Журнал высшей нервной деятельности им. И.П. Павлова, 2022, T. 72, № 1, стр. 100-116
Вклад наружной части бледного ядра в осцилляторную активность моторных нейросетей в экспериментальной модели болезни Паркинсона
М. В. Морозова 1, Е. С. Бражник 1, И. Е. Мысин 1, Л. Б. Попова 2, Н. И. Новиков 1, *
1 ФГБУЦ, Институт теоретической и экспериментальной биофизики
Пущино, Россия
2 НИИ физико-химической биологии им. А.Н. Белозерского, МГУ им. М.В. Ломоносова
Москва, Россия
* E-mail: nikolay_novikov@hotmail.com
Поступила в редакцию 20.04.2021
После доработки 20.06.2021
Принята к публикации 05.10.2021
- EDN: NREVKB
- DOI: 10.31857/S0044467722010063
Аннотация
Аберрантные синхронные β-осцилляции в моторных нейросетях при дефиците дофамина (ДА) ассоциируют с двигательными нарушениями при БП. Источники и механизмы их развития не ясны. Цель работы состояла в определении роли GPe, центрального звена BG, в генерации и передаче β-осцилляций в моторных нейросетях на модели БП у крыс. Анализ ЛПП в записях из MCx и ядер BG выявил наибольшую мощность и когерентность β-осцилляций (30–36 Гц) в MCx и SNr полушарий с ДА-дефицитом, тогда как их выраженность в dStr и GРe и когерентность с MCx и SNr были значительно ниже. Помимо β-осцилляций, усиление когерентных γ-осцилляций в диапазоне частот 50–56 Гц показано исключительно в dStr и GPe при ДА-дефиците, а их кратковременное появление у контрольных крыс совпадало с возникновением трудностей при ходьбе. Стимуляция ДА-рецепторов леводопой снижала синхронизацию в нейросетях полушарий с ДА-дефицитом и восстанавливала нормальную локомоцию. Различия между двумя типами активности (β- и γ-осцилляции) в записях из GРe при ДА-дефиците свидетельствуют о сложности организации моторных нейросетей, контролирующих в норме различные аспекты локомоции.
СПИСОК СОКРАЩЕНИЙ
| 6-OHDA | – 6-гидроксидофамин гидробромид; |
| БП | – болезнь Паркинсона; |
| ГАМК | – гамма-аминомасляная кислота; |
| ДА | – дофамин; |
| ЛД | – леводопа; |
| ЛПП | – локальный полевой потенциал; |
| MCx | – моторный отдел коры мозга; |
| Ark-GPe | – аркипаллидальные нейроны бледного ядра; |
| BG | – базальные ганглии; |
| dStr | – дорзальный отдел латерального стриатума; |
| dMSN | – проекционные нейроны прямого пути стриатума; |
| iMSN | – проекционные нейроны непрямого пути стриатума; |
| FoG (freezing of the gait) | – застывание, акинезия; |
| FSI | – высокочастотные интернейроны стриатума; |
| GPe | – наружная часть бледного ядра; |
| GPi | – внутренняя часть бледного ядра; |
| Pro-GPe | – прототипные нейроны бледного ядра; |
| SNc | – компактная часть черной субстанции; |
| SNr | – ретикулярная часть черной субстанции; |
| STN | – субталамическое ядро. |
ВВЕДЕНИЕ
Гибель дофаминергических клеток черной субстанции при болезни Паркинсона (БП) связывают с появлением синхронной осцилляторной активности в моторных нейросетях, которые тесно связаны с контролем движения. Предполагается, что эта активность является причиной возникновения двигательных нарушений при БП (Brown, 2006; Pogosyan et al., 2010; Sharott et al., 2014; Neumann et al., 2016). Усиленная активность в β-диапазоне при БП регистрируется в моторных областях неокортекса и ядрах базальных ганглиев (BG) у пациентов с паркинсонизмом (Brittain et al., 2014) и у животных с экспериментальной моделью БП (Sharott et al., 2005; Brazhnik et al., 2012; Dejean et al., 2012). Механизмы возникновения и распространения патологической активности в моторных нейросетях, контролирующих локомоцию, до конца не изучены.
Базальные ганглии – это сложноорганизованная сеть ядер переднего мозга, которые играют ведущую роль в контроле двигательной активности. В последние десятилетия эта функция BG интенсивно исследуется на животных моделях БП. Однако понимание того, как происходит обмен информацией между ядрами и каковы механизмы контроля движений, отсутствует (Rodriquez-Sabate et al., 2019). BG поражаются при БП в первую очередь, и больше всего страдает функция ядер, имеющих в норме дофаминергическую иннервацию, – стриатума и наружной части бледного ядра (GPe) (Gauthier et al., 1999; Smith Y., Villalba, 2008; Eid, Parent, 2015; Mallet et al., 2019). Входные ядра BG, dStr и STN, получают мощную глутаматергическую иннервацию от коры и таламуса и направляют ее в структуры, контролирующие движение. Установлено, что dStr и STN посылают тормозные и активационные сигналы соответственно к наружному сегменту бледного ядра (GPe) и выходному звену BG – внутреннему сегменту бледного ядра (GPi) и ретикулярной части черной субстанции (SNr) (Parent, Hazrati, 1995).
GPe рассматривают как ключевое звено в нейросети BG, контролирующей локомоцию. В действительности это ядро получает информацию от моторных областей коры и практически от всех ядер BG. Оно, в свою очередь, осуществляет контроль активности компонентов сети за счет обширных проекций на все ядра BG (Kita, 2007; Hegeman et al., 2016), моторный таламус (Yasukawa et al., 2004; Mastro et al., 2014) и моторные отделы коры (Chen et al., 2015; Grewal et al., 2018; Abecassis et al., 2019; Karube et al., 2019). Кроме того, реципроктные связи между активационными нейронами субталамического ядрa (STN) и тормозными нейронами GPe, возможно, формируют своего рода центральный пейсмейкер BG, который вовлечен в генерацию залповой β-активности и ее распространение у животных с экспериментальной БП (Bevan et al., 2002; Tachibana et al., 2011; Nevado-Holgado et al., 2014; de la Crompe et al., 2020).
В настоящее время данные об участии GPe в аномальной нейросетевой активности и о его вкладе в процесс развития двигательных нарушений при БП весьма ограничены. Большинство экспериментальных работ выполнено на анестезированных животных, поэтому реальный вклад GPe в развитие моторной дисфункции еще предстоит выяснить. В нашей работе локальную полевую активность в GPe регистрировали одновременно с записью ЛПП в основных структурах моторной нейросети у бодрствующих животных во время ходьбы в круговом тредмилле в контроле и при экспериментальной БП.
МЕТОДИКА
Все экспериментальные процедуры были одобрены комиссией по этике ИТЭБ и соответствовали Директиве Европейского парламента и Совета (2010/63/EU). Сделано все возможное для уменьшения количества использованных в работе животных и минимизации их дискомфорта.
Животные и хирургические операции. Опыты проводили на крысах-самцах аутобредной линии Вистар (300–350 г). Животные содержались в виварии в индивидуальных клетках в контролируемых условиях (22–24°C, 12 ч цикл свет/темнота) и имели доступ к еде и воде без ограничения.
Крыс анестезировали тилетамином-золепамом (Золетил, 18 мг/кг) и ксилазином (12 мг/кг), вводимыми внутримышечно. Для поддержания нормальной температуры тела животных использовали термоплатформу, частоту сердечных сокращений контролировали пульсоксиметром (Oxy9Vet Plus, Bionet, S. Korea). Животное помещали в стереотаксис, местный анестетик (1%-й мепивакаин гидрохлорид) вводили в зону намечаемого разреза. Для разрушения ДА-синтезирующих клеток SNc (n = 29) в область левого медиального переднемозгового пучка (координаты: AP = 4.6 мм, ML = 1.2 мм, DV = –8.3 мм) вводили 6 мкг нейротоксинa 6-OHDA (Sigma-Aldrich) в объеме 3 мкл физиологического раствора, содержащего 0.1% аскорбиновой кислоты, со скоростью 1 мкл/мин. Для защиты норадренергических нейронов за 20 мин до инъекции 6-OHDA вводили дезметилимипрамин (15 мг/кг, в/бр). Контрольной группе крыс (n = 7) вместо нейротоксина вводили физиологический раствор.
Для регистрации ЛПП использовали имплант, состоящий из пучка пяти стальных изолированных электродов (диаметр 50 мкм), с одного из которых удаляли изоляцию на расстоянии 0.5 мм от кончика (локальный референтный электрод). Исследование проведено на 3 группах крыс: 1-я группа – контрольные животные (n = 7) и 2 группы животных с односторонним повреждением ДА-нейронов левого полушария мозга (n = 29). У животных с повреждением ДА-нейронов (n = 8) билатерально имплантировали электроды в наружную часть бледного ядра интактного и поврежденного полушарий (GPe: AP = = 0.9 мм, ML = 3.0 мм, DV = –6.5 мм). Остальным крысам с повреждением ДА-нейронов (n = 21) и контрольным (n = 7) электроды вживляли в левую половину полушария в 5/6 слой MCx (AP = –2.0 мм, ML = 2.7 мм, DV = –2.0 мм), SNr (AP = 5.7 мм, ML = 2.2 мм, DV = –8.0 мм), dStr (AP = 0.15 мм, ML = 3.5 мм, DV = –4.5 мм) и GPe (см. выше). Общим заземлением служил винт в кости над мозжечком.
Анализ двигательной активности в круговом тредмилле. Регистрацию ЛПП в звеньях моторной нейросети проводили в состоянии покоя и во время ходьбы крыс в круговом тредмилле. За основу прибора взята конструкция, представленная в работе Avila et al. (2010). Перед проведением операции животных тренировали ходить в направлении по и против часовой стрелки при скорости вращения платформы 9 оборотов в минуту (15–20 мин, 3 раза в день, в течение 3–4 дней). Kрысы с односторонним повреждением могли идти в направлении против часовой стрелки (влево, в сторону поврежденного полушария). Однако при ходьбе по часовой стрелке (вправо, направление в сторону интактного полушария) животные испытывали затруднения вплоть до полной неспособности идти при вращении платформы тредмилла. Для количественной оценки двигательной активности в контроле и у крыс с односторонним ДА-дефицитом, а также определения влияния леводопы применяли метод, основанный на расчете величины отношения числа шагов, сделанных задней внутренней лапой, к числу шагов, совершенных задней наружной лапой, при ходьбе животного в тредмилле в направлении по (вправо) и против (влево) часовой стрелки (Brazhnik et al., 2016). Подсчет количества шагов проводили в пяти 30-секундных проходках, и полученные величины отношения усредняли по каждому направлению движения, а также вычисляли % завершенных проходок.
Регистрация локальных полевых потенциалов. Регистрацию ЛПП проводили на бодрствующих крысах в дневное время. Животных помещали в тредмилл за 5 мин до начала опыта для адаптации. Сигналы ЛПП усиливали в 1000 раз (Grass Instruments Semiconductor) Model 12 Neurodata Acquisition System, США). Для оцифровки сигнала использовали 16-канальный PCIDAS 1200JR (с разрешением A/D 330 КГц, при уровне оцифровки 1000 Гц) и фильтровали в диапазоне 3–200 Гц для последующей обработки с помощью программы Datapac 2k2 (Run, США). Записи с каждого электрода реферировали к локальному референтному электроду. Регистрацию активности в отделах моторной нейросети проводили в состоянии покоя и при ходьбе на 7, 14 и 21-й день после имплантации электродов. На 28–35-й день после 10-минутной записи активности во время ходьбы крысам вводили леводопу (5 мг/кг, пк) с последующей регистрацией ЛПП в течение 20–60 мин. Видеорегистрация ходьбы в тредмилле была синхронизирована с записью активности ЛПП.
Спектральный анализ локальных полевых потенциалов. Для анализа электрической активности взяты записи, не имевшие двигательных артефактов. Записи экспортировали в программу Spike2 (CED, UK) с последующим анализом с помощью скриптов, написанных для Spike2 и MATLAB. Для расчета мощности и когерентности брали 60 с эпохи и использовали оконное преобразование Фурье (ширина окна 1 с, смещение 250 мс). Данные, полученные с 2 электродов в каждой структуре, усредняли. Гистограммы спектральной плотности строили в диапазоне 10–100 Гц с разрешением 1 Гц, вычисляли ведущую частоту (Гц), интегральную мощность ритмов (мВ2/Гц) и когерентность между структурами в исследуемых диапазонах частот. Частоты пиков в спектрах мощности и когерентности считали значимыми, если они удовлетворяли критериям, описанным ранее (Brazhnik et al., 2016). При анализе ЛПП использовали величины значимых пиков спектров в β- (25–40 Гц) и γ- (45–90 Гц) частотных диапазонах. Мощность и среднее значение когерентности в спектрах определяли от величины пика частоты +/–3 Гц, т.е. при расчете для β-осцилляций использовали диапазон частот 30–36 Гц, а для γ- осцилляций – 50–56 Гц. Мощность рассчитывали как сумму площадей шести 1-герцевых столбцов, окружающих значимый пик, плюс площадь пика. Величину когерентности определяли как среднее значение когерентности в 7 столбцах.
Гистологический контроль. После окончания экспериментов у животных под глубоким наркозом проводили электролитическую маркировку мест отведения. Крыс перфузировали 4%-м раствором формальдегида в фосфатном буфере. На срезах мозга толщиной 40 микрон верифицировали положение электродов и определяли содержание тирозингидроксилазы (TH) в стриатуме и SNc и SNr, используя первичные кроличьи поликлональные антитела (Pel-Freez Biologicals, USA), вторичные биотинилированные антикроличьи IgG, с обработкой срезов авидин-биотин-пероксидазным комплексом (ABC-kit, Vector Labs, USA) и выявлением окраски с помощью раствора диаминобензидина с перекисью водорода (DAB-kit, Vector Labs, USA). Снижение содержания TH (на ~98%) отмечено в SNc полушарий с ДА-дефицитом.
Статистическая обработка результатов. Результаты представлены средней величиной ± стандартная ошибка. Результаты поведенческого теста (ходьба в тредмиле) в контроле (ДА+), при дефиците дофамина (ДА–) и после введения леводопы (ЛД+) анализировались методом двухфакторного дисперсионного анализа (2-way ANOVA) с последующим применением апостериорного анализа Холма-Шидака (Holm-Sidak post hoc). Для анализа изменения мощности и когерентности осцилляций и выявления различий между группами данных использовали однофакторный дисперсионный анализ Краскела–Уоллиса и тест Данна (Kruskal–Wallis one-way ANOVA; Dunn’s post hoc). Для сравнения двух групп данных применяли U-критерий Манна–Уитни (Mann–Whitney U-test). При статистическом анализе использовали программу SigmaPlot 12.1 (SyStat Software) на уровне значимости p < 0.05.
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЙ
1. Одновременная регистрация активности GPe в контрольном полушарии и полушарии с повреждением дофаминсинтезирующих нейронов черной субстанции. Экспериментальная модель БП на грызунах с односторонним повреждением ДА-нейронов SNc позволяет сравнить активность в здоровом и поврежденном полушариях у животных во время ходьбы. Запись ЛПП у крыс в состоянии покоя или при ходьбе в направлении по или против часовой стрелки осуществляли с электродов, имплантированных в GPe обоих полушарий, начиная с 14-го дня после операции (8 животных). Осцилляторная активность в GPe полушарий с ДА-дефицитом отличалась от таковой в контрольном (рис. 1). Ритмическая активность в контрольном GPe была представлена осцилляциями в широком диапазоне γ-частот (45–90 Гц) (рис. 1 (а)). Эти осцилляции возникали при ходьбе и не наблюдались в состоянии покоя. В то время как в записях активности GPe полушарий с ДА-дефицитом во время ходьбы были выявлены два выраженных спектральных пика: первый в β-диапазоне частот (25–40 Гц, пик 33.6 ± 0.6 Гц), второй в γ-дипазоне (45–60 Гц, пик 51.1 ± 0.4 Гц) (рис. 1 (б)). Мощность ЛПП в β-диапазоне частот в GPe полушарий с ДА-дефицитом превышала величину этого показателя в контрольных полушариях в два раза (11.7 ± 2.2 × × 10–6 мВ2/Гц и 6.1 ± 0.8 × 10–6 мВ2/Гц, p = = 0.036, U-тест). Отметим, что в полушариях с ДА-дефицитом осцилляции в β- и γ-диапазонах нередко регистрировались с разных электродов одного и того же пучка, хотя в ряде случаев в записях с одного электрода присутствовали осцилляции в обоих частотных диапазонах. Учитывая это, можно разделить записи ЛПП из GPe полушарий с ДА-дефицитом на две группы (с частичным перекрытием частот) – с доминантными β- (рис. 1 (б1), n = 4) и γ-осцилляциями (рис. 1 (б2)), n = 4).
Рис. 1.
Одновременная регистрация ЛПП в GPe контрольного ДА(+) и полушария с ДА-дефицитом ДА(–). (а, б): спектры мощности и примеры осцилляций в β- (30–36 Гц) и γ- (50–56 Гц) частотных диапазонах в покое (П) и при ходьбе. На вставках представлены примеры ЛПП, отфильтрованных в β- и γ-частотных диапазонах. (б1): спектры мощности и образцы ЛПП с доминантными β-осцилляциями. (б2): то же в записях с доминантными γ-осцилляциями. Пики обозначены стрелками. (в, г): динамика мощности осцилляций в GPe при ходьбе. Цифрами обозначены 1-минутные интервалы времени.
Fig. 1. Simultaneous recordings of LFP activity from the GPe in control (DA+) and DA-depleted (DA–) hemispheres. (а, б): averaged GPe LFP power spectra and examples LFP recordings filtered in β- (30–36 Hz) and γ- (50–56 Hz) frequency ranges with FIR-digital band pass filters. (б1) and (б2) represent LFP power spectra in the GPe and traces of band pass filtered LFP activity from the channels with dominant β- or γ-oscillations from the DA(–) hemisphere. (в, г): dynamic of total LFP power in β- or γ-frequency ranges in the GPe from rest (R) and walking in the two hemispheres. Numbers above bars represent consequent 60-s epochs of walking.
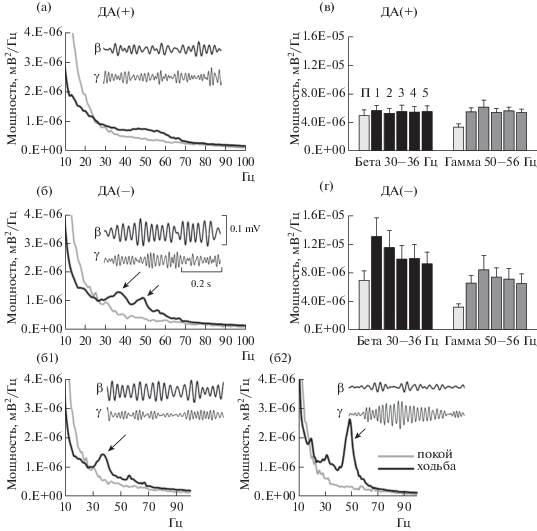
Изменения мощности осцилляций в β- и γ-частотных диапазонах в полушариях с ДА-дефицитом имели противоположную направленность (рис. 1(г)). Наибольшая мощность β-осцилляций отмечена в начале движения, и она существенно снижалась при ходьбе. В отличие от β, γ-осцилляции первоначально имели широкий частотный диапазон (45–90 Гц, пик 76.7 ± 1.9 Гц), и они быстро замещались осцилляциями в узком диапазоне частот, 50–56 Гц (пик 51.1 ± 0.4 Гц) с одновременным возрастанием их мощности. Как в контрольном полушарии, так и в полушарии с ДА-дефицитом cмещение происходило в течение 1–2 мин после начала ходьбы животного. Oтметим, что осцилляции с частотой 50–56 Гц в GPe в контрольных полушариях и полушариях с ДА-дефицитом были стабильны и длительны, присутствуя в течение ~80% времени в первые 200 с ходьбы (158.3 ± 6.8 с и 164.6 ± 6.3 с соответственно). Мощность γ-осцилляций в контрольном полушарии была ниже при ходьбе влево, но достоверно не отличалась от таковой на поврежденной стороне. При движении вправо, когда животные испытывали затруднения, осцилляции в диапазоне ~50 Гц возникали в обоих полушариях раньше, чем при ходьбе влево. При этом мощность γ-осцилляций была выше в полушариях с ДА-дефицитом при ходьбе вправо (8.5 ± 2.5 × 10–6 мВ2/Гц), чем при ходьбе влево (6.9 ± 2.3 × 10–6 мВ2/Гц, p = = 0.013, U-тест).
2. Осцилляторная активность в моторных нейросетях в контрольной группе и у животных с односторонним дефицитом ДА. Для оценки роли GPe в механизмах возникновения и передачи β- и γ-осцилляций в моторных сетях после повреждения ДА-синтезирующих клеток регистрировали ЛПП в MCx и ядрах BG в контроле и у крыс с односторонним ДА-дефицитом. Величины мощности осцилляторной активности в двух частотных диапазонах (β и γ) в GPe сравнивали с мощностью осцилляций в MCx, dStr и SNr, a величины когерентности ЛПП в парах MCx – dStr, MCx – GPe, dStr – GPe, GPe – SNr – со значением когерентности в паре MCx – SNr.
2.1. Усиление мощности осцилляций в моторных сетях при дефиците дофамина. В данной работе при анализе ЛПП в отделах нейросети MCx – BG выявлены значительные различия в активности между двумя группами животных, контрольной (ДА+) и с ДА-дефицитом (ДА–) (рис. 2 (а, б)). Установлено, что в полушариях с ДА-дефицитом β-осцилляции (30–36 Гц) возникали во время ходьбы и доминировали в MCx и SNr в 100% случаев, и в 50–88% присутствовали в dStr и GPe (рис. 2 (в)). Наибольшая величинa мощности β-осцилляций, нормализованная к осцилляциям в состоянии покоя, выявлена в MCx и SNr (MCx: 4.2 ± 0.3, n = 21; SNr: 3.3 ± 0.4, n = 21), а ее величины в dStr и GPe были значительно ниже (dStr: 1.4 ± 0.8, n = 12; GPe: 1.5 ± 0.8, n = = 21; p < 0.001, Kruskal–Wallis test). Заметим, что β-осцилляции отсутствовали во всех структурах в состоянии покоя (рис. 2 (б)) и в записях у контрольных животных (рис. 2 (а)). Величины мощности осцилляций в β-диапазоне в записях ЛПП из ядер BG и MCx полушарий с ДА-дефицитом при ходьбе были достоверно выше их величин в контрольных полушариях: MCx (p < 0.001, n = 23 и 7), SNr (p < 0.001, n = 20 и 7), GPe (p < 0.01, n = 24 и 7), dStr (p < 0.01, n = 12 и 5 соответственно; U-тест; рис. 2 (г)).
Рис. 2.
β- и γ-Осцилляции в MCx и ядрах BG в контроле (ДА+) и полушариях с ДА-дефицитом (ДА–). (а, б): спектры мощности осцилляций в отделах моторной нейросети в покое и при ходьбе. Стрелками обозначены пики в β- и γ-частотных диапазонах. (в): наличие ЛПП с осцилляциями в ДА(+) и ДА(–) группах. (г): Нормализованные к состоянию покоя мощности β- и γ-осцилляций в отделах нейросети при ходьбе. *р < 0.05, выше в MCx и SNr в сравнении с dStr and GPe в ДА(–); #р < 0.01, выше в ДА(–) в сравнении с ДА(+).
Fig. 2. β- and γ-oscillatory activity in the MCx and BG in control group (DA+) and rats with DA-deficit (DA–). (а, б): averaged LFP power spectra in motor circuits in DA(+) and DA(–) groups during rest and treadmill walking. Arrows show peaks in LFP spectra associated with β- or γ-oscillations. (в): histograms indicate % LFP power spectra with prominent oscillations in each node of motor circuits in DA(+) and DA(–) groups. (г): normalized to rest total LFP power of oscillations in the MCx and BG nuclei in DA(+) and DA(–) during walking. *р < 0.05, higher in MCx and SNr relative to dStr and GPe in DA(–); #р < 0.01, higher in DA(–) relative to DA(+).
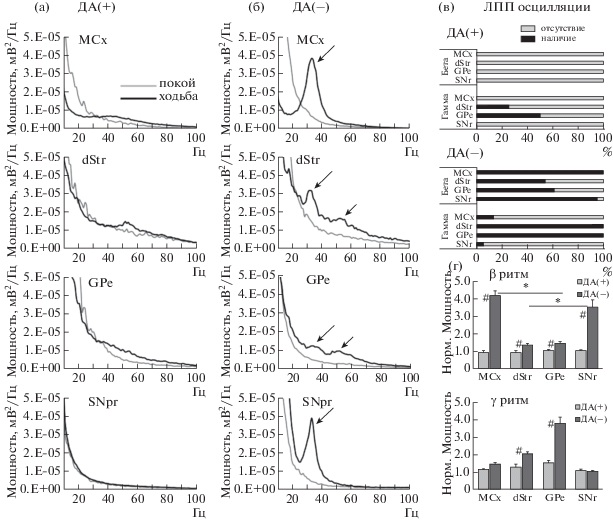
Кроме β-осцилляций в dStr и GPe полушарий с ДА-дефицитом выявлено усиление осцилляций в узком γ-частотном диапазоне (50–56 Гц) при ходьбе (рис. 2 (б)). В записях из MCx и SNr γ-осцилляции у животных с ДА-дефицитом не обнаружены (рис. 2 (б)), так же как и в контрольной группе (рис. 2 (а)). При этом в записях из dStr и GPe в контрольной группе во время ходьбы преимущественно регистрировались осцилляции низкой мощности в широком диапазоне γ-частот (45–90 Гц) с краткими эпизодами 50–56 Гц γ-осцилляций. Oсцилляции в γ-диапaзоне (50–56 Гц) отмечены в 50% записей из GPe и 33% из dStr в контроле, но не обнаружены в MCx и SNr (рис. 2 (в)). Заметим, что в GPe и dStr контрольной группы длительность эпизодов осцилляций с частотой 50–56 Гц в течение первых 200 с ходьбы была в 6.5 раза меньше, чем у животных с ДА-дефицитом (24.5 ± ± 11.2 с и 159.1 ± 6.5 с соответственно). При этом нормализованная мощность γ-осцилляций (50–56 Гц) в dStr и GPe была выше в полушариях с ДА-дефицитом по сравнению с контрольными (dStr: 2.1 ± 0.1 и 1.3 ± 0.2 соответственно, p = 0.023, n = 12 и 7; GPe: 3.9 ± 0.4 и 1.6 ± 0.1 соответственно, p < 0.001, n = 24 и 7; U-тест; рис. 2 (г)).
2.2. Когерентность осцилляций в моторной нейросети. Возрастание мощности β- и γ-активности в полушариях с ДА-дефицитом сопровождалось увеличением когерентности осцилляций в этих частотных диапазонах между компонентами моторной нейросети (MCx – dStr – GPe – SNr) (рис. 3). Увеличение когерентности β-осцилляций в полушариях с ДА-дефицитом представлено спектрами когерентности (рис. 3 (а, б)), гистограммами, демонстрирующими наличие (%) когерентных осцилляций во всех парах структур изучаемой нейросети (рис. 3 (б)), и графиками средних величин когерентности (рис. 3 (г)). В состоянии покоя и у контрольных животных когерентные β-осцилляции отсутствуют (рис. 3 (а, б)). При ходьбе величина когерентности β-осцилляций возрастала в полушариях с ДА-дефицитом во всех парах изучаемых компонентов нейросети (p < 0.001, U-тест). При этом oчевидно, что когерентность в паре MCx – SNr значительно выше (0.51 ± 0.03), чем в парах MCx – dStr (0.16 ± 0.05), MCx – GPe (0.1 ± ± 0.02), GPe – SNr (0.11 ± 0.03) и GPe – dStr (0.09 ± 0.02) (p < 0.001, Kruskal–Wallis test) (рис. 3 (а, г)).
Рис 3.
Усиление синхронизации между отделами моторной нейросети в полушариях с ДА-дефицитом. (а, б): спектры когерентности между отделами нейросети в покое и при ходьбе. Стрелки указывают пики когерентности в 2 частотных диапазонах. (в): наличие когерентных ЛПП в парах структур нейросети в ДА(+) и ДА(–) группах. (г): когерентность между отделами нейросети при ходьбе. *р < 0.05, выше в парах MCx – SNr (β ритм) и dStr – GPe (γ ритм) в ДА(–) группе; §р < 0.05, выше для dStr – GPe (γ ритм) в ДА(+) группе; #р < 0.01, выше в ДА(–) в сравнении с ДА(+).
Fig. 3. Increases in LFP coherence between different elements in the motor circuits after loss of dopamine. (а, б) averaged LFP coherence spectra in motor circuits in DA(+) and DA(–) groups during rest and walking. Arrows show peaks in LFP coherence spectra associated with γ-oscillations. (в): histograms indicate the % coherence spectra in pairs from motor circuits with β- or γ-oscillations in DA(+) and DA(–) groups during walking. (г): averaged LFP coherence in pairs from motor circuits in DA(+) and DA(–) groups during walking. *р < 0.05, higher in MCx – SNr pair in DA(–) group; *р < 0.05, highest in MCx-SNr (β-rhythm) and dStr – GPe (γ-rhythm) in DA(–) group; §р < 0.05, highest in dStr – GPe in DA(+) group; #р < 0.01 higher in DA(–) relative to DA(+).
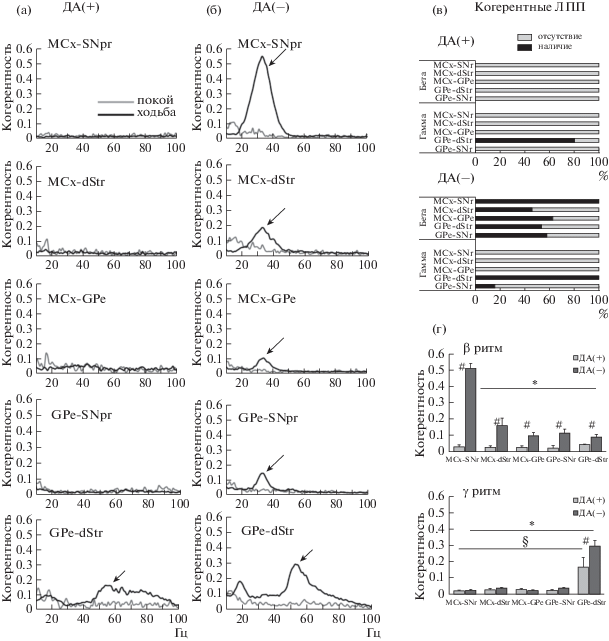
В паре GPe – dStr полушарий с ДА-дефицитом, помимо когерентных β-осцилляций, в 100% записей выявлены значительные когерентные γ-осцилляции в узком диапазоне частот (50–56 Гц) (рис. 3 (а, б)). Когерентность γ-осцилляций между dStr и GPe на 14–21-й день после введения нейротоксина была высокой (0.30 ± 0.03) (рис. 3 (б, г)), и ее величина повышалась к 28–35-му дню до 0.39 ± 0.03. В контроле в 80% записей из dStr и GPe регистрировались когерентные осцилляции в широком диапазоне γ-частот (45–90 Гц), наличие которых подтвержденo спектрами когерентности (рис. 3 (а)) и представлено на графиках их средних величин (рис. 3 (г)). При этом расчетные величины когерентности для диапазона частот в контроле (0.17 ± 0.06) были выше порогового уровня, но достоверно ниже соответствующих величин в полушариях с ДА-дефицитом (0.30 ± 0.03, p < 0.05, t-тест). В других парах структур моторной сети как в контроле, так и при ДА-дефиците когерентные γ-осцилляции в диапазоне 50–56 Гц отсутствовали.
2.3. Влияние стимуляции дофаминергических рецепторов. Для того чтобы показать, что возникновение синхронизованной в β-частотном диапазоне активности в ядрах BG связано с утратой способности нейронов SNc синтезировать ДА, мы вводили леводопу. Опыты выполнены через 21–35 дней после повреждения ДА-синтезирующих нейронов (n = 23). Леводопа значительно снижала мощность и когерентность β-осцилляций в MCx и ядрах BG при ходьбе, как и выраженность и когерентность γ-осцилляций в dStr и GPe в диапазоне 50–56 Гц. При этом в записях из GPe у 6 из 13 животных с ДА-дефицитом снижение мощности 50–56 Гц γ-осцилляций (пик 53.1 ± 0.6 Гц) сопровождалось одновременным восстaновлением паттерна 45–90 Гц γ-осцилляций (пик 73.1 ± 1.7 Гц), сходным с записями ЛПП в контроле. Спектры мощности осцилляций до и после введения леводопы представлены на рис. 4 (а), а спектры когерентности – на рис. 4 (б). Значительное снижение когерентности осцилляций наблюдалось при ДА-дефиците во всех парах моторной нейросети и подтверждалось уменьшением числа спектров с когерентными осцилляциями (рис. 4 (в)), а также снижением средних величин когерентности (рис. 4 (г)).
Рис. 4.
Влияние стимуляции ДA-рецепторов при введении леводопы (ЛД+) на осциляциии в моторной нейросети и выраженность двигательных нарушений при ДA-дефиците. (а): спектры мощности до и после ЛД+. (б): спектры когерентности между отделами нейросети при тех же условиях. (в): наличие когерентных ЛПП до и после ЛД+. (г): когерентность между отделами нейросети в ДA(–) группе до и после ЛД+. *р < 0.05, выше в парах MCx – SNr (β ритм) и dStr – GPe (γ ритм) в ДА(–) группе; #p < 0.05, снижение после ЛД+. (д): Оценка двигательного дефицита при ходьбе в круговом тредмилле. Сторона повреждения и пораженные лапы обозначены белыми кружками. (и): отношение числа шагов при ходьбе влево и вправо в ДA(+), ДА(–) и ЛД(+). #p < 0.05, ниже при ходьбе влево в ДA(–). (ж): % завершенных 30-секундных проходок.
Fig. 4. Effects of levodopa (LD+)-induced dopamine receptors stimulation on LFP synchronization and treadmill walking in DA(–) group. (а): LFP power spectra before and after LD(+). (б): LFP coherence spectra in motor circuit under the same conditions. (в): Histograms indicate the % coherence spectra with oscillations before and after LD(+). (г): Averaged LFP coherence before and after LD(+). *р < 0.05, the same as in Fig. 3 (г); #р < 0.05, higher before relative to LD(+). (д): Experimental design for motor behavior scoring. Affected paws and DA(–) hemisphere are indicated by open circles. (и): Step count ratios during walking in DA(+), DA(–) groups and following LD(+). # p < 0.05, lower as walking to the right in DA(–) group. (ж): % completed walking trials.
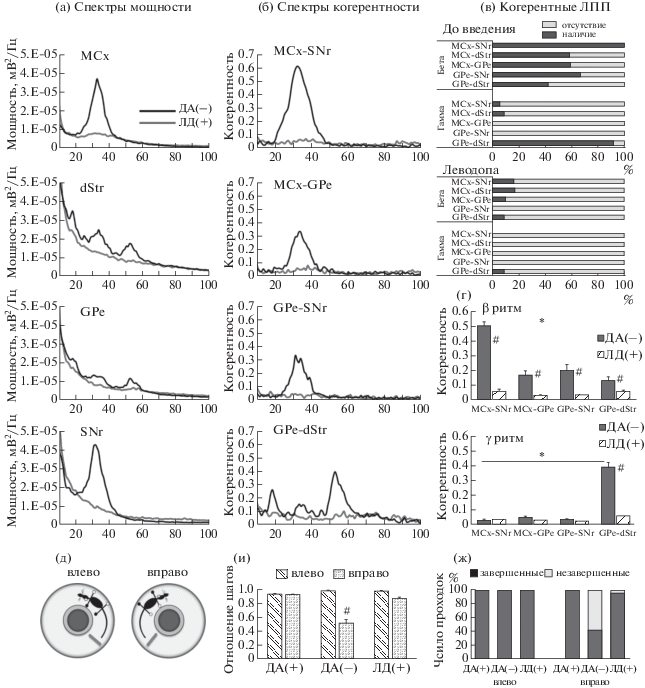
Используя круговой тредмилл, можно количественно оценить степень двигательных нарушений и выявить эффект заместительной терапии леводопой у животных с ДА-дефицитом. Крысы контрольной группы идут равномерно в круговом тредмилле (рис. 4 (д)) влево и вправо, что подтверждается одинаковыми величинами отношения числа шагов при ходьбе (0.93 ± 0.01 и 0.98 ± 0.01 соответственно, рис. 4 (и), см. ДА+) и 100%-й завершенностью 30-секундных проходок (рис. 4 (ж), см. ДА+). После одностороннего повреждения ДА-нейронов величина отношения числа шагов при ходьбе вправо значительно уменьшалась (0.51 ± 0.05, p < 0.001, 2-way ANOVA; рис. 4 (и), см. ДА–), а также снижалось до 42% число завершенных 30-секундных проходок (рис. 4 (ж), см. ДА–). Леводопа снижала мощность β- и γ-осцилляций в звеньях моторной нейросети, и животные могли идти в направлении по часовой стрелке (вправо), а отношение числа шагов при ходьбе вправо и влево было соответственно 0.87 ± ± 0.02 и 0.97 ± 0.01, p > 0.05 (рис. 4 (и), см. ЛД+). При этом число завершенных 30-секундных проходок вправо приближалось к 100% (рис. 4 (ж), см. ЛД+).
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
В экспериментальной модели БП мы изучали роль GPe в возникновении и распространении синхронизованной осцилляторной активности в моторных нейросетях, появляющейся после повреждения ДА-синтезирующих клеток SNc. В контрольной группе и у крыс с БП регистрировали ЛПП в MCx и ядрах BG в покое и при ходьбе. Анализ ЛПП в MCx и ядрах BG показал, что в структурах полушарий с ДА-дефицитом при ходьбе, но не в состоянии покоя или в интактном мозге, возникают аберрантные β-осцилляции (30–36 Гц). Мощность и когерентность осцилляций были наибольшими в MCx и SNr, в то время как выраженность осцилляций в dStr и GPe была ниже, как и величины их когерентности с MCx и SNr. Помимо β-осцилляций выявлена синхронная γ-активность в диапазоне 50–56 Гц при ходьбе, исключительно в dStr и GPe. В MCx и SNr осцилляции в γ-диапазоне отсутствовали. В ЛПП dStr и GPe у контрольных животных при ходьбе регистрировались осцилляции низкой мощности и в более широком диапазоне γ-частот (45–90 Гц).
Вклад GPe в процесс генерации и распространения β-частотных осцилляций. β-Осцилляции, регистрируемые у пациентов с БП, часто ассоциируют с возникновением таких симптомов, как тремор, ригидность, акинезия и брадикинезия (Weinberger et al., 2009). Предполагается, что в генерации β-активности участвует реципроктная микросеть STN – GPe (Plenz, Kita, 1999; Mallet et al., 2008; Tachibana et al., 2011; Koelman, Lowery, 2019), а моторный неокортекс способствует усилению их мощности и распространению этой активности в нейросети BG – таламус – MCx (Sharott et al., 2018; West et al., 2018; Baaske et al., 2020; de la Crompe et al., 2020). GPe имеет обширные реципроктные связи с моторными отделами неокортекса и всеми ядрами BG (Chen et al., 2015; Saunders et al., 2015; Abecassis et al., 2019). В силу центрального расположения оно может вносить существенный вклад в осцилляторную активность в моторных нейросетях, контролирующих локомоцию (рис. 5). В последнее время отношение к роли GPe в работе моторных нейросетей существенно изменилось – от оценки его как простого передаточного звена информационного потока от моторной коры и стриатума к выходным ядрам – SNr/GPi (Albin et al., 1989; Hernandez et al., 2015) до признания его центральным и интегративным звеном в системе BG (Kita, 2007; Gittis et al., 2014; Mastro et al., 2017; de la Crompe et al., 2020; Dong et al., 2021).
Рис. 5.
Схема связей между отделами неокортекса и ядрами базальных ганглиев у крыс. GPe входит в состав двух основные моторных нейросетей: MCx – базальные ядра – таламо-кортикальная сеть (сплошная линия), включающая Рro-GPe нейроны как ключевой элемент непрямого стриопаллидарного пути, и FСx – стриопаллидарная сеть (пунктирная линия), включающая Аrk-GPe-нейроны с их возвратными тормозными проекциями к FSI и MSNs стриатума. STN контролирует активность Рro и Аrk GPe-нейронов. Сокращения: DA –дофаминергические; GABA – ГАМК-ергические и Glu – глутаматергические проекции, FCx – фронтальная кора.
Fig. 5. Schematic representation of main connections of the BG – neocortex network of the rodent brain. GPe connectivity comprises the two major neuronal motor circuits: (1) the MCx – BG – thalamocortical with Рro-GPe and (2) the FCx – striato – pallidal with Аrk-GPe, which are interlinked with the STN. Dopaminergic (DA) – GABAergic (GABA), and glutamatergic (Glu) projections are depicted by diamond, arrow and circuit heads, respectively. FCx – frontal neocortex.
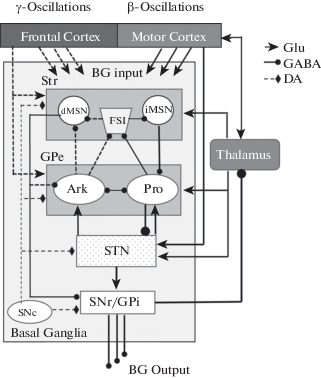
Предполагается, что дихотомическая организация нейронных популяций в GPe играет заметную роль в генерации и проведении β-осцилляций при БП (Mallet et al., 2012; Mastro et al., 2014, 2017; Abdi et al., 2015; Pamukcu et al., 2020). Детально описаны две группы нейронов в GPe: быстроразряжающиеся прототипные (Pro-GPe) и низкочастотные аркипаллидальные (Ark-GPe). Активности этих групп нейронов находятся в противофазе. Каждая субпопуляция нейронов GPe вносит специфический вклад в контроль двигательной активности. Pro-GPe нейроны, экспрессирующие парвальбумин и транскрипционные факторы Nkx2.1 и Lhx6 (Abdi et al., 2015; Dodson et al., 2015; Hernandes et al., 2015; Abrahao, Lovinger, 2018), в основном проецируются на субталамическое ядро и выходные ядра BG. Предполагается, что они участвуют в генерации β-активности в паре GPe – STN и ее распространении через выходные ядра базальных ганглиев, GPi и SNr, на моторные ядра таламуса и неокортекс (Saunders et al., 2015; Mallet et al., 2019).
В целом не ясно, какие звенья моторной сети вносят принципиальный вклад в процесс генерации и распространения β-активности: MCx и ее проекции к BG (Sharott et al., 2018), микросети STN – GPe (Bevan et al., 2002; Tachibana et al., 2011; Koelman, Lowery, 2019) и dStr – GPe (McCarthy et al., 2011; Corbit et al., 2016) или более широкая нейросеть, включающая таламус (Nevado-Holdago et al., 2014; Brazhnik et al., 2016; Singh, 2018). Результаты нашей работы показывают, что когерентныe β-осцилляции присутствуют во всех звеньях нейросети BG – MCx при дефиците ДА, и их мощность со временем возрастает. Данные, полученные при регистрации активности в MCx и ядрах BG, выявили наибольшую мощность β-осцилляций в MCx и SNr, напрямую связанных со структурами, контролирующими локомоцию. С другой стороны, мощность β-осцилляций в dStr, получающего информацию от моторной коры и GPe, была низкой, как и величины когерентности β-осцилляций в этих ядрах с активностью в MCx и SNr в β-частотном диапазоне.
В соответствии с ранними работами о наличии аберрантной β-активности в MCx и SNr (Brazhnik et al., 2012; 2016), а также в STN у крыс с ДА-дефицитом (Delaville et al., 2015), результаты этого исследования во многом подтверждают предположение о ключевой роли гиперпрямого пути от MCx к STN в генерации и распространении патологической β-активности по моторной сети (Nambu et al., 2002; Polyakova et al., 2020). Данные о том, что мощность β-осцилляций в MCx и SNr наибольшая, подтверждают предположение о преимущественном вкладе гиперпрямого пути (MCx – STN – SNr) в передачу β-активности, в то время как участие dStr и GPe в генерации и распространении β-ритмов минимально. Сильным аргументом в пользу этого предположения служит и тот факт, что β-активность в моторной коре является ведущей по отношению к β-активности в субталамическом ядре у пациентов с БП (Litvak et al., 2011; Sharott et al., 2018) и у животных с экспериментальной БП (Sharott et al. 2005; Polyakova et al., 2020). Отметим, что моторный таламус, получающий моносинаптические связи от SNr и посылающий прямые связи к MCx, является необходимым звеном в BG – таламо-кортикальной цепи, вовлеченной в проведение β-осцилляций (Nevado-Holgado et al., 2014; Brazhnik et al., 2016).
Вклад нейронной сети GPe – dStr в процесс усиления γ-осцилляций при дефиците дофамина. Мы показали, что при ходьбе у крыс с дефицитом ДА в GPe и dStr регистрируется устойчивая ритмическая активность в узком γ-диапазоне 50–56 Гц. Мощность этих осцилляций была значительна, как и величина когерентности, по сравнению с контрольной группой, где в ЛПП выявлены осцилляции низкой мощности в широком диапазоне γ-частот (45–90 Гц). Отметим, что в записях MCx и SNr в полушариях с ДА-дефицитом γ-осцилляции 50–56 Гц отсутствовали. Какова же природа γ-осцилляторной активности у животных с экспериментальной БП?
Ключевым компонентом сети ядер BG считается стриато-паллидальный путь, анатомические проекции которого хорошо изучены, но его роль до сих пор не ясна. Проекционные нейроны стриатума (MSNs) получают информацию от MCx и передают ее на выходное звено BG, GPi/SNr, через прямой стриато-нигральный путь (dMSNs), или к тем же ядрам опосредованно с переключением в GPe (Pro-GPe нейроны) – стриато-паллидальный непрямой путь (iMSNs). Вторая популяция GPe нейронов, Ark-GPe, экспрессирующие проэнкефалин (Mallet et al., 2012) и транскрипционныe факторы FoxP2 и Npas1 (Abdi et al., 2015; Hernandez et al., 2015; Hegeman et al., 2016; Abrahao, Lovinger, 2018; Pamukcu et al., 2020), получает сигнал по коллатералям от проходящего dMSNs (Ketzef, Silberberg, 2021) и за счет обратных проекций к стриатуму способна подавлять или усиливать активность его нейронов и, таким образом, регулировать степень их участия в передаче информации на выходные ядра BG, GPi/SNr и в контроле локомоции (Glajch et al., 2016; Mallet et al., 2016; Chuhma, 2021).
Нами установлено, что активность в GPe у животных с ДА-дефицитом представлена по крайней мере двумя частотными диапазонами – бета (30–36 Гц) и гамма (50–56 Гц). Природа синхронной γ-активности в диапазоне 50–56 Гц заслуживает особого внимания в связи с тем, что ее значительное усиление совпадает по времени с возникновением β-осцилляций у животных с БП. Кроме того, γ-активность 50–56 Гц в полушариях с ДА-дефицитом, как и в случае с β-осцилляциями, эффективно устранялась или значительно снижалась при введении леводопы с восстaновлением контрольного паттерна активности (45–90 Гц) и нормальной локомоции, что указывает на очевидную связь усиления этого ритма с нарушением двигательной функции у животных с БП.
Результаты морфологических исследований позволяют предположить, что обратные проекции от Ark-GPе-нейронов к стриатуму контролируют активность проекционных нейронов (MSNs) и высокочастотных интернейронов (FSI) стриатума (Berke, 2009; Mastro et al., 2014; 2017). Показано, что спайковая активность FSI имеет высокую корреляцию с ЛПП стриатума в диапазоне γ-частот (см. Berke, 2009), а их локальная оптостимуляция способна запустить осцилляции в γ-частотном диапазоне (Cardin et al., 2009). Наличие γ-осцилляций обнаружено также у части проекционных нейронов стриатума (Lemaire et al., 2012). Данные, полученные на моделях моторных сетей с аберрантной синхронизацией, сходной с наблюдаемой при БП, свидетельствуют, что для возникновения и проведения по нейронной сети BG осцилляторной активности необходимо взаимодействие между GPe- и FSI-нейронами стриатума (Corbit et al., 2016). Это в определенной степени подтверждает участие GPe в проведении γ-осцилляций к dStr и их усиление при ДА-дефиците.
Результаты клинических наблюдений свидетельствуют, что нарушения процессов планирования и осуществления действия (исполнительная функция) вносят значительный вклад в возникновение специфической двигательной симптоматики при БП – застывание (FoG), неспособность начать движениe (акинезия) или изменить его направление. В работах последних лет показана связь между развитием FoG и дисфункцией фронтальных и префронтальных отделов неокортекса при БП (Maidan et al., 2015, Belluscio et al., 2019). Активность во фронтальных и префронтальных отделах неокортекса у контрольных животных характеризуется наличием γ-осцилляций в диапазоне 40–90 Гц (см. обзор Berke, 2009), которые могут контролировать функцию проекционных отделов мозга, в частности, ядер BG (Smith et al., 2016; Choi et al., 2019; McGregor et al., 2019). Усиление мощности и длительности γ-осцилляций (50–56 Гц), регистрируемых в dStr и GPe у животных с ДА-дефицитом, по времени совпадает с появлением двигательных нарушений (акинезии, застывания), что может быть специфической характеристикой дисфункции в сети фронтальная кора – стриато-паллидальная система при БП.
На основании представленных результатов можно считать, что при дефиците дофамина меняется баланс в функционировании отдельных элементов нейронных сетей, включающих различные отделы неокортекса и ядер BG. Следствием этого являются возникновение и распространение когерентных осцилляций в моторных нейросетях. Выявленные различия между двумя типами активности в GPe с ДА-дефицитом (β- и γ-осцилляции) предполагают наличие в нем двух нейронных популяций, Pro-GPe и Ark-GPe, которые участвуют в работе разных моторных сетей, контролирующих различные аспекты процесса движения. Высокосинхронизованная β-активность, обнаруженная при БП во всех звеньях MСx – BG – таламо-кортикальной нейросети и наиболее выраженная в MCx и SNr, в основном ассоциируется с возникновением брадикинезии. В то время как усиленная по мощности и продолжительности 50–56 Гц γ-активность в dStr и GPe у крыс с ДА-дефицитом может быть характеристикой состояния кортико-стриато-паллидальной нейросети, связанной с появлением специфических двигательных симптомов (акинезия, застывание) в ситуациях, когда нарушена исполнительная функция.
ВЫВОДЫ
1. B норме активность MCx и ядер базальных ганглиев, включая GPe, не синхронизированa, что обеспечивает координированную работу моторных нейросетей, контролирующих локомоцию.
2. Утрата ДА при БП сопровождается появлением когерентных осцилляций в MCx, GPe и другиx ядраx BG и приводит к нарушениям движения.
3. При дефиците ДА активность в GPe представлена осцилляциями в двух частотных диапазонах, β (30–36 Гц) и γ (50–56 Гц), что указывает на наличие в GPe по крайней мере двух, отличающихся по свойствам, нейронных групп.
4. β-Осцилляции, связанные с развитием брадикинезии, замедленностью движений и мышечной ригидностью при БП, имеют наибольшую мощность в MCx и SNr, а в GPe и dSTR их мощность значительно ниже, что предполагает минимальную роль этих ядер в передаче β-ритма в моторных нейросетях.
5. С другой стороны, усиление когерентной 50–56 Гц γ-активности в GPe и dSTR, но не в MCx и SNr, может быть связано с другими типами двигательных нарушений при БП – акинезией, неспособностью начать движение.
6. Наличие β- и γ-осцилляторной активности в GPe полушарий с ДА-дефицитом свидетельствует о сложности организации моторных нейросетей, контролирующих в норме различные аспекты локомоции.
Список литературы
Abdi A., Mallet N., Mohamed F.Y., Sharott A., Dodson P.D., Nakamura K.C., Suri S., Avery S.V., Larvin J.T., Garas F.N., Garas S.N., Vinciati F., Morin S., Bezard E., Baufreton J., Magill P.J. Prototypic and Arkypallidal Neurons in the Dopamine-Intact External Globus Pallidus J. Neurosci. 2015. 17: 6667–6688.
Abecassis Z.A., Berceau B.L., Win P.H., García D., Xenias H.S., Cui Q., Pamukcu A., Cherian S., Hernández V.M., Chon U., Lim B.K., Kim Y., Justice N.J., Awatramani R., Hooks B.M., Gerfen C.R., Boca S.M., Chan C.S. Npas1+-Nkx2.1+ Neurons Are an Integral Part of the Cortico-pallido-cortical Loop. J. Neurosci. 2020. 4: 743–768.
Abrahao K.P., Lovinger D.M. Classification of GABAergic neuron subtypes from the globus pallidus using wild-type and transgenic mice. J. Physiol. 2018. 596: 4219–4235.
Albin R.L., Young A.B., Penney J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989. 12: 366–375.
Avila I., Parr-Brownlie L.C., Brazhnik E., Castañeda E., Bergstrom D.A., Walters J.R. Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat. Exp. Neurol. 2010. 2: 307–319.
Baaske M.K., Kormann E., Holt A.B., Gulberti A., McNamara C.G., Pötter-Nerger M., Westphal M., Engel A.K., Hamel W., Brown P., Sharott A. Parkinson’s disease uncovers an underlying sensitivity of subthalamic nucleus neurons to beta-frequency cortical input in vivo. Neurobiol. Dis. 2020. 146: 105–119.
Belluscio V., Stuart S., Bergamini E., Vannozzi G., Mancini M. The Association between Prefrontal Cortex Activity and Turning Behavior in People with and without Freezing of Gait. Neuroscience. 2019. 416: 168–176.
Berke J.D. Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur. J. Neurosci. 2009. 30: 848–859.
Bevan M.D., Magill P.J., Terman D., Bolam J.P., Wilson C.J. Review. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002. 10: 525–531.
Brazhnik E., Cruz A.V., Avila I., Wahba M.I., Novikov N., Ilieva N.M., McCoy A.J., Gerber C., Walters J.R. State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats. J. Neurosci 2012. 32: 7869–7880.
Brazhnik E., McCoy A.J., Novikov N., Hatch C.E., Walters J.R. Ventral medial thalamic nucleus promotes synchronization of increased high beta oscillatory activity in the basal ganglia-thalamocortical Network of the hemiparkinsonian rat. J. Neurosci. 2016. 36: 4196–4208.
Brittain J.S., Sharott A., Brown P. The highs and lows of beta activity in cortico-basal ganglia loops. Eur. J. Neurosci. 2014. 39: 1951–1959.
Brown P. Bad oscillations in Parkinson’s disease J. Neural Transm. 2006. 70: 27–30.
Cacciola A., Milardi D., Bertino S., Basile G.A., Calamuneri A., Chillemi G., Rizzo G., Anastasi G., Quartarone A. Structural connectivity-based topography of the human globus pallidus: Implications for therapeutic targeting in movement disorders. Mov. Disord. 2019. 7: 987–996.
Cardin J.A., Carlén M., Meletis K., Knoblich U., Zhang F., Deisseroth K., Tsai L.H., Moore C.I. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009. 459: 663–667.
Chen M.C., Ferrari L., Sacchet M.D., Foland-Ross L.C., Qiu M-H., Gotlib I.H., Fuller P.M., Arrigoni E., Lu J. Identification of a direct GABAergic pallidocortical pathway in rodents. Eur. J. Neurosci. Mov Disord. 2015. 30: 293–295.
Choi K., Holly E., Davatolhagh M.F., Beier K.T., Fuccillo M.V. Integrated anatomical and physiological mapping of striatal afferent projections. Eur J Neurosci. 2019; 49 (5): 623–636.
Chuhma N. Functional Connectome Analysis of the Striatum with Optogenetics. Adv Exp Med Biol. 2021. 1293: 417–428.
Corbit V.L., Whalen T.C., Zitelli K.T., Crilly S.Y., Rubin J.E., Gittis A.H. Pallidostriatal projections promote β oscillations in a dopamine-depleted biophysical network model. J. Neurosci. 2016. 2: 5556–5571.
de la Crompe B., Aristieta A., Leblois A., Elsherbiny S., Boraud T., Mallet N.P. The globus pallidus orchestrates abnormal network dynamics in a model of Parkinsonism. Nat. Commun. 2020. 11: 1570–1584.
Dejean C.A., Le Moine C., Bioulac B., Gross C.E., Boraud T. Evolution of the dynamic properties of the cortex-basal ganglia network after dopaminergic depletion in rats. Neurobiol. Dis. 2012. 2: 402–413.
Delaville C., McCoy A.J., Gerber C.M., Cruz A.V., Walters J.R. Subthalamic nucleus activity in the awake hemiparkinsonian rat: relationships with motor and cognitive networks. J. Neurosci. 2015. 17: 6918–6930.
Dodson P.D., Larvin J.T., Duffell J.M., Garas F.N., Doig N.M., Kessaris N., Duguid I.C., Bogacz R., Butt S.J., Magill P.J. Distinct developmental origins manifest in the specialized encoding of movement by adult neurons of the external globus pallidus. Neuron. 2015. 86: 501–513.
Eid L., Parent M. Morphological evidence for dopamine interactions with pallidal neurons in primates. Front. Neuroanat. 2015. 9: 1–14.
Gauthier J., Parent M., Levesque M., Parent A. The axonal arborization of single nigrostriatal neurons in rats. Brain Res. 1999. 834: 228–232.
Gittis A.H., Berke J.D., Bevan M.D., Chan C.S., Mallet N., Morrow M.M., Schmidt R. New roles for the external globus pallidus in basal ganglia circuits and behavior. J. Neurosci. 2014. 34: 15178–15183.
Glajch K.E., Kelver D.A., Hegeman D.J., Cui Q., Xenias H.S., Augustine E.C., Hernández V.M., Verma N., Huang T.Y., Luo M., Justice N.J., Chan C.S. Npas1+ Pallidal Neurons Target Striatal Projection Neurons. J. Neurosci. 2016. 36: 5472–5488.
Grewal S.S., Holanda V.M., Middlebrook E.Y. Corticopallidal Connectome of the Globus Pallidus Externus in Humans: An Exploratory Study of Structural Connectivity Using Probabilistic Diffusion Tractography. Am. J. Neuroradiol. 2018. 11: 2120–2125.
Hegeman D.J., Hong E.S., Hernández V.M., Chan C. The External Globus Pallidus: Progress and Perspectives. Eur. J. Neurosci. 2016. 10: 1239–1265.
Hernández V.M., Hegeman D.J., Cui Q., Kelver D.A., Fiske M.P., Glajch K.E., Pitt J.E., Huang T.Y., Justice N.J., Chan C.S. Parvalbumin+ Neurons and Npas1+ Neurons Are Distinct Neuron Classes in the Mouse External Globus Pallidus. J. Neurosci. 2015. 35: 11830–11847.
Karube F., Takahashi S., Kobayashi K., Fujiyama F. Motor cortex can directly drive the globus pallidus neurons in a projection neuron type-dependent manner in the rat. Elife. 2019; 8: e49511.
Ketzef M., Silberberg G. Differential Synaptic Input to External Globus Pallidus Neuronal Subpopulations In Vivo. Neuron. 2021 Feb 3; 109 (3): 516–529.
Kita H. (2007). Globus pallidus external segment. Prog. Brain Res. 2007. 160: 111–133.
Koelman L.A., Lowery M.M. Beta-Band Resonance and Intrinsic Oscillations in a Biophysically Detailed Model of the Subthalamic Nucleus-Globus Pallidus Network. Front. Comput. Neurosci. 2019. 13: 1–24.
Lemaire N., Hernández L.F., Hu D., Kubota Y., Howe M.W., Graybiel A.M. Effects of dopamine depletion on LFP oscillations in striatum are task- and learning-dependent and selectively reversed by L-DOPA. Proc. Natl. Acad. Sci. U S A. 2012. 109: 18126–18131.
Litvak V., Jha A., Eusebio A., Oostenveld R., Foltynie T., Limousin P., Zrinzo L., Hariz M.I., Friston K., Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease. Brain. 2011.134: 359–374.
Maidan I., Bernad-Elazari H., Gazit E., Giladi N., Hausdorff J.M., Mirelman A. Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: an fNIRS study of transient motor-cognitive failures. J. Neurol. 2015. 4: 899–908.
Mallet N., Delgado L., Chazalon M., Miguelez C., Baufreton J. Cellular and Synaptic Dysfunctions in Parkinson’s Disease: Stepping Out of the Striatum Cells. 2019. 9: 1005.
Mallet N., Micklem B.R., Henny P., Brown M.T., Williams C., Bolam J.P., Nakamura K.C., Magill P.J. Dichotomous organization of the external globus pallidus. Neuron 2012. 74: 1075–1086.
Mallet N., Pogosyan A., Marton L.F., Bolam J.P., Brown P., Magill P.J. Parkinsonian Beta Oscillations in the External Globus Pallidus and Their Relationship with Subthalamic Nucleus Activity. J. Neurosci. 2008. 52: 14245–14258.
Mallet N., Schmidt R., Leventhal D., Chen F., Amer N., Boraud T., Berke J.D. Arkypallidal Cells Send a Stop Signal to Striatum. Neuron. 2016. 89: 308–316.
Mastro K.J., Bouchard R.S., Holt H.A., Gittis A.H. Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J. Neurosci. 2014. 34: 2087–2099.
Mastro K.J., Zitelli K.T., Willard A.M., Leblanc K.H., Kravitz A.V., Gittis A.H. Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice. Nat. Neurosci. 2017. 20: 815–823.
McCarthy M.M., Moore-Kochlacs C., Gu X., Boyden E.S., Han X., Kopell N. Striatal origin of the pathologic beta oscillations in Parkinson’s disease. Proc. Natl. Acad. Sci. U S A. 2011. 108: 11620–11625.
McGregor M.M., McKinsey G.L., Girasole A.E., Bair-Marshall C.J., Rubenstein J.L.R., Nelson A.B. Functionally Distinct Connectivity of Developmentally Targeted Striosome Neurons. Cell Rep. 2019. 29(6): 1419–1428.
Nambu A., Tokuno H., Takada M. Functional significance of the cortico-subthalamo-pallidal 'hyperdirect' pathway. Neurosci Res. 2002. 2:111–117.
Neumann W.J., Degen K., Schneider G.H., Brücke C., Huebl J., Brown P., Kühn A.A. Subthalamic synchronized oscillatory activity correlates with motor impairment in patients with Parkinson’s disease. Mov. Disord. 2016. 31:1748–1751.
Nevado-Holgado A.J., Mallet N., Magill P.J., Bogacz R. Effective connectivity of the subthalamic nucleus–globus pallidus network during Parkinsonian oscillations. J. Physiol. 2014. 592 (Pt 7): 1429–1455.
Pamukcu A., Cui Q., Xenias H.S., Berceau B.L., Augustine E.C., Fan I., Chalasani S., Hantman A.W., Lerner T.N., Boca S.M., Chan C.S. Parvalbumin (+) and Npas1(+) Pallidal Neurons Have Distinct Circuit Topology and Function. J. Neurosci. 2020. 40: 7855–7876.
Parent A., Hazrati L.N. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Brain Res. Rev. 1995. 1: 91–127.
Plenz D., Kital S.T. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999. 6745: 677–682.
Pogosyan A., Yoshida F., Chen C.C., Martinez-Torres I., Foltynie T., Limousin P., Zrinzo L., Hariz M.I., Brown P. Parkinsonian impairment correlates with spatially extensive subthalamic oscillatory synchronization. Neuroscience. 2010. 171 (1): 245–257.
Polyakova Z., Chiken S., Hatanaka N., Nambu A. Cortical Control of Subthalamic Neuronal Activity through the Hyperdirect and Indirect Pathways in Monkeys. J. Neurosci. 2020. 39: 7451–7463.
Rodriguez-Sabate C., Morales I., Monton F., Rodriguez M. The influence of Parkinson’s disease on the functional connectivity of the motor loop of human basal ganglia. Parkinsonism Relat Disord. 2019. 63: 100–105.
Saunders A., Oldenburg I.A., Berezovskii V.K., Johnson C.A., Kingery N.D., Elliott H.L., Tiao X., Gerfen C.R., Sabatini B.L. A direct GABAergic output from the basal ganglia to frontal cortex. Nature. 2015. 521: 85–89.
Sharott A., Gulberti A., Hamel W., Köppen J.A., Münchau A., Buhmann C., Pötter-Nerger M., Westphal M., Gerloff C., Moll C.K.E., Engel A.K. Spatio-temporal dynamics of cortical drive to human subthalamic nucleus neurons in Parkinson’s disease. Neurobiol. Dis. 2018. 112: 49–62.
Sharott A., Magill P.J., Harnack D., Kupsch A., Meissner W., Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur. J. Neurosci. 2005. 5: 1413–1422.
Sharott A., Gulberti A., Zittel S., Tudor J.A.A., Fickel U., Munchau A., Köppen J.A., Gerloff C., Westphal M., Buhmann C., Hamel W., Engel A.K., Moll C.K.E. Activity parameters of subthalamic nucleus neurons selectively predict motor symptom severity in Parkinson’s disease. J. Neurosci. 2014. 34: 6273–6285.
Singh A. Oscillatory activity in the cortico-basal ganglia-thalamic neural circuits in Parkinson’s disease. Eur. J. Neurosci. 2018. 8: 2869–2878.
Smith J.B., Klug J.R., Ross D.L., Howard C.D., Hollon N.G., Ko V.I., Hoffman H., Callaway E.M., Gerfen C.R., Jin X. Genetic-based dissection unveils the inputs and outputs of striatal patch and matrix compartments. Neuron. 2016; 91 (5): 1069–1084.
Smith Y., Villalba R. Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov. Disord. 2008. 3: S534–547.
Tachibana Y., Iwamuro H., Kita H., Takada M., Nambu A. Subthalamo-pallidal interactions underlying parkinsonian neuronal oscillations in the primate basal ganglia. Eur. J. Neurosci. 2011. 34: 1470–1484.
Weinberger M., Hutchison W.D., Dostrovsky J.O. Pathological subthalamic nucleus oscillations in PD: can they be the cause of bradykinesia and akinesia? Exp. Neurol. 2009. 1: 58–61.
West T.O., Berthouze L., Halliday D.M., Litvak V., Sharott A., Magill P.J., Farmer S.F. Propagation of beta/gamma rhythms in the cortico-basal ganglia circuits of the parkinsonian rat. J. Neurophysiol. 2018. 5: 1608–1628.
Yasukawa T., Kita T., Xue Y., Kita H. Rat intralaminar thalamic nuclei projections to the globus pallidus: a biotinylated dextran amine anterograde tracing study. J. Comp. Neurol. 2004. 2: 153–167.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


