentate gyrus are heterogeneous. Their nomenclature is based on the location of the cell body and the axon terminal field. For example, MOPP cells have a cell body in the MOL and terminals in the OML and MML, where the terminals of the perforant path are located. HICAP cells (interneurons with a hilar cell body) innervate the IML, where the commissural/associational projection from mossy cells is located. The neurons that innervate the granule cell somata or the axon initial segments are called perisomatic-targeting cells. Two of the most common cell types in this group are basket cells, which make basket-like endings around the granule cells and often have a pyramidal-shaped soma that is located at the border of the GCL and HIL, and axo-axonic (AA) cells. AA cells are often present near or in the GCL, and innervate granule cell axon initial segments. Several GABAergic neuron subtypes innervate granule cell dendrites. The most common of these are cells in the HIL that innervate the OML and MML (HIPP cells, hilar cells that project to the terminal zone of the perforant path). An example of a neurogliaform cell (NG) that innervates the molecular layer is shown. There are some types of GABAergic neurons that have an axon that innervates more than one layer (here labelled ‘other’), and some interneurons innervate each other, such as calretinin-expressing hilar cells (CR). (в) Organization of the mossy cell axon. The axon of a single ventral mossy cell is illustrated schematically. Near the soma, the local ipsilateral branches of the mossy cell axon make synapses in the hilus (HIL) and the inner molecular layer (IML). Far from the soma, the distant ipsilateral or distant contralateral branches of the axon project primarily to the IML. (г) Distant (left) and local (right) ipsilateral circuitry. At distant ipsilateral locations, the axon primarily makes synapses on spines; because there are few spines on dentate gyrus interneurons, it is likely that the mossy cell axon in the IML innervates granule cells. In addition, the mossy cell axon extends hilar, outer molecular layer (OML) and middle molecular layer (MML) collaterals. It is not clear whether mossy cell terminals in the OML and MML make synapses, or what cell types mossy cells target in the HIL (indicated by question marks). At local ipsilateral locations, the mossy cell axons collateralize in the HIL, especially near the soma. There, they are likely to contact interneurons because of the numerous interneuron dendrites present in this region and the absence of granule cell dendrites. Mossy cells also make local projections to the IML, but these are not as numerous as those to distant sites. GCL, granule cell layer; SGZ, subgranular zone. Adapted from Scharfman, 2016.
Кроме образования гигантских синапсов, еще одним характерным свойством мшистых волокон является высокое содержание в них везикулярного цинка (Cole et al., 1999). “Свободный” цинк (Zn2+) вместе с глутаматом содержится лишь в определенной субпопуляции везикул (Haug, 1967; Kay, 2003; Vogt et al., 2000; Qian and Noebels, 2005; Lavoie et al., 2011); по причине такой двойственной нейрохимической природы при описании этих синапсов применяется термин “глюцинергические” (“gluzinergic”) (Frederickson, 1989; Frederickson, Bush, 2001). Показано, что цинк модулирует общую возбудимость сети гиппокампа, влияя на выделение глутамата; в отсутствие везикулярного цинка экзоцитоз пузырьков замедляется (Lavoie et al., 2011). Предполагается, что, поскольку везикулярный цинк играет важную роль в динамике выделения передатчика, он может влиять на кодирование информации в сети гиппокампа (Lavoie et al., 2011).
Мшистые клетки (“mossy cells”) ЗИ находятся только в хилусе ЗИ, и число их невелико: у крысы их насчитывается около 10 000 (Amaral, 1978; Amaral et al., 1990, 2007; Scharfman, Myers, 2012). В отличие от гранулярных и других основных нейронов гиппокампа, они не образуют клеточного слоя, а разбросаны в хилусе, что затрудняет исследование этих клеток in vivo. Их характерным признаком является наличие нескольких шиповидных выростов (thorny excrescenses) на телах нейронов и на проксимальных дендритных участках (Amaral, 1978; Ribak et al., 1985; Frotscher et al., 1991) (рис. 2 (а)), благодаря которым их называют “колючими” клетками (термин “мшистый”, используемый как для мшистых клеток, так и для мшистых волокон гранулярных клеток, может вызывать путаницу; тем не менее он уместен, учитывая “мшистый” вид обоих).
По данным (Amaral, 1978) дендриты мшистых клеток ограничены хилусом; однако впоследствии было обнаружено, что некоторые из мшистых клеток имеют единственную дендритную ветвь, пронизывающую молекулярный слой ЗИ (Soltesz, Mody, 1994; Scharfman, 1995b). Основными постсинаптическими мишенями аксонов мшистых клеток являются дендриты гранул (Blasco-Ibáñez, Freund, 1997). Окончания мшистых клеток у мышей могут быть визуализированы по содержанию в них кальретинина (Rovira-Esteban et al., 2020); у крыс мшистые клетки обнаруживают кальцитонин (Freund et al., 1997).
Основная организация шиповидных выростов мшистых клеток и аксональных бутонов гранул схожа в хилусе и поле СА3: гигантские бутоны находятся в оппозиции к шиповидным выростам на мшистых клетках или пирамидных нейронах, а меньшие бутоны контактируют с интернейронами (рис. 1 (а), рис. 1 (в)). Малые бутоны аксонов гранул находятся на коллатералях мшистых волокон в хилусе, где они контактируют с интернейронами хилуса и дистальными дендритами мшистых клеток. Малые бутоны также найдены на расширениях филаментов, исходящих из гигантских бутонов.
Интернейроны зубчатой извилины разнообразны; их обычно классифицируют по местоположению сом и проекций аксонов. Основная популяция интернейронов хилуса, так называемые HIPP-клетки, – это клетки, проецирующиеся к терминальной зоне перфорантного пути (Halasy, Somogyi, 1993; Han et al., 1993; Freund, Buzsaki, 1996) (рис. 2 (б)). Интернейроны с телами на границе хилуса или в полиморфном слое имеют восходящие дендриты и аксон; они называются HICAP-клетками, иннервирующими внутренний молекулярный слой ЗИ (Han et al., 1993; Freund, Buzsaki, 1996). Интернейроны с сомой в среднем молекулярном слое и с дендритами и аксоном во внешних двух третях молекулярного слоя (в терминальной зоне перфорантного пути) называются MOPP-клетками (Han et al., 1993). Интернейроны ЗИ были классифицированы также в соответствии с экспрессией ими кальций-связывающих белков (таких как парвальбумин или кальбиндин (Sloviter, 1989)) или нейропептидов (включая соматостатин, нейропептид Y и холецистокинин (Houser, 2007)). Среди интернейронов парвальбумин (ПВ)-содержащие клетки принадлежат к двум популяциям быстроразряжающихся ГАМКергических клеток: корзинчатые (basket) интернейроны, которые образуют аксо-соматические контакты, и аксо-аксональные (АА) интернейроны, образующие синапсы на аксонах гранулярных клеток (Halasy, Somogyi, 1993; Han et al., 1993; Freund, Buzsáki, 1996) (см. рис. 2 (б)). Большинство ПВ-интернейронов имеют пирамидальную форму. Хотя большинство интернейронов ЗИ проецируются локально, некоторые из них, экспрессирующие ПВ или нейропептид Y, проецируются в более отдаленные области, такие как контралатеральная ЗИ (Goodman, Sloviter, 1992; Deller et al., 1995).
Корзинчатые ПВ-нейроны ЗИ считаются ключевыми элементами, участвующими в осуществлении функций гиппокампа как в норме, так и в патологии (Sloviter et al., 2003). Эти клетки перисоматически иннервируются аксонами, образующими возбуждающие синапсы; на их сому и апикальный дендрит поступают также тормозящие воздействия (Ribak, Seress, 1983). В работе (Rovira-Esteban et al., 2020) произведен тщательный анализ происхождения возбуждающих и тормозных сигналов на ПВ-интернейронах в гранулярном слое у мыши, на основе которого сделан вывод, что перисоматическая возбуждающая иннервация ПВ-нейронов в гранулярном слое сильнее по сравнению с тормозной и происходит преимущественно от полулунных гранулярных клеток.
1.2. СВЯЗИ ЗУБЧАТОЙ ИЗВИЛИНЫ
1.2.1. Афферентные связи
ЗИ является основной гиппокампальной мишенью для путей, несущих сенсорную информацию множества модальностей о внешнем мире; она считается первым переключением внутригиппокампальной трисинаптической петли: ЗИ–СА3–СА1. Источники и нейрохимический характер афферентных сигналов к этой структуре представлен в суммарном виде на рис. 1 (в). Кортикальный вход в ЗИ – перфорантный путь (ПП) – идет из слоя II энторинальной коры, которая формирует большинство глутаматергических афферентных входов во внешнем и срединном молекулярном слоях ЗИ; он несет пространственную, а также иную сенсорную информацию в эти разные слои ЗИ соответственно (Amaral, 1978; Steward, Scoville, 1976; Amaral, 1978; Amaral et al., 2007; Witter, 2007; Hunsaker et al., 2007).
Второй главный внешний афферентный путь к ЗИ образован субкортикальными структурами. Эти пути сформированы волокнами нейронов, находящихся в медиальной септальной области (МС-ДБ) (холинергических, ГАМКергических и глутаматергических), супрамамиллярном ядре (глутаматергических и ГАМКергических), медианном ядре шва (серотонинергических), голубом пятне (норадренергических) и вентральной тегментальной области (дофаминергических); еще один путь образован комиссуральной проекционной системой (Amaral, Campbell, 1986; Swanson et al., 1987; Leranth, Frotscher, 1987; Nyakas et al., 1987; Gaykema et al., 1990; Leranth, Brown et al., 2005; Colom et al., 2005; Etter, Krezel, 2014; Hashimotodani et al., 2018; Salib et al., 2019). Пути от МС-ДБ идут неодинаково к разным областям ЗИ: к дорзальной части волокна идут почти исключительно от ДБ, в то время как к вентральной – от МС и ДБ (Nyakas et al., 1987). Клетки супрамамиллярного ядра избирательно иннервируют разные нейроны ЗИ: они образуют моносинаптические связи с гранулярными клетками и ГАМКергическими интернейронами, но не с мшистыми клетками. При этом в синапсах одновременно выделяются глутамат и ГАМК (Hashimotodani et al., 2018). Таким образом, ЗИ получает входы как от неокортекса, так и от субкортикальных структур. Выяснение того, как ЗИ интегрирует эти входы, важно для понимания информационных процессов в ЗИ.
1.2.2. Выходные пути зубчатой извилины
ЗИ уникальна в том отношении, что она является исключительно внутренним реле гиппокампа: аксоны клеток ЗИ уходят не дальше, чем к контралатеральной его области (Raisman et al., 1965; Blackstad, et al., 1970; Amaral, 1978; Ribak et al., 1985).
Гранулярные нейроны ЗИ посредством мшистых волокон образуют синапсы как на пирамидных нейронах поля СА3, так и на мшистых клетках хилуса, а также на ГАМКергических интернейронах (Ribak et al., 1985; Frotscher et al., 1991; Soltesz et al., 1993; Wenzel et al., 1997; Buckmaster et al., 1996; Acsady et al., 1998) (рис. 1 (а), рис. 1 (в), справа)). Количество иннервируемых одним мшистым волокном пирамидных клеток достигает 15–20 (Amaral et al., 2007). Гранулы также контактируют посредством аксонных коллатералей с таким же количеством (15–20) мшистых клеток и с ~100–150 ГАМКергических интернейронов в хилусе и поле СА3 (Acsády et al., 1998). Интересно, что гигантские бутоны могут образовывать филоподиальные расширения (filamentous extentions), благодаря которым мшистые волокна формируют в слое люцидум моносинаптический возбуждающий вход к интернейронам, находящимся в этом слое (Acsády et al. 1998) (рис. 1 (в)). Тот факт, что число синапсов мшистых волокон на ГАМКергических нейронах на порядок превышает количество синапсов на пирамидных клетках CA3, позволяет предположить, что этот путь оказывает сильные ингибирующие эффекты.
Входом к гранулярным нейронам во внутреннем молекулярном слое являются аксоны мшистых клеток (Ribak et al., 1985; Buckmaster et al., 1996; Scharfman, Myers, 2012) (рис. 2 (г)); при этом гранулярные нейроны и мшистые клетки связаны двусторонними связями (Ribak et al., 1985; Frotscher et al., 1991; Wenzel et al., 1997). Гранулы получают также возвратные коллатерали от пирамидных клеток поля СА3 (Zimmer, 1971; Lynch et al., 1973).
Мшистые клетки образуют сложные внутренние проекции (рис. 2 (в), рис. 2 (г)). Большие проекции (известные как “дальние” или “интраламеллярные”) заканчиваются далеко от тела клетки как в ипсилатеральной, так и в контралатеральной ЗИ (Zimmer, 1971; Berger et al., 1981; Ribak et al., 1985; Frotscher et al., 1991; Buckmaster et al., 1996; Scharfman, Myers, 2012). Терминали этой отдаленной проекции образуют синапсы во внутреннем молекулярном слое; электронная микроскопия показывает, что они в первую очередь иннервируют дендритные шипики гранулярных клеток (Buckmaster et al., 1996). Аксоны мшистых клеток также отдают коллатерали в хилусе и распространяются во все слои ЗИ (Scharfman, Schwartzkroin, 1988; Buckmaster et al., 1996; Scharfman, Myers, 2012). Таким образом, мшистые клетки иннервируют все слои зубчатой извилины, как локальные, так и отдаленные ее части.
Недавно было показано, что проекции аксонов мшистых клеток в дорзальном и вентральном гиппокампе значительно различаются. Мшистые клетки в дорзальной и вентральной частях селективно метили Cre-зависимыми маркерами при использовании двух линий трансгенных мышей. После односторонней маркировки мшистых клеток в вентральной ЗИ плотная полоса волокон была видна во внутренней четверти молекулярного слоя и простиралась с двух сторон по всей ростро-каудальной протяженности ЗИ. Напротив, после маркировки мшистых клеток в дорзальной ЗИ проекции были распределены более диффузно: на уровне маркировки волокна присутствовали во внутреннем молекулярном слое, но они постепенно расширялись в средний молекулярный слой и, наиболее вентрально, образовывали отчетливую полосу в этом слое (Houser et al., 2021).
Необходимо отметить также, что, по недавним данным, гранулярные нейроны, мшистые клетки и локальные интернейроны в пределах ЗИ обнаруживают сложный паттерн возвратных связей, сила которых изменяется в онтогенезе (Shi et al., 2019).
1.3. ФИЗИОЛОГИЧЕСКИЕ СВОЙСТВА НЕЙРОНОВ ЗУБЧАТОЙ ИЗВИЛИНЫ
1.3.1. Характеристики активности различных нейронов ЗИ
В ранних работах сведения о характере активности нейронов ЗИ были крайне противоречивыми, что лишь частично зависело от точности размещения регистрирующих электродов; в основном это объяснялось тем, что мшистые клетки и большие интернейроны, находящиеся в субгранулярном слое, генерируют высокоамплитудные спайки, которые из-за объемного распространения могут регистрироваться в гранулярном слое. Это приводило к ошибочному объединению различных по природе клеток в одну группу (см. Henze, Buzsáki, 2007). Позднее в работе (Senzai, Buzsáki, 2017), проведенной на мышах в свободном поведении, авторы, основываясь на предыдущих исследованиях (Bragin et al., 1995; Penttonen et al., 1997), разработали нейрофизиологический метод классификации нейронов, который был подтвержден ими при использовании трансгенных мышей и оптогенетического метода. Они смогли сначала отделить основные глутаматергические нейроны ЗИ от интернейронов, имеющих характерную пачечную активность. Затем, для разделения гранулярных и мшистых клеток, учитывали три основные характеристики: (1) расположение (глубина) регистрируемых клеток, определяемое по реверсии локального полевого потенциала; (2) относительная частота спайков в состояниях медленного сна и бодрствования; и (3) форма потенциала действия. Гранулярные клетки (их тела) располагались по глубине вблизи реверсии потенциала, имели возрастающую частоту разрядов в состоянии медленного сна по сравнению с бодрствованием и несимметричную острую форму потенциала действия. В отличие от них, мшистые клетки находились значительно ниже глубины реверсии потенциала, имели близкую частоту разрядов в состояниях медленного сна и бодрствования и более симметричную форму потенциала действия. Гранулярные нейроны во время бодрствования в основном имели низкую активность (редкие разряды); напротив, для мшистых клеток свойственна более высокочастотная активность (Bragin et al., 1995; Penttonen et al., 1997; GoodSmith et al., 2017; Senzai, Buzsaki 2017; Meier et al., 2020).
1.3.2. Характер межнейронных взаимодействий в зубчатой извилине и гиппокампе
Взаимодействие гранулярных нейронов и пирамидных клеток поля СА3 гиппокампа. При стимуляции гранулярных нейронов (с частотой от 0.1 до 100 Гц, пачками стимулов длительностью 1 с) и регистрации ответов одиночных нейронов в поле СА3 гиппокампа было выявлено, что у разных клеток ответы могли существенно различаться. У большинства нейронов наблюдались ответы “стимул-спайк” с постепенным вовлечением в ответ, но латентные периоды таких ответов сильно варьировали, от 4 до 35–40 мс. Характерным было наличие достаточно узкого и относительно низкого частотного оптимума (10–30 Гц), а при повторных стимуляциях – длительное сохранение потенцированного ответа (в течение 12 ч и более). После окончания стимуляции наблюдалось торможение нейронной активности различной длительности, от 300 мс до 3 с; иногда оно могло достигать 5–8 с. У 9% нейронов таких ответов не наблюдалось, но регистрировалось подавление спонтанной активности либо диффузная активация (Брагин, Виноградова, 1973; Кичигина, Брагин, 1976). Эти различия в характере ответов авторы объясняли морфологическими особенностями данной системы связей, показанными в работе (Hamlyn, 1963). Впоследствии причины таких различий ответов стали более понятными. Во-первых, в гранулярном слое, который стимулировался в упомянутых работах, находятся интернейроны (Freund, Buzsaki, 1996), раздражение которых может быть причиной тормозных ответов у некоторых клеток СА3, что подтвердилось данными, полученными (Acsady et al., 1998). Кроме того, хотя мшистые волокна, идущие от гранулярных нейронов, образуют прямой путь к CA3, однако коллатерали аксонов клеток CA3 проецируются обратно к ЗИ (Scharfman, 2007) и простираются далеко в хилус (Ishizuka et al., 1990; Li et al., 1994). Эти возвратные проекции могут влиять на нейронные цепи как в ЗИ, так и в CA3 (Penttonen et al., 1997; Lisman et al., 2005), что, по-видимому, и является причиной большого разброса латентных периодов и различной длительности тормозного последействия в ответах нейронов СА3 при стимуляции гранулярного слоя. Интересно, что в работе на срезах гиппокампа показано, что основным эффектом залповых разрядов в поле CA3 является ГАМКергическое ингибирование гранулярных клеток (Scharfman, 1994). Известно, что пирамидные нейроны CA3 иннервируют мшистые клетки и интернейроны хилуса (у большинства интернейронов ЗИ есть сома или дендриты в хилусе) (Scharfman et al., 1991, 1994; Kneisler, Dingledine, 1995). Таким образом, при активации CA3 наиболее вероятно включение цепи: пирамидная клетка – мшистая клетка – интернейрон – гранулярная клетка; возможен также путь: пирамидная клетка – пирамидная клетка – интернейрон – гранулярная клетка (Scharfman, 1994). Примечательно, что при блокировании ГАМКергического ингибирования выявляется надежный возбуждающий путь пирамидная клетка – мшистая клетка – гранулярная клетка (Scharfman, 1994). Все эти данные могут объяснять как большой разброс латентных периодов, так и различную длительность тормозного последействия в ответах нейронов СА3 при стимуляции гранулярного слоя ЗИ в работах (Брагин, Виноградова, 1973; Кичигина, Брагин, 1976).
Влияния мшистых клеток на гранулярные нейроны. Эксперименты по выяснению характера действия мшистых клеток на гранулярные нейроны ЗИ начались в 1980-х годах с выяснения того, являются ли мшистые клетки возбуждающими или тормозящими (Buzsáki, Eidelberg, 1981; Douglas et al., 1983). Одна из проблем в этом аспекте заключалась в том, что было сложно избирательно активировать мшистые нейроны с помощью стимулирующих электродов: аксоны и дендриты мшистых клеток пространственно близки к другим клеткам ЗИ и аксональным проекциям, которые влияют на нее. Одна из стратегий обойти эту проблему заключалась в регистрации пар моносинаптически связанных мшистых и гранулярных нейронов (Scharfman, 1995). Этот подход показал, что моносинаптический вход мшистых клеток к гранулам генерирует ВПСП, который, однако, может быть обнаружен только в одном из двух случаев: либо при блокаде ГАМКергического торможения, либо при деполяризации постсинаптическиго гранулярного нейрона (Scharfman, 1995). Таким образом, было установлено, что мшистые клетки могут возбуждать гранулы посредством прямого воздействия (Scharfman, 1995; Larimer, Strowbridge, 2008). Исследования, проводимые на срезах гиппокампа с повреждением мшистых клеток или молекулярного слоя (Jackson, Scharfman, 1996; Wright, Jackson, 2014), представили дополнительные доказательства того, что прямое влияние мшистых клеток на гранулы является возбуждающим. Однако мшистые клетки активируют также интернейроны ЗИ, которые в свою очередь тормозят гранулы (Scharfman, 1995; Larimer, Strowbridge, 2008). При этом мшистые клетки могут активировать ГАМКергические интернейроны широко и синхронно и, следовательно, увеличивать ГАМКергическое ингибирование гранул. Тем не менее убедительные эксперименты на бодрствующих животных, доказывающие это, долгое время отсутствовали. В связи с этим становилось все более важным найти способ селективного тестирования мшистых клеток; важным достижением в этом аспекте стало создание линии трансгенных мышей, которые относительно специфично экспрессируют Cre-рекомбиназу (Cre) в мшистых клетках. В этой линии был использован промотор Crlr для управления экспрессией Cre (Jinde et al., 2012). Этих мышей скрещивали с мышами, несущими рецептор дифтерийного токсина, который вводили взрослым мышам для уничтожения мшистых клеток; через неделю после инъекции токсина мшистые клетки погибали. У этих животных гранулярные нейроны демонстрировали гипервозбудимость в ответ на стимуляцию перфорантного пути. Это подтвердило гипотезу о том, что в норме ингибирование гранулярных нейронов является основной функциональной ролью мшистых клеток (Jinde et al., 2012). Такое утверждение согласуется также с предположением об опосредованной мшистыми клетками фазовой синхронизации гранул (Soltesz et al., 1993; Henze, Buzsáki, 2007).
Проблема о характере действия мшистых клеток на гранулярные нейроны отчасти также решалась при избирательной активации мшистых клеток с помощью оптогенетики в опытах ex vivo, проведенных на срезах гиппокампа (Hsu et al., 2016). Результаты показали, что in vivo ингибирующее действие мшистых клеток может быть сильнее их возбуждающих эффектов.
При хилусном возбуждении (hilar up-states) (Williams et al., 2007; Larimer and Strowbridge, 2010) полулунные гранулярные клетки, специфически иннервирующие ПВ-содержащие интернейроны, выполняют критическую роль в управлении активностью нейронных ансамблей в ЗИ, наряду с мшистыми клетками (Larimer, Strowbridge, 2010). Во время состояния хилусного возбуждения полулунные гранулярные нейроны могут эффективно и одновременно вызывать разряды мшистых клеток и ПВ-баскетных интернейронов, приводя тем самым к параллельному возбуждению дендритов гранулярных клеток во внутреннем молекулярном слое и ингибированию перисоматической области гранулярных нейронов. Эти данные находят подтверждение в недавних результатах, полученных in vivo, показывающих, что разряды гранулярных клеток довольно редки в разных условиях, тогда как мшистые клетки обычно сильно активированы (GoodSmith et al., 2017; Senzai, Buzsáki, 2017). Предполагается, что полулунные гранулярные нейроны, хотя они немногочисленны, являются ключевыми сетевыми элементами в функции ЗИ, поскольку эти нейроны могут одновременно управлять разрядами мшистых клеток хилуса и ПВ-интернейронов; в течение этого временного окна сильное энторинальное возбуждающее воздействие может вызвать активацию только у ограниченной популяции гранулярных клеток, преодолевая их перисоматическое торможение с помощью возбуждения мшистых клеток.
1.3.3. Нейрогенез в зубчатой извилине
Одним из важнейших свойств ЗИ является ее способность к нейрогенезу в течение всей жизни (van Praag et al., 2002). Предполагается, что нейрогенез в ЗИ у взрослых особей – это непрерывный процесс, который поддерживает функционирование гиппокампа у многих видов млекопитающих, в том числе у человека (Eriksson et al., 1998; Spalding et al., 2013). Он заключается в генерации новых функциональных нейронов из нервных стволовых клеток посредством амплификации промежуточных предшественников и нейробластов, а также интеграции этих новых нейронов в существующие нервные цепи. Нейрогенез в ЗИ у взрослых обеспечивает значительную степень структурной и функциональной пластичности в трисинаптической цепи гиппокампа ЗИ–СА3–СА1. Взрослые нейральные стволовые клетки (радиальные глиальные клетки) присутствуют в субгранулярной зоне ЗИ.
Доказательства нейрогенеза были впервые получены у грызунов (Altman, 1962, 1963); впоследствии это было подтверждено у людей и низших приматов (Kuhn et al., 1996; Kempermann et al., 1997; Seki, Arai, 1998; Roy et al., 2000; Knoth et al., 2010; Mathews et al., 2017). Значительный прогресс в понимании нейрогенеза у взрослых был достигнут на моделях грызунов благодаря возможности использования новых технологий, включая создание трансгенных мышей.
Нейрогенез у взрослых начинается с деления нейральных предшественников в субгранулярной зоне ЗИ. Предшественники мигрируют в гранулярный слой, обычно оставаясь в его внутренней трети (Esposito, 2005). Многие из этих новорожденных нейронов не доживают до зрелости. Существуют по крайней мере две важные критические точки для их выживания: первая – в течение первых нескольких дней после рождения, а вторая – примерно через три недели (Ming, Song, 2011).
Различные формы активации окружающей среды стимулируют покоящиеся радиальные глиальные клетки и способствуют их пролиферации. Активные радиальные глиальные клетки самообновляются, а также генерируют ряд промежуточных нейральных предшественников, которые впоследствии дифференцируются в нейробласты и, наконец, дают начало гранулярным нейронам ЗИ, а также, в меньшей степени, астроцитам. Эти процессы, включая пролиферацию, дифференцировку, миграцию, распространение нейритов и синаптическую интеграцию, регулируются рядом сигналов из локальных нейронных цепей, в частности, глутаматергическими и ГАМКергическими входами (Ge et al., 2007; Bergami, et al., 2015; Alvarez et al., 2016). Глутаматергические входы через NMDA-рецепторы имеют решающее значение для выживания незрелых нейронов (Tashiro et al., 2006); выжившие нейроны функционально интегрируются в существующие цепи в течение одного месяца (Jessberger, Kempermann, 2003). Осцилляторная активность в гиппокампе может регулировать интеграцию молодых нейронов ЗИ в нейронные сети гиппокампа посредством облегчения передачи ГАМК-сигналов. Важно отметить, что обогащение окружающей среды оказывает существенное влияние на выживание и интеграцию новых гранулярных клеток у взрослых, хотя оно ограничивается первыми тремя неделями после рождения нейронов (Tashiro et al., 2007). В частности, обогащенная среда стимулирует ГАМКергические входы от парвальбумин-положительных интернейронов, способствуя интеграции и созреванию молодых нейронов ЗИ (Alvarez et al., 2016). В то же время стресс и старение уменьшают нейрогенез в ЗИ у взрослых посредством передачи сигналов от коры надпочечников, продуцирующих стероидные гормоны (Kuhn et al., 1996; Gould et al., 1997; Snyder et al., 2011).
Первичный вход к незрелым гранулам обеспечивает окружающая ГАМК (Ge et al., 2006), после чего в течение первых двух недель клеточной жизни развиваются синаптические связи от локальных тормозных интернейронов (Esposito et al., 2005; Deshpande, 2013). По мере развития незрелых гранул они посылают аксоны для контактов с нейронами поля CA3 (Faulkner et al., 2008; Toni et al., 2008); функциональные связи с CA3 могут наблюдаться через четыре–шесть недель (Restivo, 2015). Созревающие гранулы также образуют дендриты в молекулярном слое для получения входа от энторинальной коры через перфорантный путь (Esposito et al., 2005; Deshpande, 2013). Правильная интеграция новых гранул зависит от активности существующих в ЗИ нейронных цепей в период созревания.
В течение периода времени от четырех до шести недель после рождения новые гранулы электрофизиологически отличаются от своих зрелых собратьев. В исследованиях на переживающих срезах незрелые гранулы (в возрасте 40 дней) реагируют на более широкий диапазон входных сигналов (Marin-Burgin et al., 2012) и демонстрируют повышенную возбудимость и пластичность, которая отличает их от менее пластичной и относительно низковозбудимой популяции зрелых гранул (в возрасте 60 дней) (Espósito et al., 2005; Ge et al., 2007; Schmidt-Hieber et al., 2004; Mongiat et al., 2009). Вероятно, это обеспечено тем, что незрелые гранулы получают меньшее синаптическое торможение по сравнению со зрелыми гранулярными нейронами (Dieni et al., 2015). Это физиологическое состояние вновь рожденных клеток, отличное от обычных гранул, предполагает, что незрелые гранулы играют уникальную роль в сети ЗИ: по-видимому, они снижают возбудимость соседних зрелых гранулярных нейронов. Используя потенциал-зависимые красители, (Ikrar et al., 2013) обнаружили, что устранение нейрогенеза приводит к более широкому распространению деполяризации после стимуляции. В то же время усиление нейрогенеза (за счет удаления проапоптотического гена Bax) приводит к уменьшению плотности шипиков и снижению ВПСП в зрелых гранулах; напротив, подавление (нокдаун) нейрогенеза усиливает ВПСП в зрелых клетках (Adlaf et al., 2017). Эти результаты предполагают, что некоторые существующие синаптические контакты могут перераспределяться от зрелых к незрелым нейронам, поскольку последние интегрируются в нейронную сеть ЗИ. Общее время достижения зрелого морфологического и электрофизиологического фенотипа вновь рожденных гранул у грызунов составляет приблизительно восемь недель.
Включение новых клеток в нейронную сеть гиппокампа – замечательный пример пластичности. Сохранение у млекопитающих такого энергозатратного процесса генерации и отбраковки новых нейронов предполагает, что вновь рожденные гранулы должны иметь некоторые важные функции, в частности, в обучении и памяти, выполнение которых невозможно лишь с помощью гранулярных клеток, рожденных в процессе развития. Существуют данные о том, что незрелые гранулярные клетки включаются преимущественно в нейронные цепи, поддерживающие пространственную память (Kee et al., 2007); при этом изменения в генерации незрелых гранулярных клеток коррелируют с силой ДП в зубчатой извилине (Park et al., 2015). Известно также, что подавление нейрогенеза нарушает обратное обучение (reversal learning) в водном лабиринте Морриса (Garthe et al., 2009), исполнение задачи активного избегания (Burghardt et al., 2012; Park et al., 2015) и распознавание местоположения на основе сенсорных экранов (Swan et al., 2014). В то же время усиление нейрогенеза может улучшать способность распознавать местоположение в задаче с сенсорным экраном (Creer et al., 2010) или в аналогичных задачах с использованием подкрепления в виде удара слабым электрическим током (выработка условнорефлекторного страха) (Sahay et al., 2011). Сообщалось также, что, хотя усиление нейрогенеза при возрастании подвижности (бега) приводило к менее устойчивой памяти о местоположении платформы в водном лабиринте Морриса, тем не менее бег увеличивал скорость обратного обучения (Epp et al., 2016).
Недавно было высказано предположение, что нейрогенез у взрослых может играть роль не только в приобретении новой информации, но и в ее забывании (Akers et al., 2014). Результаты, полученные в приведенной работе, предполагают, что высокие темпы нейрогенеза в раннем постнатальном периоде способствуют детской амнезии, а усиление нейрогенеза у взрослых может способствовать забыванию. Однако почему тогда у большинства млекопитающих сохраняется нейрогенез, если он способствует забыванию? Не объясняется ли это тем, что объем памяти ограничен, и в связи с этим есть необходимость дестабилизации и удаления некоторых старых следов памяти для включения новых воспоминаний в специфическую сеть? В настоящее время гипотеза о том, что нейрогенез взрослых способствует забыванию, является спорной: в этой области нет единого мнения о том, является ли забывание отдельным процессом, принципиально отличающимся от пластичности.
1.3.5. Синаптическая пластичность в зубчатой извилине
Именно в ЗИ впервые было обнаружено такое замечательное свойство нейронных связей, как долговременная потенциация (ДП), определяемая как длительное повышение эффективности синаптической передачи. В 1966 г. в экспериментах на кроликах было обнаружено, что высокочастотная стимуляция перфорантного пути (ПП) вызывала увеличение первоначального ответа гранулярных клеток ЗИ на одиночные стимулы, наносимые на этот путь с исходными параметрами (Lomo, 1966). В 1973 г. это явление было более детально описано Блиссом и Ломо (Bliss, Lomo, 1973). Практически одновременно с этим открытием наши соотечественники обнаружили ДП в поле СА3 гиппокампа кролика при раздражении гранулярного слоя ЗИ, являющейся первым звеном кортикального входа в гиппокамп в его трисинаптической цепи (Брагин, 1973; Брагин, Виноградова, 1973). Достигнутый уровень потенциации мог сохраняться до 12 ч и более (несколько дней).
Позднее в экспериментах Брагина и Виноградовой было неожиданно выявлено, что стимуляция ПП или мшистых волокон приводила не только к развитию ДП, но и к параллельному подавлению реакций нейронов гиппокампа на сенсорные стимулы (Виноградова, 1975; Брагин и др., 1976). При этом была выявлена отрицательная корреляция между степенью ДП и уровнем реактивности на сенсорные раздражители. В то же время за пределами потенцированного сегмента гиппокампа сенсорные реакции нейронов были вполне нормальными (Брагин и др., 1976). Таким образом, потенцирование синапсов имитировало эффект привыкания, как если бы первоначально новые сенсорные стимулы стали знакомыми и не включали внимание. Авторы рассматривали это явление как дополнительный механизм селективной обработки информации и защиту от интерферирующих сигналов.
Интересно, что ДП в системе “гранулярные нейроны–пирамидные клетки СА3” посредством мшистых волокон не зависит от активации ионотропных глутаматных NMDA-рецепторов (Grover, Teyler, 1990), хотя эти рецепторы участвуют в кратковременной пластичности синапсов в данной системе связи (Lituma et al., 2021). В то же время в других отделах гиппокампа NMDA-рецепторы участвуют в осуществлении ДП (Buonomano, 1999; Honoré et al., 2021). Это подтверждает мнение, что активация NMDA-рецепторов может быть критична для индукции многих форм ДП, но не необходима для всех ее форм (Lynch, 2004). Относительно участия метаботропных глутаматных mGluR1-рецепторов для осуществления ДП с участием мшистых волокон мнения расходятся. У мутантных мышей, лишенных mGluR1, одна группа исследователей сообщала, что ДП отсутствовала (Conquet et al., 1994), но это не было подтверждено результатами, полученными второй группой авторов (Hsia et al., 1995; Martin, Morris, 1997).
Вход гранулярных нейронов к мшистым клеткам обнаруживает ДП с характеристиками, сходными с таковыми в системе связи “гранулярные нейроны–пирамидные клетки CA3” (Lysetskiy et al., 2005). Однако вопрос о том, существует ли ДП в синапсах, образуемых мшистыми клетками на гранулярных нейронах (т.е. в системе связи в обратном направлении), имеются разногласия. Одно исследование in vivo показало ДП полевых потенциалов, зарегистрированных во внутреннем молекулярном слое (т.е. вблизи гранулярных клеток) после высокочастотной стимуляции хилуса (Hetherington et al., 1994). Но другое исследование, тоже in vivo, не обнаружило ДП в синапсах “мшистые клетки – гранулярные нейроны” (Kleschevnikov, Routtenberg, 2003).
До настоящего времени продолжается изучение ДП в синапсах “ПП–гранулярные нейроны”, начатое Блиссом и Ломо (Lomo, 1966; Bliss, Lomo, 1973; Bliss, Collingridge, 1993), которые частично проясняют некоторые упомянутые выше вопросы. Обнаружено, в частности, что стимуляция ПП вызывает ДП не только в синапсах, образуемых этим входом на гранулярных клетках, но и гетеросинаптическую ДП ответа, вызываемого во внутреннем молекулярном слое, указывая, что вход мшистых клеток к гранулярным нейронам может быть усилен (Kleschevnikov, Routtenberg, 2003). Другое исследование in vivo показало, что ДП в синапсах “ПП–гранулярные нейроны” ведет к ДП в синапсах “контралатеральные мшистые клетки–гранулярные клетки” (Alvarez-Salvado et al., 2014). Это исследование показало, что ДП в синапсах “ПП–гранулярные нейроны” индуцирует пластичность “нижележащих” мшистых клеток и, таким образом, оказывается пермиссивной для ДП в синапсах “мшистые клетки–гранулярные нейроны”. Было высказано предположение, что входы мшистых клеток и перфорантного пути к гранулярным нейронам кооперативны (Namgung et al., 1997; Kleschevnikov, Routtenberg, 2003). Более поздние исследования подтвердили, что мшистые клетки могут способствовать или даже быть необходимыми для ДП в синапсах “ПП–гранулярные клетки”. Так, имиджинг потенциалов в срезах гиппокампа показал, что цепь “гранулярный нейрон–мшистая клетка–гранулярный нейрон” необходима для ДП в синапсах “ПП–гранулярная клетка” (Wright, Jackson, 2014). Кроме того, ранее было выявлено, что экспрессия белка GAP43 (нейромодулина) была увеличена в мшистых клетках в ответ на высокочастотную стимуляцию ПП, что могло поддерживать устойчивость ДП (Namgung et al., 1997). Таким образом, подтверждается предположение о кооперативности входов мшистых клеток и перфорантного пути к гранулярным нейронам (Namgung et al., 1997; Kleschevnikov, Routtenberg, 2003).
Интересно, что нейротрофический фактор головного мозга (НТФ) при его внутригиппокампальном введении приводит к усилению синаптического ответа в ЗИ (Messaoudi et al., 1998). Более того, ДП может быть вызвана повышением уровня НТФ, подобно тому, как она индуцируется тетанической стимуляцией ПП; при этом НТФ-вызванная ДП, как и при стимуляции ПП, обеспечивается активацией NMDA-рецепторов и обнаруживает зависимость от ERK и CREB (Messaoudi et al., 2002). Параллельно с индукцией ДП в ЗИ было выявлено увеличение мРНК и белка НТФ после обучения в тесте пассивного избегания. Было также показано, что поведение в водном лабиринте нарушалось у крыс, которым интрацеребровентрикулярно вводили антитела против НТФ (Mu et al., 1999). Более того, подавление экспрессии НТФ в ЗИ нарушало обучение в парадигме пассивного избегания (Nosten-Bertrand et al., 1996).
Интересно, что ДП в ЗИ облегчается, как показано, пространственным обучением (Kemp, Manahan-Vaughan, 2008a, 2008b), а также стимуляцией голубого пятна (locus coeruleus) (Hansen и Manahan-Vaughan, 2014). Обнаружено также, что голубое пятно активируется во время обучения, приводящего к накоплению нового опыта (Sara et al., 1994; Kitchigina et al., 1997). Таким образом, когда оно активировано, происходит активация β-адренорецепторов в ЗИ, что способствует кодированию нового опыта с помощью синаптической пластичности, что было подтверждено впоследствии (Gelinas and Nguyen, 2005; Kemp, Manahan-Vaughan, 2008a, 2008b; Hagena, Manahan-Vaughan, 2012; Goh et al., 2013). Позже было показано, что нейроны голубого пятна, экспрессирующие тирозин-гидроксилазу, могут опосредовать кодирование нового опыта и улучшение памяти в результате совместного высвобождения норадреналина и дофамина в гиппокампе (Takeuchi et al., 2016).
Дендритные шипики гранулярных клеток ЗИ – это основные мишени, куда поток информации из неокортекса поступает к гиппокампу. Синаптическая пластичность может быстро влиять на способность синапсов хранить информацию; показано, что в срединном молекулярном слое ЗИ через 30 мин после индукции ДП in vivo заметно увеличилось количество как малых, так и больших шипиков, что могло быть временным явлением (Bromer, 2018).
Кроме ДП, другая форма синаптической пластичности была выявлена в ЗИ, а именно долговременная депрессия (ДД), вызываемая в основном низкочастотной (1–2 Гц) стимуляцией медиального ПП в течение 5–15 мин. Эта форма пластичности определяется как длительное (от часов до недель) снижение эффективности синаптической передачи (Sahay et al., 2007). В зубчатой извилине ДП (Bliss, Gardner-Medwin, 1973; Douglas and Goddard, 1975) и ДД (Levy and Steward, 1979; Doyѐre et al., 1997; Abraham et al., 2001) могут одновременно индуцироваться в соседних синаптических путях, через медиальный и латеральный ПП. При изучении in vivo ДП и сопутствующая ДД в ЗИ могут сохраняться в течение многих месяцев (Abraham et al., 1994, 2002). ДД, так же, как и ДП, присутствующие в синапсах ЗИ, участвуют в когнитивных процессах, таких как обучение и память.
При использовании стимуляции ЗИ при обучении был выявлен низкий порог внутренней пластичности (Lopez-Rojas et al., 2016). Применение трехмерной электронной микроскопии выявило заметное расширение динамического диапазона размеров синапсов и снижение коэффициента вариации после индукции ДП в зубчатой извилине. Эти изменения приводили к значительному увеличению емкости хранения информации, которая, тем не менее, была значительно ниже емкости синапсов CA1 даже в контроле.
Поскольку ЗИ свойствен нейрогенез, в ней обнаружена синаптическая пластичность, на которую влияет относительный нейрональный возраст (Saxe et al., 2006; Snyder et al., 2001). В опытах ex vivo, на срезах гиппокампа, незрелые гранулярные нейроны имеют более низкий порог пластичности, чем зрелые гранулы (Ratzliff et al., 2002; Toni et al., 2002). Изменения в генерации незрелых гранулярных клеток коррелируют с силой ДП в зубчатой извилине (Park et al., 2015). Имеет место общее представление о существовании положительной корреляции между нейрогенезом и силой синаптической пластичности у взрослых (Khuu et al., 2019).
1.4. РОЛЬ ЗИ В КОГНИТИВНЫХ ФУНКЦИЯХ
Предполагается, что ЗИ участвует во многих когнитивных функциях, включая детекцию новизны (Vinogradova, 2001; Hunsaker et al., 2008), разделение и завершение паттернов (pattern separation and pattern completion) (Rolls, 2016), пространственную рабочую память (Sasaki et al., 2018), кодирование информации (Treves, Rolls, 1994; Rolls, 2018) и консолидацию памяти (Nakashiba et al., 2008; Kitamura et al., 2014; Park et al., 2016; Sasaki et al., 2018).
Участие ЗИ в способности гиппокампа определять, что является знакомым для мозга, а что является новым в окружающей среде (Vinogradova, 2001; Hunsaker et al., 2008; Aggleton et al., 2013), осуществляется благодаря тому, что ЗИ получает афференты как от стволовых структур (несущих “сырые” сигналы об окружающей среде), так и от неокортекса (передающего хранящуюся в памяти обработанную информацию). В этом процессе гиппокамп выполняет компараторную функцию (Виноградова, Дудаева, 1972; Vinogradova, 2001).
Выявление роли ЗИ в ментальных процессах основано, в частности, на результатах экспериментов с оптогенетической активацией специфичных для памяти нейронных ансамблей, или “энграмм”, в ЗИ, которая может вызывать искусственное воспроизведение памяти у мышей (Liu et al., 2012) и создавать мнемонические ассоциации между отдельными событиями (Ramirez et al., 2013). Данные об активности ЗИ во время распознавания и планирования на основе памяти (Gilbert et al., 2001; van Dijk, Fenton, 2018) и опосредованного гранулярными нейронами торможения в CA3 (Ruediger et al., 2011; Guo et al., 2018) позволяют предполагать, что активность нейронов ЗИ повышает точность во время воспроизведения памяти.
Участие ЗИ в кодировании информации и консолидации памяти. Основная гипотеза относительно роли гранулярных нейронов в кодировании информации заключается в том, что они вовлекают уникальные ансамбли пирамидных клеток CA3 во время кодирования сигналов в системе памяти, которые затем становятся ассоциированными с энторинальным входным паттерном, представляющим новую информацию (Treves, Rolls, 1994). Поскольку синаптическая пластичность постоянно усиливает внутренние и внешние связи этих клеточных ансамблей, ПП-входы могут реактивировать их, способствуя воспроизведению ассоциированной памяти без необходимости активации входов через мшистые волокна (Rolls, 2018).
Предполагается, что входы от гранулярных клеток к CA3 могут потребоваться для ранней консолидации памяти. Считают, что в поддержании памяти во время ее консолидации ключевую роль играет кратковременное увеличение двух входов к пирамидным нейронам СА3: входа от энторинальной коры (посредством ПП) и от гранулярных клеток (через мшистые волокна) (Park et al., 2016; Sasaki et al., 2018). Долгое время считалось, что информация проходит через гиппокамп однонаправленно по трисинаптической петле ЗИ–CА3–CА1; однако позже теоретические (Treves, Rolls, 1994) и экспериментальные данные (McNaughton, Barnes, 1989; Kitamura et al., 2014, 2015) изменили эту точку зрения. Во-первых, индивидуальные пирамидные клетки CA3 получают входные данные от тысяч энторинальных и других пирамидных нейронов CA3, но только от ~50 гранулярных клеток (Amaral, Ishizuka, 1990), и многие из этих клеток “молчат” во время исследовательского поведения (Chawla et al., 2005; Hainmueller, Bartos, 2018). Вследствие этого, хотя образуемые мшистыми волокнами одиночные синапсы эффективнее, чем ПП- или рекуррентно-коллатеральные входы, только залповая активность гранулярных клеток способна рекрутировать пирамидные нейроны CA3, тогда как низкочастотное возбуждение в первую очередь активирует прямое ингибирование (Henze et al., 2002; Mori et al., 2004; Zucca et al., 2017). Следовательно, существуют ситуации, когда правильное сохранение и поиск в памяти могут быть достигнуты только на основе цикла ПП–CA1 или ПП–CA3–CA1 (Nakashiba et al., 2008; Kitamura et al., 2014).
Большинство авторов из своих наблюдений делает вывод, что активность гранулярных нейронов может быть необходимой во время начального кодирования и ранней консолидации памяти в гиппокампе, но необязательна для ее воспроизведения (Lee, Kesner, 2004; Lassalle et al., 2000; Kheirbek et al., 2013; Denny et al., 2014; Madroñal et al., 2016; Park et al., 2016).
Наибольшее внимание в исследованиях когнитивной роли зубчатой извилины уделяется ее основным функциям: “разделению паттернов” (pattern separation) и “завершению паттернов” (pattern completion). Под разделением паттернов подразумевается способность сети ЗИ принимать паттерн афферентных входов от неокортекса и “разделять” его так, чтобы выходы были менее похожи, чем входы (Marr, 1971; McNaughton, Morris, 1987; Rolls, 2006, 2016; Kesner, 2007; Myers, Scharfman, 2011; Yassa, Stark, 2011; Jung et al., 2013). Например, если два паттерна активности, поступающие в ЗИ, перекрываются на 50%, а активность пирамидных клеток CA3 после воздействия этих двух паттернов перекрывается только на 20%, можно сделать вывод, что произошло разделение паттернов. Обычно гранулярные клетки ЗИ имеют удивительно низкую частоту разрядов, и уменьшение перекрытия, как полагают, частично достигается за счет разреженного кодирования в ЗИ, где низки уровни активности, что известно из электрофизиологических и иммуногистохимических данных (Chawla et al., 2005; Leutgeb et al., 2007; Jung et al., 2013; Deng et al., 2013). Эта разреженность обеспечивает способность распознавать и усиливать небольшие различия во входных сигналах, что позволяет избежать интерференции. Перекрывающиеся паттерны из кортикальных входов могут быть рассредоточены по большому количеству гранул в ЗИ с низкой активностью, которые, в свою очередь, образуют немногие, но эффективные синапсы на пирамидных клетках CA3 (McNaughton, Morris, 1987). С помощью ЗИ субъекты могут различать похожие воспоминания, потому что редкая связь между гранулами и пирамидными клетками CA3 гарантирует, что никакие две пирамидные клетки не получают входных данных от одних и тех же гранулярных нейронов (McHugh et al., 2007; Leutgeb et al., 2007). Эта предполагаемая функция поддерживается сетью ЗИ, которая состоит из входов от энторинальных нейронов (слоя II), локальных взаимодействий между гранулярными клетками, мшистыми клетками и интернейронами, и выходов к пирамидным клеткам CA3. Т.к. количество гранулярных клеток (один миллион в каждом полушарии крысы) в пять раз больше, чем входных нейронов из энторинальной коры, и они не общаются друг с другом напрямую (Amaral, 1978), разветвленная связь в энторинально-гранулярной клеточной системе может сегрегировать даже незначительные, но важные различия, присутствующие во входных паттернах (McNaughton, Morris, 1987; McNaughton, Nadel, 1990). В частности, мшистые клетки получают ограниченное, но сильное сходящееся возбуждение от 40 до 100 гранулярных клеток и возвращают билатеральное, широко распространенное возбуждение в гранулы (Buckmaster et al., 1992, 1996).
Было высказано предположение, что разделение паттернов в ЗИ важно для хранения памяти в области CA3, поскольку оно позволяет сохранять аналогичную накопленную опытом информацию в различных подмножествах пирамидных клеток CA3, облегчая, таким образом, точное извлечение памяти (Yassa, Stark, 2011). Эта теория предполагает, что афферентные входные паттерны, которые подвержены разделению, поступают в ЗИ через ПП. Однако основной, не менее важный, глутаматергический вход к гранулярным клеткам обеспечивают мшистые клетки; это дает основание предполагать, что они могут посылать входные паттерны гранулярным клеткам в дополнение к перфорантному пути или вместо него (Scharfman, 2016). На поведенческом уровне предполагаемое проявление разделения паттернов – это улучшение различения очень похожих событий или окружающих условий.
Завершение паттернов (pattern copmpletion) соотносят с извлечением из сохраненной памяти, когда предъявляется часть (фрагмент) исходного входного сигнала, подаваемого при его запоминании (McNaughton, Morris, 1987; Hunsaker et al., 2008; Nakashiba et al., 2012). Разделение паттернов и завершение паттернов часто обсуждаются, когда речь идет о ЗИ и CA3 соответственно, и их связи посредством мшистых волокон. В срезах гиппокампа основным эффектом активации CA3 является ГАМКергическое ингибирование гранулярных клеток (Scharfman, 1994). Однако достаточно длительная задержка между потенциалом действия пирамидных клеток и началом торможения гранулярных клеток (10–20 мс) предполагает, что простой путь: “пирамидная клетка–интернейрон–гранулярная клетка” – неактивен; вместо этого путь: “пирамидная клетка–мшистая клетка–интернейрон–гранулярная клетка” или путь: “пирамидная клетка–пирамидная клетка–интернейрон–гранулярная клетка” – по-видимому, более вероятны (Scharfman, 1994a). Примечательно, что надежный дисинаптический путь возбуждения: “пирамидная клетка–мшистая клетка–гранулярная клетка” – выявляется, когда ГАМКергическое ингибирование блокируется (Scharfman, 1994).
Обратное проецирование от CA3 к ЗИ может влиять на сети в ЗИ и CA3 (Penttonen et al., 1997; Lisman et al., 2005), и существование этого пути может играть значительную роль в обработке информации. Действительно, использование компьютерного моделирования показало, что эта обратная проекция играет решающую роль в способности вычислительной модели выполнять моделируемое разделение и завершение паттернов (Myers, Scharfman, 2011).
Роль нейронов, вновь рожденных у взрослых, в процессах “разделения поведенческих паттернов” была продемонстрирована путем подавления нейрогенеза и оценки способности различать похожие контексты при обусловливании страхом (Tronel et al., 2012; Nakashiba et al., 2012; Danielson et al., 2016), в частности, близлежащие местоположения в радиальном лабиринте (Clelland et al., 2009) и пары “объект–местоположение” (Bekinschtein et al., 2014). Вновь рожденные нейроны наиболее наглядно влияют на успешность при выполнении задач, когда представляется новая или противоречивая информация, то есть условия, при которых, как ожидается, в ЗИ будут направляться перекрывающиеся паттерны сенсорной информации и в ней будет происходить разделение паттернов. Действительно, подавление нейрогенеза нарушает обратное обучение в водном лабиринте Морриса (Garthe et al., 2009), выполнение задачи активного избегания (Burghardt et al., 2012; Park et al., 2015) и распознавание местоположения на основе сенсорных экранов (Swan et al., 2014).
ЗАКЛЮЧЕНИЕ
Многочисленные исследования зубчатой извилины свидетельствуют о ее важной роли в функционировании гиппокампа. ЗИ, являясь его внутренним реле, необходима для осуществления таких когнитивных функций, как распознавание новизны, кодирование информации и консолидация памяти, в которых гиппокамп играет ключевую роль. Это обеспечивается чрезвычайной синаптической пластичностью связей, а именно пластическими изменениями в синапсах, образуемых аксонами перфорантного пути на гранулярных нейронах, и в синапсах, образуемых аксонами гранулярных клеток на мшистых клетках и на нейронах поля СА3 гиппокампа. Однако относительно наличия длительной потенциации в синапсах, образуемых мшистыми клетками на гранулярных нейронах, существуют противоречивые сообщения. Кроме этого, количественные данные о входах и выходах мшистых клеток неполны как для грызунов, так и, особенно, для приматов. Окончательно не выяснены функции мшистых клеток. Имеются разногласия относительно специфической роли происходящего в ЗИ нейрогенеза для когнитивных функций у взрослых млекопитающих и человека. Пока еще существует определенный разрыв между исследованиями нейронной сети зубчатой извилины и изучением зависимого от ЗИ поведения, поэтому главной целью в будущем относительно функций ЗИ, как предполагается, будет заполнение этого разрыва.
Список литературы
Брагин А.Г. Характер ответов пирамид поля СА3 гиппокампа на электрическую стимуляцию зубчатой фасции. Лимбическая система мозга. Под ред А.Н. Черкашина и К.Н Культас, Пущино, 1973, стр. 141–160.
Брагин А.Г., Виноградова О.С. Явление хронической потенциации в кортикальном афферентном входе пирамид поля СА3 гиппокампа. Физиологические механизмы памяти. Пущино-на-Оке: Изд-во НЦБИ Пущино. 1973. С. 8–24.
Брагин А.Г., Виноградова О.С., Емельянов В.В. Пространственная организация нейронов поля СА3 гиппокампа на электрическую стимуляцию зубчатой фасции. Журнал высшей нервной деятельности им. И.П. Павлова. 1976. 26(3), 605–611.
Виноградова О.С. Гиппокамп и память. Москва, Наука, 1975.
Виноградова О.С., Дудаева К.И. О компараторной функции гиппокампа. Доклады АН СССР. 1972. 202(1), 241–244.
Кичигина В.Ф., Брагин А.Г. Функциональные характеристики основных внутренних систем связей гиппокампа. Нейрофизиология 1976. 6(3): 259–266.
Abraham W.C., Christie B.R., Logan B., Lawlor P., Dragunow M. Immediate early gene expression associated with the persistence of heterosynaptic long-term depression in the hippocampus. Proc. Natl. Acad Sci. USA. 1994. 91: 10049–10053.
Abraham W.C., Logan B., Greenwood J.M., Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J. Neurosci. 2002. 22: 9626–9634.
Abraham W.C., Mason-Parker S.E., Bear M.F., Webb S., Tate W.P. Heterosynaptic metaplasticity in the hippocampus in vivo: A BCM-like modifiable threshold for LTP. Proc. Natl. Acad. Sci. USA 2001. 98: 10924–10929.
Acsady L., Kamondi A., Sik A., Freund T., Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998. 18: 3386–3403.
Adams B., Lee M., Fahnestock M., Racine R. Long-term potentiation trains induce mossy fiber sprouting. Brain Res. 1997. 775: 193–197.
Aizenman E., Stout A.K., Hartnett K.A., Dineley K.E., McLaughlin B., Reynolds I.J. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J. Neurochem. 2000. 75: 1878–1888.
Aggleton J.P., Brown M.W., Albasser M.M. Contrasting brain activity patterns for item recognition memory and associative recognition memory: insights from immediate-early gene functional imaging. Neuropsychologia. 2013. 50: 3141–3155.
Althaus A., Zhang H., Parent J. Axonal plasticity of age-defined dentate granule cells in a rat model of mesial temporal lobe epilepsy. Neurobiol. Dis. 2016. 86: 187–96.
Alvarez-Salvado E., Pallares V., Moreno A., Canals S. Functional MRI of long-term potentiation: imaging network plasticity. Phil. Trans. R. Soc. B. 2014. 369: 20130152.
Amaral D.G. A golgi study of cell types in the hilar region of the hippocampus in the rat. J. Comp. Neurol. 1978. 15: 851–914.
Amaral D.G., Campbell M.J. Transmitter systems in the primate dentate gyrus. Hum. Neurobiol. 1986. 5: 169–180.
Amaral D.G., Dent J.A. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J. Comp. Neurol. 1981. 195: 51–86.
Amaral D.G., Ishizuka N., Claiborne B. Neurons, numbers and the hippocampal network. Prog. Brain Res. 1990. 83: 1–11.
Amaral D.G., Scharfman H.E., Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 2007. 163: 3–22.
Axmacher N., Elger C.E., Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain J. Neurol. 2008. 131: 1806–1817.
Beck H., Blumcke I., Kral T., Clusmann H., Schramm J., Wiestler, O.D., Elger, C.E. Properties of a delayed rectifier potassium current in dentate granule cells isolated from the hippocampus of patients with chronic temporal lobe epilepsy. Epilepsia. 1996. 37. 892–901.
Bekinschtein P., Kent B.A., Oomen C.A., Clemenson G.D., Gage F.H., Saksida L.M., Bussey T.J. Brain-derived neurotrophic factor interacts with adult-born immature cells in the dentate gyrus during consolidation of overlapping memories. Hippocampus. 2014. 24: 905–911.
Bekirov I.H., Nagy V., Svoronos A., Huntley G.W., Benson D.L. Cadherin-8 and N-cadherin differentially regulate pre- and postsynaptic development of the hippocampal mossy fiber pathway. Hippocampus 2008. 18: 349–63.
Berger T.W., Semple-Rowland S., Bassett J.L. Hippocampal polymorph neurons are the cells of origin for ipsilateral association and commissural afferents to the dentate gyrus. Brain Res. 1981. 224: 329–336.
Binder D.K., Croll S.D., Gall C.M., Scharfman H.E. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001. 24: 47–53.
Bittencourt S., Covolan L., Hamani C., Longo B., Faria F., Freymuller E., Ottersen O.P., Mello L.E. Replacement of asymmetric synaptic profiles in the molecular layer of dentate gyrus following cycloheximide in the pilocarpine model in rats. Front Psychiatry. 2015. 6: 157.
Blackstad T.W., Brink K., Hem J., Jeun B. Distribution of hippocampal mossy fibers in the rat. An experimental study with silver impregnation methods. J. Compar. Neurol. 1970. 138: 433–447.
Blackstad T.W., Kjaerheim A. Special axo-dendritic synapses in the hippocampal cortex: electron and light microscopic studies on the layer of mossy fibers. J. Comp. Neurol. 1961. 117: 133–159.
Blasco-Ibáñez J.M., Freund T.F. Distribution, ultrastructure, and connectivity of calretinin immunoreactive mossy cells of the mouse dentate gyrus. Hippocampus. 1997. 7: 307–320.
Bliss T.V., Gardner-Medwin A.R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J. Physiol. 1973. 232: 357–374.
Bliss T.V., Collingridge G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993. 361: 31–39.
Bliss T.V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973. 232: 331–356.
Blümcke I., Zuschratter W., Schewe J.C., Suter B., Lie A.A., Riederer B.M., Meyer B., Schramm J., Elger C.E., Wiestler O.D. Cellular pathology of hilar neurons in Ammon’s horn sclerosis. The Journal of Comparative Neurology. 1999. 414: 437–453.
Bragin A., Jandó G., Nádasdy Z., van Landeghem M., Buzsáki G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J. Neurophysiol. 1995. 73: 1691–1705.
Bromer C., Bartol T.M., Bowden J.B., Hubbard D.D., Hanka D.C., Gonzalez P.V., Kuwajima M., Mendenhall J.M., Parker P.H., Abraham W.C., Sejnowski T.J., Harris K.M. Long-term potentiation expands information content of hippocampal dentate gyrus synapses. Proc. Natl. Acad. Sci. U S A. 2018. 115(10): E2410-E2418.
Bronzino J.D., Kehoe P., Mallinson K., Fortin D.A. Increased extracellular release of hippocampal NE is associated with tetanization of the medial perforant pathway in the freely moving adult male rat. Hippocampus. 2001. 11: 423–429.
Brown R.A., Walling S.G., Milway J.S., Harley C.W. Locus ceruleus activation suppresses feedforward interneurons and reduces β-γ electroencephalogram frequencies while it enhances θ frequencies in rat dentate gyrus. J. Neurosci. 2005. 25: 1985–1991.
Buckmaster P. Does mossy fiber sprouting give rise to the epileptic state? In: Scharfman H., Buckmaster P., editors. Issues in Clinical Epileptology: A View From the Bench, Advances in Experimental Medicine and Biology. Vol. 813. Dordrecht: Springer. 2014.
Buckmaster P.S., Strowbridge B.W., Kunkel D.D., Schmiege D.L., Schwartzkroin P.A. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus. 1992. 2: 349–362.
Buckmaster P.S., Abrams E., Wen X. Seizure frequency correlates with loss of dentate gyrus GABAergic neurons in a mouse model of temporal lobe epilepsy. J. Comp. Neurol. 2017. 525(11): 2592–2610.
Buckmaster P.S., Wenzel H.J., Kunkel D.D., Schwartzkroin P.A. Axon arbors and synaptic connections of hippocampal mossy cells in the rat in vivo. J. Comp Neurol. 1996. 366: 271–292.
Buonomano D.V. Distinct Functional Types of Associative Long-Term Potentiation in Neocortical and Hippocampal Pyramidal Neurons. J. Neurosci. 1999. 19(16): 6748–6754.
Burgess N., Maguire E., O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron 2002. 35: 625–641.
Burghardt N.S., Park E.H., Hen R., Fenton A.A. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012. 22: 1795–1808.
Chawla M.K., Guzowski J.F., Ramirez-Amaya V., Lipa P., Hoffman K.L., Marriott L.K., Worley P.F., McNaughton B.L., Barnes C.A. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005. 15: 579–586.
Chicurel M.E., Harris K.M. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J. Comp. Neurol. 1992. 325: 169–182.
Clelland C.D., Choi M., Romberg C., Clemenson G.D. Jr., Fragniere A., Tyers P., Jessberger S., Saksida L.M., Barker R.A., Gage F.H., Bussey T.J. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009. 325: 210–213.
Cole T.B., Wenzel H.J., Kafer K.E., Schwartzkroin P.A., Palmiter R.D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. U S A. 1999. 96: 1716 –1721.
Colling S., Khana M., Collinge J., Jefferys J. Mossy fibre reorganization in the hippocampus of prion protein null mice. Brain Res. 1997. 755: 28–35.
Colom L.V., Castañeda M.T., Reyna T., Hernandez S., Garrido-Sanabria E. Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse. 2005. 58: 151–164.
Conquet F., Bashir Z.I., Davies C.H., Daniel H., Ferraguti F., Bordi F., Franz-Bacon K., Reggiani A., Matarese V., Conde F., Collingridge G.L. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994. 372: 237–243.
Cossart R., Dinocourt C., Hirsch J.C., Merchan-Perez A., De F.J., Ben-Ari Y., Esclapez M., Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat. Neurosci. 2001. 4: 52–62.
Coulter D.A., Carlson G.C. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog. Brain Res. 2007. 163: 235–243.
Cronin J., Obenaus A., Houser C., Dudek F. Electrophysiology of dentate granule cells after kainate-induced synaptic reorganization of the mossy fibers. Brain Res. 1992. 573: 305–310.
Danielson N.B., Kaifosh P., Zaremba J.D., Lovett-Barron M., Tsai J., Denny C.A., et al. Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding. Neuron. 2016. 90: 101–112.
Das A., Wallace G.C., Holmes C., McDowell M.L., Smith J.A., Marshall JD, Bonilha L., Edwards J.C., Glazier S.S., Ray S.K., Banik N.L. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience. 2012. 220: 237–246.
Deller T., Nitsch R., Frotscher M. Phaseolus vulgaris–leucoagglutinin tracing of commissural fibers to the rat dentate gyrus: evidence for a previously unknown commissural projection to the outer molecular layer. J. Comp. Neurol. 1995. 352: 55–68.
Deller T., Katona I., Cozzari C., Frotscher M., Freund T.F. Cholinergic innervation of mossy cells in the rat fascia dentata. Hippocampus. 1999. 9: 314–320.
Deng W., Mayford M., Gage F.H. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. Elife. 2013. 2: e00312.
Dengler C.G., Coulter D.A. Normal and epilepsy-associated pathologic function of the dentate gyrus. Prog. Brain Res. 2016. 226: 155–178.
Denny C.A., Kheirbek M.A., Alba E.L., Tanaka K.F., Brachman R.A., Laughman K.B., Tomm N.K., Turi G.F., Losonczy A., Hen R. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014. 83: 189–201.
van Dijk M.T., Fenton A.A. On How the Dentate Gyrus Contributes to Memory Discrimination. Neuron. 2018. 98: 832–845.e5.
Douglas R.M., Goddard G.V. Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 1975. 86: 205–215.
Doyère V., Srebro B., Laroche S. Heterosynaptic LTD, depotentiation in the medial perforant path of the dentate gyrus in the freely moving rat. J. Neurophysiol. 1997. 77: 571–578.
El Bahh B., Lespinet V., Lurton D., Coussemacq M., Le Gal La Salle G, Rougier A. Correlations between granule cell dispersion, mossy fiber sprouting, and hippocampal cell loss in temporal lobe epilepsy. Epilepsia. 1999. 40: 1393–1401.
Elmer E., Kokaia Z., Kokaia M., Lindvall O., McIntyre D. Mossy fibre sprouting: evidence against a facilitatory role in epileptogenesis. Neuroreport. 1997. 8: 1193–1196.
Engel Jr.J. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001. 42: 796–803.
Etter G., Krezel W. Dopamine D2 receptor controls hilar mossy cells excitability. Hippocampus. 2014. 24: 725–732.
Ezrokhi V.L., Zosimovskii V.A., Korshunov V.A., Markevich V.A. Restoration of decaying long-term potentiation in the hippocampal formation by stimulation of neuromodulatory nuclei in freely moving rats. Neuroscience. 1999. 88: 741–753.
Frederickson C.J. Neurobiology of zinc and zinc containing neurons. Int. Rev. Neurobiol. 1989. 31: 145–238.
Frederickson C.J., Bush A.I. Synaptically released zinc: physiological functions and pathological effects. Biometals. 2001. 14: 353–366.
Freund T.F. GABAergic septal and serotonergic median raphe afferents preferentially innervate inhibitory interneurons in the hippocampus and dentate gyrus. Epilepsy Res. Suppl. 1992. 7: 79–91.
Freund T.F., Buzsaki, G. Interneurons of the hippocampus. Hippocampus. 1996. 4: 347–470.
Freund T.F., Hajos N., Acsady L., Gorcs T.J., Katona I. Mossy cells of the rat dentate gyrus are immunoreactive for calcitonin gene-related peptide (CGRP). Eur. J. Neurosci. 1997. 9: 1815–1830.
Fricke R., Prince D. Electrophysiology of dentate gyrus granule cells. J. Neurophysiol. 1984. 51: 195–209.
Frotscher M., Seress L., Schwerdtfeger W.K., Buhl E. The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J. Comp. Neurol. 1991. 312: 145–163.
Hainmueller T., Bartos M. Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature. 2018. 558: 292–296.
Hagena H., Manahan-Vaughan D. Learning-facilitated longterm depression and long-term potentiation at mossy fiber-CA3 synapses requires activation of b-adrenergic receptors. Frontiers Integr. Neurosci. 2012. 6: 23.
Halasy K., Somogyi P. Subdivisions in the multiple GABAergic innervation of granule cells in the dentate gyrus of the rat hippocampus. Eur. J. Neurosci. 1993. 5: 411–429.
Halabisky B., Parada I., Buckmaster P.S., Prince D.A. Excitatory Input Onto Hilar Somatostatin Interneurons Is Increased in a Chronic Model of Epilepsy. J. Neurophysiol. 2010. 104(4): 2214–2223.
Hamlyn L.H. An electron microscope study of pyramidal neurons in the Ammon’s Horn of the rabbit. J. Anat. 1963. 97(Pt 2): 189–201.
Han Z.S., Buhl E.H., Lorinczi Z., Somogyi P. A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur. J. Neurosci. 1993. 5: 395–410.
Hansen N., Manahan-Vaughan D. Locus coeruleus stimulation facilitates long-term depression in the dentate gyrus that requires activation of b-adrenergic receptors. Cereb Cortex. 2015 Jul; 25(7): 1889–1896.
Hashimotodani Y., Karube F., Yanagawa Y., Fujiyama F., Kano M. Supramammillary Nucleus Afferents to the Dentate Gyrus Co-release Glutamate and GABA and Potentiate Granule Cell Output. Cell Rep. 2018. 25(10): 2704-2715.e4.
Haug F.M. Electron microscopical localization of the zinc in hippocampal mossy fibre synapses by a modified sulfide silver procedure. Histochemie. 1967. 8: 355–368.
Hendricks L., Chen Y., Bensen A., Westbrook G., Schnell E. Short-term depression of sprouted mossy fiber synapses from adult-born granule cells. J. Neurosci. 2017. 37: 5722–5735.
Heng K, Haney M, Buckmaster P. High-dose rapamycin blocks mossy fiber sprouting but not seizures in a mouse model of temporal lobe epilepsy. Epilepsia. 2013. 54: 1535–1341.
Henze D.A., Wittner L., Buzsáki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat. Neurosci. 2002. 5: 790–795.
Henze D.A., Buzsáki G. Hilar mossy cells: functional identification and activity in vivo. Prog. Brain Res. 2007. 163: 199–216.
Hetherington P.A., Austin K.B., Shapiro M.L. Ipsilateral associational pathway in the dentate gyrus: an excitatory feedback system that supports N-methyl-D-aspartate–dependent long-term potentiation. Hippocampus. 1994. 4: 422–438.
Hofmann M.E., Frazier C.J. Muscarinic receptor activation modulates the excitability of hilar mossy cells through the induction of an afterdepolarization. Brain Res. 2010. 1318: 42–51.
Holtmaat A., Gorter J., De Wit J., Tolner E., Spijker S., Giger R., Lopes da Silva F.H., Verhaagen J. Transient downregulation of Sema3A mRNA in a rat model for temporal lobe epilepsy. A novel molecular event potentially contributing to mossy fiber sprouting. Exp. Neurol. 2003. 182: 142–150.
Honoré E., Khlaifia A., Bosson A., Lacaille J.-C. Hippocampal Somatostatin Interneurons, Long-Term Synaptic Plasticity and Memory. Front. Neural. Circuits. 2021. 15: 687558.
Houser C.R. Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog. Brain Res. 2007. 163: 217–232.
Houser C.R., Peng Z., Wei X., Huang C.S., Mody I. Mossy Cells in the Dorsal and Ventral Dentate Gyrus Differ in Their Patterns of Axonal Projections. J. Neurosci. 2021. 41(5): 991–1004.
Hsia A.Y., Salin P.A., Catillo P.E., Abeliovich A., Tonegawa S., Nicoll R.A. Evidence against a role for metabotropic glutamate receptors in mossy fibre LTP: the use of mutant mice and pharmacological antagonists. Neuropharmacology. 1995. 34: 1567–1572.
Hsu T.T., Lee C.T., Tai M.H., Lien C.C. Differential recruitment of dentate gyrus interneuron types by commissural versus perforant pathways. Cereb. Cortex. 2016. 26: 2715–2727.
Hunsaker M.R., Rosenberg J.S., Kesner R.P. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008. 18: 1064–1073.
Hunsaker M.R., Mooy G.G., Swift J.S., Kesner R.P. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behav. Neurosci. 2007. 121(4): 742–750.
Igarashi K.M., Lu L., Colgin, L.L., Moser M.B., Moser E.I. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature. 2014. 510: 143–147.
Ikegaya Y. Abnormal targeting of developing hippocampal mossy fibers after epileptiform activities via L-type Ca2+channel activation in vitro. J. Neurosci. 1999. 19: 802–812.
Ishizuka N., Weber J., Amaral D.G. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J. Comp. Neurol. 1990. 295: 580–623.
Garthe A., Behr J., Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009. 4: e5464.
Gaykema R.P., Luiten P.G., Nyakas C., Traber J. Cortical projection patterns of the medial septum-diagonal band complex. J. Comp. Neurol. 1990. 293: 103–124.
Gelinas J.N., Nguyen P.V. Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J. Neurosci. 2005. 25: 3294–3303.
Gilbert P.E., Kesner R.P., Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001. 11: 626–636.
Gloor P. The Temporal Lobe and Limbic System. New York, NY: Oxford University Press (1997).
Goh J.J., Manahan-Vaughan D. Hippocampal long-term depression in freely behaving mice requires the activation of beta-adrenergic receptors. Hippocampus. 2013. 23: 1299–1308.
Gorter J., van Vliet E., Aronica E., Lopes da Silva F. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatinimmunoreactive neurons. Eur. J. Neurosci. Biobehav. Rev. 2001. 13: 657–669.
Goodman J.H., Sloviter R.S. Evidence for commissurally projecting parvalbumin-immunoreactive basket cells in the dentate gyrus of the rat. Hippocampus. 1992. 2: 13–21.
GoodSmith D., Chen X., Wang C., Kim S.H., Song H., Burgalossi A., Christian K.M., Knierim J.J. Spatial representations of granule cells and mossy cells of the dentate gyrus. Neuron. 2017. 93(3): 677–690.e5.
Grover L.M., Teyler T.J. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990. 347: 477–479.
Guo N., Soden M.E., Herber C., Kim M.T., Besnard A., Lin P., Ma X., Cepko C.L., Zweifel L.S., Sahay A. Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nat. Med. 2018. 24: 438–449.
Jinde S., Zsiros V., Jiang Z., Nakao K., Pickel J., Kohno K., Belforte J.E., Nakazawa K. Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation. Neuron. 2012. 76: 1189–1200.
Jung H.J., Lee J.M., Yang S.H., Young S.G., Fong L.G. Nuclear lamins in the brain - new insights into function and regulation. Mol. Neurobiol. 2013. 47: 290–301.
Jung M.W., McNaughton B.L. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993. 3: 165–182.
Kay A.R., Tóth K. Is zinc a neuromodulator? Sci. Signal. 2008. 1: re3.
Kemp A., Manahan-Vaughan D. Beta-adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb. Cortex 2008a. 18: 1326–1334.
Kemp A., Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb. Cortex 2008b. 18: 968–977.
Kesner R.P. A behavioral analysis of dentate gyrus function. Prog. Brain Res. 2007. 163: 567–576.
Khuu M.A., Pagan C.M., Nallamothu T., Hevner R.F., Hodge R.D., Ramirez J.-M., Garcia III A.J. Intermittent Hypoxia Disrupts Adult Neurogenesis and Synaptic Plasticity in the Dentate Gyrus. J. Neurosci. 2019. 39(7): 1320–1331.
Kheirbek M.A., Drew L.J., Burghardt N.S., Costantini D.O., Tannenholz L., Ahmari S.E., Zeng H., Fenton A.A., Hen R. Differential Control of Learning and Anxiety along the Dorsoventral Axis of the Dentate Gyrus. Neuron. 2013. 77: 955–968.
Kitamura T, Pignatelli M., Suh J., Kohara K., Yoshiki A., Abe K., Tonegawa S. Island cells control temporal association memory. Science. 2014. 343: 896–901.
Kitamura T., Sun C., Martin J., Kitch L.J., Schnitzer M.J., Tonegawa S. Entorhinal Cortical Ocean Cells Encode Specific Contexts and Drive Context-Specific Fear Memory. Neuron. 2015. 87: 1317–1331.
Kitchigina V., Vankov A., Harley C., Sara S.J. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur. J. Neurosci. 1997. 9: 41–47.
Koyama R., Yamada M.K., Fujisawa S., Katoh-Semba R., Matsuki N., Ikegaya Y. Brain-derived neurotrophic factor induces hyperexcitable reentrant circuits in the dentate gyrus. J. Neurosci. 2004. 24: 7215–7224.
Kleschevnikov A.M., Routtenberg A. Long-term potentiation recruits a trisynaptic excitatory associative network within the mouse dentate gyrus. Eur. J. Neurosci. 2003. 17: 2690–2702.
Larimer P., Strowbridge B.W. Representing information in cell assemblies: persistent activity mediated by semilunar granule cells. Nat. Neurosci. 2010. 13: 213–222.
Lassalle J.M., Bataille T., Halley H. Reversible inactivation of the hippocampal mossy fiber synapses in mice impairs spatial learning, but neither consolidation nor memory retrieval, in the Morris navigation task. Neurobiol. Learn. Mem. 2000. 73: 243–257.
Lee I., Kesner R.P. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus. 2004. 14: 66–76.
Leranth C., Hajszan T. Extrinsic afferent systems to the dentate gyrus. Prog. Brain Res. 2007. 163: 63–84.
Leranth C., Frotscher M. Cholinergic innervation of hippocampal GAD- and somatostatin-immunoreactive commissural neurons. J. Comp. Neurol. 1987. 261: 33–47.
Levy W.B., Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979. 175: 233–245.
Leutgeb J.K., Leutgeb S., Moser M.B., Moser E.I. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007. 315: 961–966.
Li X., Somogyi P., Ylinen A., Buzsáki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J. Comp. Neurol. 1994. 339: 181–208.
Lindvall O., Stenevi U. Dopamine and noradrenaline neurons projecting to the septal area in the rat. Cell Tissue Res. 1978. 190: 383–407.
Lisman J.E., Talamini L.M., Raffone A. Recall of memory sequences by interaction of the dentate and CA3: a revised model of the phase precession. Neural Netw. 2005. 18: 1191–1201.
Lituma P.J., Kwon H.-B., Alviña K., Luján R.l, Castillo P.E. Presynaptic NMDA receptors facilitate short-term plasticity and BDNF release at hippocampal mossy fiber synapses. eLife. 2021. 10: e66612.
Liu X., Ramirez S., Pang P.T., Puryear C.B., Govindarajan A., Deisseroth K., Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012. 484: 381–385.
Liu R.S., Lemieux L., Bell G.S., Sisodiya S.M., Bartlett P.A., Shorvon S.D., Sander J.W., Duncan J.S. Cerebral damage in epilepsy: a population-based longitudinal quantitative MRI study. Epilepsia. 2005. 46: 1482–1494.
Longo B., Covolan L., Chadi G., Mello L. Sprouting of mossy fibers and the vacating of postsynaptic targets in the inner molecular layer of the dentate gyrus. Exp. Neurol. 2003. 181: 57–67.
Lopez-Rojas J., Heine M., Kreutz M.R. Plasticity of intrinsic excitability in mature granule cells of the dentate gyrus. Sci. Rep. 2016. 6: 21615.
Lorente de No R. Studies on the structure of the cerebral cortex. II. Continuation of the of the study of of the ammonic system. J. Psychol. Neurol. (Leipz.). 1934. 46.113.
Lynch M.A. Long-Term Potentiation and Memory. Physiol. Rev. 2004. 84: 87–136.
Lysetskiy M., Foldy C., Soltesz I. Long- and short-term plasticity at mossy fiber synapses on mossy cells in the rat dentate gyrus. Hippocampus. 2005. 15: 691–696.
Madroñal N., Delgado-García J.M., Fernández-Guizán A., Chatterjee J., Köhn M., Mattucci C., Jain A., Tsetsenis T., Illarionova A., Grinevich V., Gross C.T., Gruart A. Rapid erasure of hippocampal memory following inhibition of dentate gyrus granule cells. Nat. Comm. 2016. 7: 10923.
Malenka R.C., Bear M.F. LTP and LTD: an embarrassment of riches. Neuron. 2004. 44: 5–21.
Malheiros J., Paiva F., Longo B., Hamani C., Covolan L. Manganese-enhanced MRI: biological applications in neuroscience. Front. Neurol. 2015. 6: 161.
Marr D. Simple memory: a theory for archicortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1971. 262: 23–81.
Mathern G., Cifuentes F., Leite J., Pretorius J., Babb T. Hippocampal EEG excitability and chronic spontaneous seizures are associated with aberrant synaptic reorganization in the rat intrahippocampal kainate model. Electroencephalogr Clin Neurophysiol. 1993. 87: 326–339.
Mitsueda-Ono T., Ikeda A., Sawamoto N., Aso T., Hanakawa T., Kinoshita M., Matsumoto R., Mikuni N., Amano S., Fukuyama H., Takahashi R. Internal structural changes in the hippocampus observed on 3-tesla MRI in patients with mesial temporal lobe epilepsy. Intern. Med. 2013. 52: 877–885.
McHugh T.J., Jones M.W., Quinn J.J., Balthasar N., Coppari R., Elmquist J.K., Lowell B.B., Fanselow M.S., Wilson M.A., Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007. 317(5834): 94–99.
McNamara J. Cellular and molecular basis of epilepsy. J. Neurosci. 1994. 14: 3413–3425.
McNaughton B.L., Barnes C.A., Meltzer J., Sutherland R.J. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp. Brain Res. 1989. 76: 485–496.
McNaughton B.L., Morris R.G. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in neurosciences. 1987. 10: 408–415.
McNaughton B.L., Nadel L. Hebb-Marr networks and the neurobiological representation of action in space. In: Neuroscience and Connectionist Theory, M.A. Gluck and D.E. Rumelhart, eds. (Erlbaum), 1990. 1–63 pp.
Meier K., Merseburg A., Isbrandt D., Marguet S.L., Morellini F. Dentate gyrus sharp waves, a local field potential correlate of learning in the dentate gyrus of mice. J. Neurosci. 2020. 40: 7105–7118.
Mello L., Covolan L. Neuronal injury and progressive cell damage. In: Schwartzkroin PA, editor. Encyclopedia of Basic Epilepsy Research. Cambridge, MA; London: Academic Press. 2009. 125–128 pp.
Messaoudi E., Bardsen K., Srebro B., Bramham C.R. Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J. Neurophysiol. 1998. 79: 496–499.
Messaoudi E., Ying S.W., Kanhema T., Croll S.D., Bramham C.R. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J. Neurosci. 2002. 22: 7453–7461.
Mori M., Abegg M.H., Gähwiler B.H., Gerber U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature. 2004. 431: 453–456.
Morimoto K., Fahnestock M., Racine R.J. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog. Neurobiol. 2004. 73: 1–60.
Moser M.B., Moser E.I. Functional differentiation in the hippocampus. Hippocampus. 1998. 8: 608–619.
Mu J.S., Li W.P., Yao Z.B., Zhou X.F. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999. 835: 259–265.
Myers C.E., Scharfman H.E. Pattern separation in the dentate gyrus: a role for the CA3 backprojection. Hippocampus. 2011. 21: 1190–1215.
Nadler J.V., Perry B.W., Cotman C.W. Selective reinnervation of hippocampal area CA1 and the fascia dentata after destruction of CA3-CA4 afferents with kainic acid. Brain Res. 1980. 182: 1–9.
Nakashiba T., Young J.Z., McHugh T.J., Buhl D.L., Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008. 319: 1260–1264.
Nakashiba T., Cushman J.D., Pelkey K.A., Renaudineau S., Buhl D.L., McHugh T.J., Rodriguez Barrera V., Chittajallu R., Iwamoto K.S., McBain C.J., et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012 149: 188–201.
Namgung U., Matsuyama S., Routtenberg A. Long-term potentiation activates the GAP-43 promoter: selective participation of hippocampal mossy cells. Proc. Natl. Acad. Sci. USA. 1997. 94: 11675–11680.
Nissinen J., Lukasiuk K., Pitkänen A. Is mossy fiber sprouting present at the time of the first spontaneous seizures in rat experimental temporal lobe epilepsy? Hippocampus. 2001. 11: 299–310.
Nosten-Bertrand M., Errington M.L., Murphy K.P., Tokugawa Y., Barboni E., Kozlova E., Michalovich D., Morris R.G., Silver J., Stewart C.L., Bliss T.V., Morris R.J. Normal spatial learning despite regional inhibition of LTP in mice lacking thy-1. Nature. 1996. 379: 826–829.
Parent J.M., Elliott R.C., Pleasure S.J., Barbaro N.M., Lowenstein D.H. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann. Neurol. 2006. 59: 81–91.
Park S., Kramer E.E., Mercaldo V., Rashid A.J., Insel N., Frankland P.W., Josselyn S.A. Neuronal Allocation to a Hippocampal Engram. Neuropsychopharmacol. 2016. 41: 2987–2993.
Park E.H., Burghardt N.S., Dvorak D. Hen R., Fenton A.A. Experience-dependent regulation of dentate gyrus excitability by adult-born granule cells. J. Neurosci. 2015. 35: 11656 –11666.
Penttonen M., Kamondi A., Sik A., Acsady L., Buzsaki G. Feed-forward and feed-back activation of the dentate gyrus in vivo during dentate spikes and sharp wave bursts. Hippocampus. 1997. 7: 437–450.
Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992. 106: 274–285.
Pierce J., Melton J., Punsoni M., McCloskey D., Scharfman H. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp. Neurol. 2005. 196: 316–331.
Polli R., Malheiros J., Dos Santos R., Hamani C., Longo B., Tannús A., Mello L.E., Covolan L. Changes in hippocampal volume are correlated with cell loss but not with seizure frequency in two chronic models of temporal lobe epilepsy. Front. Neurol. 2014. 5: 111.
Raisman G., Cowan W.M., Powel T.P.S. The extrinsic afferent, commissural and association fibers of hippocampus. Brain. 1965. 88: 963–981.
Ramirez S., Liu X., Lin P.A., Suh J., Pignatelli M., Redondo R.L., Ryan T.J., Tonegawa S. Creating a false memory in the hippocampus. Science. 2013. 341: 387–391.
Ramon y Cajal S. Estructura del asfa de Ammon y fascia dentata. Ann. SOC Esp. Hist. Nat. Madrid. 1893. 22. 1.
Reyes-Garcia S.Z., Scorza C.A., Araújo N.S, Ortiz-Villatoro N.N., Prada J.A., Ricardo C., Yacubian E.M.T., Faber J., Cavalheiro E.A. Different patterns of epileptiform-like activity are generated in the sclerotic hippocampus from patients with drug-resistant temporal lobe epilepsy. Sci. Rep. 2018. 8: 7116.
Ribak C.E., Seress L. Five types of basket cell in the hippocampal dentate gyrus: a combined Golgi and electron microscopic study. J. Neurocytol. 1983. 12: 577–597.
Ribak CE, Seress L, Amaral DG. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985. 14: 835–857.
Rolls E.T. Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiol. Learn. Mem. 2016. 129: 4–28.
Rolls E.T. The storage and recall of memories in the hippocampo-cortical system. Cell Tissue Research. 2018. 373(3): 577–604.
Rolls E.T., Kesner R.P. A computational theory of hippocampal function, and empirical tests of the theory. Prog. Neurobiol. 2006. 79: 1–48.
Roth B.L. DREADDs for neuroscientists. Neuron. 2016. 89(4): 683–694.
Rovira-Esteban L., Hájos N., Nagy G.A., Crespo C., Nacher J., Varea E., Blasco-Ibáñez J.M. Semilunar Granule Cells Are the Primary Source of the Perisomatic Excitatory Innervation onto Parvalbumin-Expressing Interneurons in the Dentate Gyrus. eNeuro. 2020. 7(4): 1–17.
Ruediger S., Vittori C., Bednarek E., Genoud C., Strata P., Sacchetti B., Caroni P. Learning-related feedforward inhibitory connectivity growth required for memory precision. Nature. 2011. 473: 514–518.
Sajikumar S., Frey J.U. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol. Learn. Mem. 2004. 82: 12–25.
Sahay A., Drew M.R., Hen R. Dentate gyrus neurogenesis and depression. Prog. Brain Res. 2007. 163: 697–722.
Sahay A., Scobie K.N., Hill A.S., O’Carroll C.M., Kheirbek M.A., Burghardt N.S., Fenton A.A., Dranovsky A., Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011. 472(7344): 466–470.
Sakon J.J., Suzuki W.A. A neural signature of pattern separation in the monkey hippocampus. PNAS. 2019. 116: 9634–9643.
Salib M., Joshi A., Katona L., Howarth M., Micklem B.R., Somogyi P., Viney T.J. GABAergic Medial Septal Neurons with Low-Rhythmic Firing Innervating the Dentate Gyrus and Hippocampal Area CA3. J. Neurosci. 2019. 39(23): 4527–4549.
Sara S.J., Vankov A., Hervé A. Locus coeruleus-evoked responses in behaving rats: A clue to the role of noradrenaline in memory. Brain Res. Bull. 1994. 35: 457–465.
Saxe MD, Battaglia F., Wang J.-W., Malleret G., David D.J., Monckton J. E, A. Garcia D. R., Sofroniew M. V., Kandel E.R., Santarelli L., Hen R., Drew M. R. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. USA. 2006. 103: 17501–17506.
Scharfman H.E. EPSPs of dentate gyrus granule cells during epileptiform bursts of dentate hilar “mossy” cells and area CA3 pyramidal cells in disinhibited rat hippocampal slices. J. Neurosci. 1994. 14: 6041–6057.
Scharfman H.E. Dentate hilar cells with dendrites in the molecular layer have lower thresholds for synaptic activation by perforant path than granule cells. J. Neurosci. 1991. 11: 1660–1673.
Scharfman H.E. The CA 3 “backprojection” to the dentate gyrus. Prog. Brain Res. 2007. 163: 627–637.
Scharfman H.E. Electrophysiological evidence that dentate hilar mossy cells are excitatory and innervate both granule cells and interneurons. J Neurophysiol. 1995. 74: 179–194.
Scharfman H.E. Characteristics of spontaneous and evoked EPSPs recorded from dentate spiny hilar cells in rat hippocampal slices. J. Neurophysiol. 1993. 70: 742–757.
Scharfman H.E. The enigmatic mossy cell of the dentate gyrus. Nat. Rev. Neurosci. 2016. 17(9): 562–575.
Scharfman H.E., Goodman J., McCloskey D. Ectopic granule cells of the rat dentate gyrus. Dev. Neurosci. 2007. 29: 14–27.
Scharfman H.E., Myers C.E. Hilar mossy cells of the dentate gyrus: a historical perspective. Front. Neural. Circuits. 2012. 6: 106.
Scharfman H.E., Pierce J.P. New insights into the role of hilar ectopic granule cells in the dentate gyrus based on quantitative anatomic analysis and three-dimensional reconstruction. Epilepsia. 2012. 53(Suppl 1): 98–108.
Sensi S.L., Ton-That D., Weiss J.H. Mitochondrial sequestration and Ca2+-dependent release of cytosolic Zn2+ loads in cortical neurons. Neurobiol. Dis. 2002. 10: 100–108.
Senzai Y., Buzsáki G. Physiological properties and behavioral correlates of hippocampal granule cells and mossy cells. Neuron. 2017. 93(3): 691–704. e5.
Shibata K., Nakahara S., Shimizu E., Yamashita T., Matsuki N., Koyama R. Repulsive guidance molecule a regulates hippocampal mossy fiber branching in vitro. Neuroreport. 2013. 24: 609–615.
Shi Y., Grieco S.F., Holmes T.C., Xu X. Development of Local Circuit Connections to Hilar Mossy Cells in the Mouse Dentate Gyrus. eNeuro. 2019. 6(2) e0370-18: 1–14.
Sloviter R.S., Zappone C.A., Harvey B.D., Bumanglag A.V., Bender R.A., Frotscher M. Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J. Comp. Neurol. 2003. 459: 44–76.
Sloviter R.S. Decreased hippocampal inhibition and selective loss of interneurons in experimental epilepsy. Science. 1987. 235: 73–76.
Sloviter R.S. Feedforward and feedback inhibition of hippocampal principal cell activity evoked by perforant path stimulation: GABA-mediated mechanisms that regulate excitability in vivo. Hippocampus. 1991a. 1: 31–40.
Sloviter R.S. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991b. 1: 41–66.
Sloviter R.S. Status epilepticus-induced neuronal injury and network reorganization. Epilepsia. 1999. 40 S34–9.
Sloviter R., Bumanglag A., Schwarcz R., Frotscher M. Abnormal dentate gyrus network circuitry in temporal lobe epilepsy. In: Noebels J., Avoli M., Rogawski M., Olsen R., Delgado-Escueta A., editors. Jasper’s Basic Mechanisms of the Epilepsies. Bethesda. MD: National Center for Biotechnology Information. 2012.
Smith R.L., Mensah P., Cotman C. Tracing the dentate gyrus mossy fiber system with horseradish peroxidase histochemistry. Exp. Neurol. 1973. 40(2): 516–524.
Snyder J.S., Kee N., Wojtowicz J.M. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J. Neurophysiol. 2001. 85: 2423–2431.
Song M.Y., Tian F.F, Wang Y.Z., Huang X., Guo J.L., Ding D.X. Potential roles of the RGMa-FAK-Ras pathway in hippocampal mossy fiber sprouting in the pentylenetetrazole kindling model. Mol. Med. Rep. 2015. 11: 1738–1744.
Steward O., Scoville S.A. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J. Comp. Neurol. 1976. 169: 347–370.
Strowbridge B.W., Schwartzkroin P.A. Transient potentiation of spontaneous EPSPs in rat mossy cells induced by depolarization of a single neuron. J. Physiol. 1996. 494: 493–510.
Strüber M., Sauer J.F., Jonas P., Bartos M. Distance-dependent inhibition facilitates focality of gamma oscillations in the dentate gyrus. Nat. Commun. 2017. 8(1): 758.
Swan A.A., Clutton J.E., Chary P.K., Cook S.G., Liu G.G., Drew M.R. Characterization of the role of adult neurogenesis in touch-screen discrimination learning. Hippocampus. 2014. 24: 1581–1591.
Swanson L.W., Kohler C., Bjorklund A. Handbook of Chemical Neuroanatomy. Hokfelt T., Bjorklund A., Swanson L.W., editors. Vol. 5. Elsevier; 1987. pp. 125–277.
Sun C., Mtchedlishvili Z., Bertram E.H., Erisir A., Kapur J. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J. Comp. Neurol. 2007. 500: 876–893.
Takeuchi T., Duszkiewicz, A.J., Sonneborn A., Spooner P.A., Yamasaki M., Watanabe M., Smith C.C., Fernández G., Deisseroth K., Greene R.W., Morris R.G.M. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. 2016. 537(7620): 357–362.
Tamagnone L., Comoglio P. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell. Biol. 2000. 10: 377–383.
Tauck D., Nadler J. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J. Neurosci. 1985. 5: 1016–1022.
Toni N., Laplagne D.A., Zhao C., Lombardi G., Ribak C.E., Gage F.H., Schinder A.F. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 2008. 11: 901–907.
Treves A., Rolls E.T. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994. 4: 374–391.
Tronel S., Belnoue L., Grosjean N., Revest J.M., Piazza P.V., Koehl M., Belnoue L., Abrous D.N. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012. 22: 292–298.
Tulving E. Episodic memory: from mind to brain. Annu. Rev. Psychol. 2002. 53: 1–25.
Van Paesschen W., Revesz T., Duncan J.S., King M.D., Connelly A. Quantitative neuropathology and quantitative magnetic resonance imaging of the hippocampus in temporal lobe epilepsy. Annals of Neurology. 1997. 42: 756–766.
Vinogradova O.S. Hippocampus as comparator: Role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001. 11: 578–597.
Vogt K., Mellor J., Tong G., Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000. 26: 187–196.
Williams P.A., Larimer P., Gao Y., Strowbridge B.W. Semilunar granule cells: glutamatergic neurons in the rat dentate gyrus with axon collaterals in the inner molecular layer. J Neurosci. 2007. 27: 13756–13761.
Witter M.P. The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog. Brain Res. 2007. 163: 43–61.
Wittner L. Maglóczky Z., Borhegyi Z., Halász P., Tóth S., Eross L., Szabó Z., Freund T.F. Preservation of perisomatic inhibitory input of granule cells in the epileptic human dentate gyrus. Neuroscience. 2021. 108: 587–600.
Wright B.J., Jackson M.B. Long-term potentiation in hilar circuitry modulates gating by the dentate gyrus. J. Neurosci. 2014. 34: 9743–9753.
Wuarin J., Dudek F. Excitatory synaptic input to granule cells increases with time after kainate treatment. J. Neurophysiol. 2001. 85: 1067–1077.
Yassa M.A., Stark C.E. Pattern separation in the hippocampus. Trends Neurosci. 2011. 34: 515–525.
Zimmer J. Changes in the Timm sulfide silver staining patter of the rat hippocampus and fascia dentata following early postnatal deaferentiation. Brain Res. 1973. 64: 313–326.
Zimmer J. Long-term synaptic reorganization in rat fascia dentate deafferented at adolescent and adult stages: observations with the Timm method. Brain Res. 1974. 76: 336–242.
Yamawaki R., Thind K., Buckmaster P.S. Blockade of excitatory synaptogenesis with proximal dendrites of dentate granule cells following rapamycin treatment in a mouse model of temporal lobe epilepsy. J. Comp. Neurol. 2015. 523: 281–297.
Zimmer J. Ipsilateral afferents to the commissural zone of the fascia dentata, demonstrated in decommissurated rats by silver impregnation. J. Comp. Neurol. 1971. 142: 393–416.
Zucca S., Griguoli M., Malézieux M., Grosjean N., Carta M., Mulle C. Control of Spike Transfer at Hippocampal Mossy Fiber Synapses In Vivo by GABA A and GABA B Receptor-Mediated Inhibition. The Journal of Neuroscience. 2017. 37: 587–598.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


