Журнал высшей нервной деятельности им. И.П. Павлова, 2021, T. 71, № 5, стр. 649-666
Инвазивная регистрация фокальных потенциалов у пациентов с эпилепсией при наблюдении, представлении и выполнении движений
Е. Д. Каримова 1, 2, *, С. Е. Буркитбаев 1, 2, И. С. Трифонов 3, М. В. Синкин 4, Ф. К. Ридер 2, А. Б. Гехт 2, 5, Н. Н. Лебедева 1
1 ФГБУН Институт высшей нервной деятельности и нейрофизиологии РАН
Москва, Россия
2 ГБУЗ Научно-практический психоневрологический центр им. З.П. Соловьева, ДЗМ
Москва, Россия
3 Клинический медицинский центр МГМСУ им. А.И. Евдокимова
Москва, Россия
4 НИИ скорой помощи им. Н.В. Склифосовского
Москва, Россия
5 РНИМУ им. Н.И. Пирогова
Москва, Россия
* E-mail: e.d.karimova@gmail.com
Поступила в редакцию 24.11.2020
После доработки 16.02.2021
Принята к публикации 02.03.2021
Аннотация
Существование нейронов с “зеркальными” свойствами у человека было показано в инвазивном исследовании на пациентах с эпилепсией только в 2010 г., спустя почти 15 лет изучения зеркальной системы мозга (ЗСМ). Сложность исследования ЗСМ у человека заключается в ограничениях используемых методов оценки активности структур, таких как ЭЭГ и фМРТ. В данной работе регистрировали активность подкорковых структур мозга с помощью погружных электродов при реализации различных моторных задач у пациентов с фармакорезистентной эпилепсией. Характеристики фокальных потенциалов (local field potential, LFP) были получены для левых и правых лобных и височных областей. Мощность LFP-сигнала достоверно повышалась при выполнении социально окрашенных задач в левом лобном электроде, что может свидетельствовать о наличии нейрональных связей ЗСМ с системой базальных ганглиев, обеспечивающих контроль и затормаживание нежелательных моторных действий при восприятии, а также принимающих участие в процессе моторного ассоциативного обучения.
ВВЕДЕНИЕ
Зеркальные нейроны обладают важным свойством избирательной активации. Активность определенных зеркальных нейронов повышается как при наблюдении, так и при представлении и выполнении определенного действия. Зеркальная система мозга (ЗСМ) в связи с этим играет важнейшую роль в обучении через подражание в раннем возрасте и в социальном поведении и взаимоотношениях людей во взрослом возрасте. Основной проблемой в исследованиях зеркальных нейронов является то, что непрямые методы регистрации активности нейронов, такие как фМРТ и ЭЭГ, не обладают способностью с точностью выявить активацию зеркальных нейронов. Методы нейровизуализации позволяют указать на области активации ЗСМ, применение ЭЭГ – оценить уровень активации ЗСМ по степени десинхронизации мю-ритма, однако лишь инвазивные методы позволяют зарегистрировать активность ЗСМ напрямую. Исследование зеркальной системы мозга у пациентов, страдающих фармакорезистентной эпилепсией, с помощью погружных инвазивных электродов дает уникальную возможность приблизиться к изучению активности отдельных зеркальных нейронов, способствует пониманию их свойств.
Зеркальные нейроны впервые были обнаружены в 90-х годах итальянскими исследователями под руководством Риццолатти во время опытов с обезьянами (Gallese et al., 1996). Сразу в этом же году появилось первое исследование ЗСМ человека, опять же под руководством Риццолатти (Grafton et al., 1996). Дальнейшие исследования ЗСМ у людей продолжили уже с использованием других приемлемых методов, таких как фМРТ, ТМС и ЭЭГ. Были обнаружены новые свойства – повышение активности ЗСМ при представлении наблюдаемого двигательного акта, а также при просмотре и повторении эмоциональных выражений лица человека. Исследования с использованием ЭЭГ обнаружили взаимосвязь между десинхронизацией мю-ритма и активацией ЗСМ.
Большую часть современных исследований ЗСМ занимают работы, посвященные роли зеркальных нейронов в эмоциональных реакциях, эмпатии, понимании эмоций и мотиваций других людей. Одна из работ, посвященных роли ЗСМ в эмоциональной сфере, была выполнена Марком Якобони, разработчиком теории сети зеркальных нейронов (Carr et al., 2003). В результате были обнаружены достоверные изменения в правой островковой доле и нижней лобной коре. Дальнейшие исследования также подтвердили, что островок, имеющий обширные связи с моторными отделами мозга, рассматривается как своего рода посредник между лимбической корой и системой зеркальных нейронов (Косоногов, 2009).
Обширные исследования ЗСМ за последние 10 лет с помощью косвенных методов регистрации привели к тому, что понятие зеркальных нейронов, их функции и локализация сети значительно расширились и размылись. С другой стороны, противоречивые результаты некоторых исследований стали основанием для того, что многие ученые дискредитировали теорию зеркальных нейронов. Множество методически неверных работ, а также применение непрямых методов регистрации активности мозга породили сомнения в вопросе существования зеркальных нейронов у человека.
Однако в 2010 г. Мукамель выпустил статью, которая поставила точку в вопросе существования зеркальных нейронов у человека (Mukamel et al., 2010). Мукамель с коллегами нейрохирургами впервые зарегистрировал активность единичных нейронов у человека при исследовании ЗСМ. Для этого пациентам с фармакорезистентной эпилепсией демонстрировали стимульный материал для активации ЗСМ во время регистрации внеклеточной активности нейронов с погружных инвазивных электродов. Всего была зарегистрирована активность 1177 нейронов, 7% из которых реагировали одинаково на наблюдение, представление и выполнение одного и того же действия. Большой процент зеркальных нейронов был найден в гиппокампе, моторной и премоторной областях.
Совсем немного работ с тех пор было сделано на пациентах с вживленными электродами, хотя, если брать во внимание сложность подобных работ и уникальность пациентов, это совсем неудивительно. Насколько нам известно, пока существует только одно исследование ЗСМ, выполненное Мукамель с коллегами, в котором анализировались записи активности отдельных нейронов (Mukamel et al., 2010). Вслед за ним было еще несколько исследований.
При использовании электродов глубокой стимуляции головного мозга для прямой записи активности субталамического ядра базальных ганглиев у пациентов с болезнью Паркинсона была также выявлена двусторонняя десинхронизация бета-активности во время движения и наблюдения за движением, что предполагает субкортикальную активацию ЗСМ (Alegre et al., 2010). В 2014 г. при исследовании возможностей инвазивного интерфейса “мозг-компьютер” было показано, что и при наблюдении, и при выполнении захвата предметов субдуральные электроды регистрировали схожие паттерны активности в альфа-бета- и гамма-диапазонах (Collinger et al., 2014).
Другое исследование ЭКоГ, в котором анализировали гамма-диапазон частот и сравнивали его с десинхронизацией на более низких частотах, фокусировалось исключительно на премоторной коре во время наблюдения, при этом не использовали задачи выполнения и представления, а также не анализировали другие области этой сети (Caruana et al., 2014). В работе на макаках рассматривались локальные потенциалы поля (local field potential – LFP), зарегистрированные в премоторной и первичной моторной коре двух обезьян (Waldert et al., 2015). В данном исследовании показали, что низкочастотный сигнал LFP может модулироваться во время наблюдения за действием в этих двух областях коры головного мозга.
Сравнительное исследование сенсомоторных и речевых функций ЗСМ с использованием записи ЭКоГ было сделано в 2015 г. (Halje et al., 2015), при этом было показано, что не только моторные задачи, но и вербально-речевые вызывают десинхронизацию альфа- и бета-ритмов. Полученные изменения наблюдались в лобной, теменной, височной областях и нижней лобной извилине при наблюдении и выполнении как зрительно-моторных, так и речевых задач.
Группа соавторов под руководством Вавилони проводила исследование динамики ритмов ЭКоГ у пациентов с фармакорезистентной эпилепсией в сенсомоторных, премоторных и префронтальных областях коры в задачах на “отзеркаливание” (Babiloni et al., 2016; Babiloni et al., 2017). В результате наблюдались снижение мощности сигнала в альфа- и бета-диапазоне и повышение мощности в гамма-диапазоне частот при активации ЗСМ.
В недавней работе Перри и соавт. (Perry et al., 2018) исследовали активность ЗСМ с помощью ЭКоГ, полученной с корковых электродов – полосок и сеток, установленных над префронтальной, моторной, височной или теменной областях правого или левого полушария. В качестве стимульного материала использовалось видео с захватом трех разных предметов, которые стояли на подносе перед экраном. Результаты показали повышение мощности гамма-диапазона при наблюдении и выполнении задач. Также были обнаружены области, более активные во время паузы между заданиями.
При одновременной регистрации мультинейрональной активности (multi unit activity), фокальных потенциалов (local field potentials, LFP) и ЭЭГ от вентральной премоторной коры у обезьян было обнаружено, что активность нейронов премоторной коры достоверно коррелирует с повышением мощности гамма-диапазона LFP, а также со снижением мощности в верхнем альфа- и нижнем бета-диапазонах ЭЭГ центральных отведений (Bimbi et al., 2018). Более того, в данной работе обнаружили, что повышение гамма-активности LFP предшествует десинхронизации мю-ритма в задачах выполнения и наблюдения действия, а это значит, что зеркальные нейроны модулируют генерацию мю-ритма.
Таким образом, пока существует совсем немного работ, освещающих работу зеркальной системы мозга с использованием регистрации внеклеточной активности нейронов. Безусловно, это прежде всего связано с тем, что инвазивные методы регистрации нейрональной активности невозможно применять у здоровых людей по этическим соображениям, а проводятся подобные хирургические воздействия только у пациентов в случаях крайней медицинской необходимости.
Инвазивные методики регистрации электрической активности нейронов позволяют получить несколько видов сигналов, которые зависят от типа используемых электродов. При наложении электродов на внешнюю поверхность коры полученный сигнал представляет собой электрокортикограмму (ЭКоГ) – суммацию колебаний потенциалов, генерируемых достаточно большим объемом нейронов. ЭКоГ обычно используют в протоколе хирургического лечения эпилепсии (DiLorenzo et al., 2014; Kuruvilla, 2003). Сигнал ЭКоГ в основном является результатом активности кортикальных пирамидальных клеток, таким образом, он проходит через несколько слоев коры головного мозга и спинномозговой жидкости. Визуально сигнал ЭКоГ схож с ЭЭГ, имеет тот же спектральный состав, однако на порядок большую амплитуду, поскольку не проходит еще и костную ткань, имеющую низкую проводимость, и лишен физиологических артефактов, привносимых мышечным напряжением (Asano et al., 2005; Hashiguchi et al., 2007).
Погружные электроды, внедряемые на 4–5 см в глубину мозга, также в основном используют для предоперационного мониторинга пациентов с эпилепсией для локализации эпилептического очага, а также у пациентов с болезнью Паркинсона. Физические характеристики таких электродов позволяют регистрировать фокальные потенциалы (в международной терминологии LFP), которые являются результатом суммации колебаний потенциалов, генерируемых небольшим числом нейронов, расположенных вблизи контактов (Mitzdorf, 1985; Viswanathan, Freeman, 2007).
Принято считать, что фокальные потенциалы, или LFP, отражают активность нейронов, находящихся в непосредственной близости от регистрирующего электрода. Это предположение коренится в биофизическом объяснении сигнала LFP как пространственно взвешенного среднего значения синаптических трансмембранных токов (Mitzdorf, 1985; Viswanathan, Freeman, 2007). Действительно, исследования показали, что сигнал LFP в конкретном месте может быть хорошо предсказан спайковой активностью нейронов, зарегистрированных в достаточно большой области, окружающей электрод (Nauhaus et al., 2009). Эта точка зрения подтверждается выводом о том, что LFP сильно коррелирует с флуктуациями мембранного потенциала соседних нейронов (Poulet, Petersen, 2008) даже при отсутствии спайковой активности в регистрируемых клетках (Okun et al., 2010). Двумя наиболее важными факторами, влияющими на LFP, являются клеточно-синаптическая архитектурная организация сети и синхронность источников тока (Buzsáki et al., 2012).
Медленные колебания LFP, как полагают, связаны с синаптическими и сомато-дендритными токами (Michmizos et al., 2012). Также низкочастотные колебания LFP могут быть обусловлены сложными электрическими свойствами внеклеточного пространства. Тот факт, что внеклеточное пространство неоднородно и состоит из сложной совокупности высокопроводящих жидкостей и низкопроводящих и емкостных мембран, может оказывать сильное фильтрующее воздействие на нижние частоты (Bédard et al., 2004). Ионная диффузия, которая играет важную роль в вариациях мембранного потенциала, также может выступать в качестве фильтра нижних частот. В связи с этим спектральный состав LFP-сигнала довольно сильно отличается от ЭЭГ и ЭКоГ: в спектре превалирует как низкочастотная компонента дельта- и тета-диапазонов, так и быстрые гамма-колебания. При этом экспериментально было показано, что амплитудные и фазовые характеристики LFP-сигнала напрямую связаны с синхронной спайковой активностью окружающих нейронных сетей (Denker et al., 2007, 2011), что дает возможность оценивать их активность по динамике LFP-сигнала.
Возвращаясь к проблеме исследования зеркальной системы мозга у человека, можно предположить, что инвазивная регистрация сигнала LFP, отражающего активность близлежащих нейронных сетей, позволит приблизиться к пониманию механизмов работы зеркальных нейронов и их связей с другими системами мозга. Подобных работ до сих пор практически нет, что вполне понятно с учетом необходимости внедрения электродов в живой мозг пациента, кроме того, больше половины инвазивных работ было сделано на субдуральных электродах, регистрирующих ЭКоГ с поверхности моторной и премоторной коры. Проведение подобных исследований, безусловно, важно, поскольку возможно проанализировать изменения электрических колебаний на поверхности коры без миографических артефактов, в отличие от ЭЭГ, однако подобные работы не освещают вопрос взаимодействия зеркальных нейронов с глубинными структурами и архитектоники зеркальной системы мозга. Анализ локальных изменений потенциалов в глубинных структурах мозга, в отличие от непрямых методов исследования активности, таких как ЭЭГ и фМРТ, позволяет оценивать активность ближайших нейронных сетей. Таким образом, исследование LFP-сигнала, отражающего уровень активности определенных структур в экспериментальной парадигме, позволит значительно улучшить понимание принципов работы зеркальной системы мозга.
Целью данной работы было изучить активность нейронных сетей в височных и лобных областях правого и левого полушария при реализации задач, активирующих зеркальную систему мозга, у пациентов, страдающих эпилепсией, с использованием инвазивных погружных электродов.
МЕТОДИКА
В исследовании принимали участие 11 пациентов (10 мужчин, 1 женщина, правши, возраст от 21 года до 39 лет, в среднем 29 лет) с диагнозом “фокальная эпилепсия”, готовящихся к хирургическому удалению эпилептического очага. Все пациенты были госпитализированы в нейрохирургическое отделение Клинического медицинского центра МГМСУ им. А.И. Евдокимова (Москва); перед проведением записи пациенты заполняли форму информированного согласия о добровольном участии в исследовании. Перед операцией данной группе пациентов для определения “зоны начала приступа” и “ирритативной зоны” устанавливали глубинные электроды для проведения инвазивного видео-ЭЭГ-мониторинга, который длился в среднем около 100 ч. Электроды имплантировались в области гиппокампа правого и левого виска, также в большинстве случаев и в лобные доли, если на это были показания. Для мониторинга эпилептиформной активности использовались стерильные одноразовые электроды AD-TECH® с платиновыми контактами. Каждый погружной электрод представлял собой тонкий стержень диаметром 1.12 мм с 4 или 6 контактами. Платиновые контакты имели характерный размер 1.32 мм, располагались по длине стержня через 1 см друг от друга. Расстояние между первым и последним контактом электрода – 32 мм для четырех контактных и 52 мм для шести контактных электродов; первый по счету контакт был самым глубоким и располагался на глубине около 6 см. В лобные доли электроды проводили через нижнюю лобную извилину с кончиком в проекции головки хвостатого ядра в левое и правое полушарие. Височные электроды заводили через среднюю или верхнюю височную извилину с кончиком, расположенным в проекции головки правого гиппокампа, также в левое и правое полушарие (рис. 1). В качестве референтного и заземляющего электрода использовали электроды-штопоры, вкручивающиеся в кожу скальпа. Поскольку в основном использовались электроды с 4 контактами, при этом они располагались на расстоянии 1 см друг от друга, начиная с самого кончика электрода, который заводился в глубинные структуры мозга, наибольший вклад в сигнал вносили подкорковые структуры. Контроль локализации инвазивных электродов осуществляли с помощью компьютерной томографии (КТ).
Рис. 1.
Компьютерная томография (а) и 3D-реконструкция (б) черепа одного из пациентов с внедренными инвазивными височными и лобными электродами.
Fig. 1. Computed tomography (a) and 3D reconstruction (б) of the skull for one patient with embedded invasive temporal and frontal electrodes.
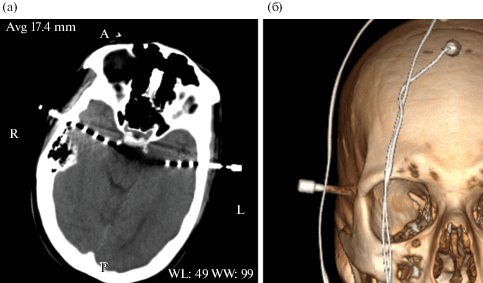
Исследование активности зеркальной системы мозга проводилось не ранее, чем спустя 5 ч после операции по внедрению электродов, когда пациент уже находился в полном сознании и проявлял адекватную активность. Впоследствии, когда была определена локализация очага каждого пациента, из пула данных исключались электроды этой доли мозга.
Методика проведения исследования активации ЗСМ должна включать в себя задачи наблюдения и выполнения (также часто используется представление) биологических жестов, для чего был смонтирован видеоролик, включающий в себя фрагменты с различными движениями руки (пробы на “отзеркаливание”), а также фрагмент с небиологическим движущимся объектом (шарик, который катается по столу). На видеоролике все движения выполняет актер в рубашке, в объектив при этом попадают только руки, предплечья и часть стола, за которым находится актер (лица при этом не видно). Были выбраны три различных движения: простой моторный акт (сжимание кисти правой руки), эмоциональное движение, задействующее обе руки (хлопки в ладоши), целенаправленное движение (захват и перемещение чашки). Пациенту давали инструкцию сначала наблюдать демонстрацию движения (задача “наблюдение”), затем представить, как он выполняет это движение (задача “представление”), затем повторить самостоятельно это движение (задача “выполнение”). Длительность каждой задачи составляла 10 с, между задачами были паузы, во время которых исследователь напоминал, какой будет следующая задача. В качестве фона перед демонстрацией движений регистрировали активность в состоянии спокойного бодрствования с открытыми глазами в том же положении тела. Также в видеоролик был включен 10-секундный фрагмент со статической картиной, где этот же актер не показывает никаких движений (задача наблюдения “статической картины”). Демонстрация статической картины проводилась для того, чтобы дифференцировать реакцию на “картинку” и на социальное движение. Демонстрацию видеоролика каждому пациенту проводили 1–3 раза в зависимости от готовности и желания пациента, всего длительность исследования не превышала 15 мин.
Видеоролик демонстировали с монитора ноутбука диагональю 17 дюймов, который располагался на подставке перед пациентом, расстояние от экрана монитора до лица пациентов составляло 40–50 см. Пациенты в это время располагались на своей больничной койке, спинку койки приводили в полувертикальное положение, до начала эксперимента пациентов приводили в максимально удобное положение для просмотра видео с экрана монитора и выполнения движений правой рукой. Перед началом эксперимента пациентам разъясняли суть исследования, давали необходимые инструкции, после чего они подписывали информированное согласие.
Регистрация биопотенциалов с погружных электродов проводилась с частотой дискретизации 2 кГц (у 3 пациентов), 8 кГц (у 1 пациента) и 4 кГц (у 7 пациентов) в зависимости от технических требований используемого компьютера, затем применялись полосовой фильтр 1–300 Гц и фильтр сетевой наводки 50 Гц с гармониками, с использованием свободной программы EDF browser. Из анализа исключали электроды с сильно зашумленным сигналом. Для каждого пациента по каждой пробе были получены спектральные значения мощности дельта- (1–4 Гц), тета- (4–8 Гц), альфа-(8–13 Гц), бета-(13–35 Гц) и гамма-(35–300 Гц) ритмов с помощью быстрого преобразования Фурье по двухсекундным эпохам анализа, затем полученные значения мощности спектра усредняли для каждой пробы. Далее рассчитывали логарифмированные относительные значения мощности ритмов, производя деление значений мощности ритмов в активной пробе к значению мощности в фоне ln(Pзадача/Pфон) для каждого контакта погружных электродов. Логарифмирование натуральным логарифмом применялось для приближения распределения к нормальному. Абсолютные и относительные значения мощности ритмов анализировали с помощью дисперсионного анализа (Statistica v. 7.0, StatSoft © Inc., США) с такими факторами, как “глубина электрода”, “полушарие” и “локализация электрода” в зависимости от того, располагались ли регистрирующие электроды в правом или левом полушарии мозга и в височной или лобной доле. Также отдельно проводился многофакторный анализ с учетом фактора “вид движения” в зависимости от типа наблюдаемого и выполняемого движения руки.
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЙ
Поскольку регистрируемый сигнал с погружных электродов достаточно сильно отличается по спектральному составу от сигнала, получаемого методом ЭЭГ, на рис. 2 представлены некоторые характеристики регистрируемого сигнала. Рисунок 2 (а) демонстрирует соотношение долей каждого ритма в усредненном спектре фонового сигнала для левого и правого полушария. Дисперсионный анализ, проведенный с целью выявить влияние факторов положения электрода или глубины контакта на соотношения долей ритмов в сигнале, показал только эффект правого и левого полушария: F(4, 376) = 8.79, p < 0.001. Мощность низких частот была выше в сигнале, зарегистрированном в правом полушарии, а мощность высоких частот выше в левом полушарии (достоверно для дельта-, бета-ритмов, апостериорный тест Тьюки, р < < 0.05). Предварительный сравнительный анализ изменений сигнала в разных контактах каждого погружного электрода не показал каких-либо значимых различий, в связи с чем сигналы от разных контактов одного и того же электрода в дальнейшем анализе рассматривались совместно.
Рис. 2.
Характеристики регистрируемых сигналов с погружных электродов: (а) – на графике представлено процентное соотношение доли каждого ритма в усредненном спектре регистрируемых сигналов с погружных электродов отдельно для левого и правого полушария; вертикальные планки соответствуют 95%-му доверительному интервалу; * – различия в значениях для левого и правого полушария достоверно отличаются по дельта- и бета-ритмам, апостериорный тест Тьюки: р < 0.01, p = 0.03 соответственно; (б) – трехсекундный фрагмент регистрируемых сигналов с 5 контактов правого височного погружного электрода одного из пациентов после фильтрации; (в) – пример типичной динамики мощности ритмов во время проведения эксперимента у одного из пациентов правого височного электрода – изменения мощности происходят синхронно во всех частотных диапазонах.
Fig. 2. Characteristics of recorded signals from submerged electrodes: (a) – the graph shows the percentage of each rhythm in the average spectrum of recorded signals from submerged electrodes separately for the left and right hemispheres; the vertical bars correspond to the 95% confidence interval; * – differences in values for the left and right hemispheres significantly differ in delta and beta rhythms, post-hoc Tukey test: p < 0.01, p = 0.03, respectively; (б) – a three-second fragment of recorded signals from 5 contacts of the right temporal immersion electrode for one patient after filtration; (в) – an example of a typical dynamics of the rhythms power during an experiment in the right temporal electrode – power changes occur synchronously in all frequency ranges.
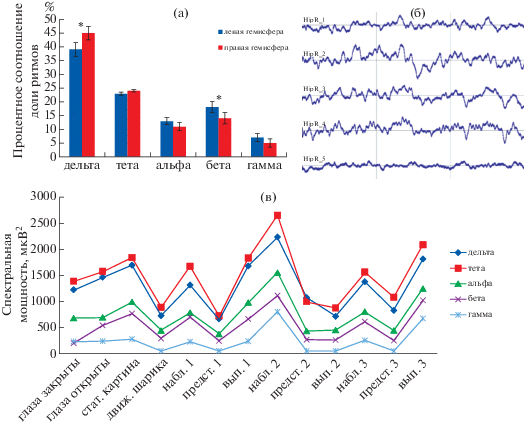
На рис. 2 (б) приведен в качестве примера трехсекундный фрагмент регистрируемых сигналов правого височного электрода с 5 контактов на разной глубине (более глубокий под номером 1). Рисунок 2 (в) демонстрирует динамику абсолютных мощностей ритмов при проведении исследования у одного из пациентов с правого височного электрода. Особенностью регистрируемых сигналов с погружных электродов было синхронное изменение всех спектральных ритмов при реализации различных задач, что, например, совершенно нетипично для ЭЭГ-сигнала.
Для анализа изменения мощности ритмов при реализации задач по сравнению с фоном использовались логарифмированные относительные значения мощности ритмов (рис. 3). Дисперсионный анализ с учетом факторов “ритм”, “полушарие” и “локализация” выявил достоверное для каждой задачи влияние сочетания факторов “полушарие” × “локализация” (p < 0.01, дисперсионный анализ проводился отдельно для каждой задачи). Иллюстрирует эти изменения рис. 3, на котором представлены относительные мощности каждого ритма лобных и височных электродов в левом и правом полушарии в задачах: “наблюдение”, “выполнение”, наблюдение “статической картины”, “наблюдение движения шарика” (значение в фоне соответствует нулю на оси у).
Рис. 3.
Относительные изменения мощности ритмов в различных полушариях и разных долях мозга при наблюдении и выполнении моторных движений, а также при наблюдении статической картины с неподвижным актером и при наблюдении видео с движением шарика – небиологического объекта. Значения в фоне соответствуют нулю по оси у. Вертикальные планки соответствуют 95%-му доверительному интервалу.
Fig. 3. Relative changes in the rhythms power in different hemispheres and different parts of the brain when observing and performing motor movements, as well as when observing a static picture and a video with the movement of a ball (a non-biological object). The values in the background correspond to zero on the y-axis. The vertical bars correspond to a 95% confidence interval.
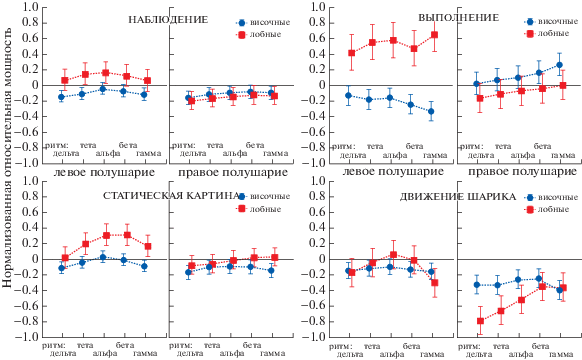
Поскольку дисперсионный анализ не выявил влияния фактора “ритм” на динамику показателей мощности ритмов относительно фона, изменения происходили синхронно, при этом в самом сигнале не было выявлено четких ритмических паттернов, дальнейший анализ проводился с использованием показателя суммарной мощности сигнала, которая отражала бы изменения амплитуды сигнала LFP.
Дисперсионный анализ относительных значений суммарной мощности спектра проводился по протоколу повторных измерений (пробы “статическая картина”, “наблюдение”, “представление”, “выполнение”, “движение шарика”) с учетом факторов “локализация” и “полушарие” (рис. 4). Было выявлено достоверное влияние (F(4.692) = 8.98, р < < 0.001) взаимодействия всех факторов. Апостериорный тест Тьюки выявил значительную долю попарных достоверных различий между значениями относительной мощности сигнала. Рисунок 4 иллюстрирует изменения суммарной мощности LFP-сигнала в различных задачах и областях мозга.
Рис. 4.
Динамика суммарной мощности спектра LFP-сигнала в различных полушариях и областях
при реализации различных проб. Вертикальные планки соответствуют 95%-му доверительному
интервалу. * – достоверные различия мощности в левой лобной области по сравнению с
пробой “движение шарика”, апостериорный тест Тьюки, р < 0.05.  – достоверные различия мощности в левой лобной области по сравнению с пробой “статическая
картина”, апостериорный тест Тьюки, р < 0.05.
– достоверные различия мощности в левой лобной области по сравнению с пробой “статическая
картина”, апостериорный тест Тьюки, р < 0.05.
Fig. 4. Dynamics of the total power of the LFP spectrum in different hemispheres and regions
during the implementation of various samples. The vertical bars correspond to a 95%
confidence interval. * – significant power differences in the left frontal region
compared to the “ball movement” test, a posteriori Tukey test, p < 0.05.  – significant power differences in the left frontal region compared to the " static
picture " test, a posteriori Tukey test, p < 0.05.
– significant power differences in the left frontal region compared to the " static
picture " test, a posteriori Tukey test, p < 0.05.
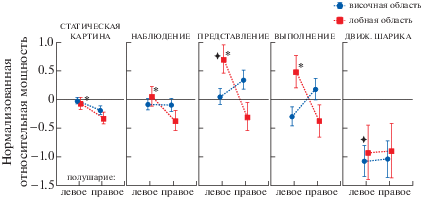
В задачах на активацию ЗСМ (“наблюдение”, “представление”, “выполнение”) изменения происходят в левой лобной доле – мощности ритмов повышаются (достоверные отличия в левом лобном электроде во всех трех задачах от значений в пробе “движение шарика”, апостериорный тест Тьюки, p < < 0.001). Наибольшее повышение мощности было зарегистрировано с левого лобного электрода в задачах “представление” и “выполнение”. В то же время при наблюдении небиологического движения (движущегося шарика) значительно снижается суммарная мощность сигнала относительно фона (достоверные попарные отличия с другими задачами во всех областях, кроме правой лобной доли, апостериорный тест Тьюки, p < 0.001). При наблюдении статической картины неподвижного актера повышения суммарной мощности сигнала не происходило, однако достоверных попарных отличий с задачей “наблюдение” (апостериорный тест Тьюки) обнаружено не было.
Далее для того, чтобы выявить, какой тип движения вызывал наибольшие изменения мощности сигнала, был проведен дисперсионный анализ с учетом факторов “вид движения”, “полушарие” и “локализация” отдельно для задач “наблюдение” и “выполнение” движений. Поскольку в задачах “наблюдение” и “выполнение” не было выявлено различий в динамике разных ритмов, дисперсионный анализ выполнялся также для суммарной мощности спектра. Дисперсионный анализ показал значимое влияние как одного фактора – “вид движения”, так и взаимодействия всех факторов: “вид движения” × “полушарие” × “локализация” – и при наблюдении, и при выполнении (табл. 1). Апостериорный тест Тьюки выявил достоверные отличия (р < < 0.05) относительной суммарной мощности сигнала в левом полушарии между лобными и височными электродами в задаче “выполнение” (во всех типах движения) и в задаче “наблюдение” (в движениях “хлопки” и “чашка”).
Таблица 1.
Показатели дисперсионного анализа с учетом влияния отдельно фактора “вид движения” и взаимодействия факторов “вид движения” × “ полушарие” × × “локализация” при наблюдении и выполнении задач Table 1. Indicators of ANOVA taking into account the factors: “type of the movement” and factors interaction “type of the movement” × “hemisphere” × “localization”
| Задача | Наблюдение | Выполнение |
|---|---|---|
| Влияние фактора | ||
| “вид движения” | F(2, 1428) = 5.21, p = 0.005 | F(2, 376) = 14.85, p < 0.001 |
| “вид движения” × × “полушарие” × × “локализация” | F(2, 1428) = 20.48, p < 0.001 | F(4, 376) = 3.89, p = 0.02 |
Рисунок 5 иллюстрирует результаты дисперсионного анализа с учетом трех вышеперечисленных факторов – изменения суммарной мощности спектра при реализации различных типов движений: наблюдении и выполнении сжимания кисти, хлопков и перемещения чашки. На рис. 5 можно проследить, при каких типах движений происходят более значимые изменения суммарной мощности сигнала, в отличие от рис. 4. Видно, что наибольшие изменения, а также различия в динамике в височных и лобных долях происходили при наблюдении и выполнении наиболее сложной задачи с перемещением чашки. Повышение суммарной спектральной мощности относительно фона (ноль по оси у на графиках) происходило при наблюдении и выполнении движений “хлопки” и “перемещение чашки” в левых лобных областях, а также при выполнении наиболее простого движения “сжимание кисти”. Также было выявлено увеличение суммарной мощности сигнала при выполнении задач “хлопки” и “перемещение чашки” в правой лобной области.
Рис. 5.
Динамика суммарной мощности спектра в различных полушариях и областях при наблюдении и выполнении различных типов моторных движений – сжимания кисти, хлопков и перемещения чашки. Вертикальные планки соответствуют 95%-му доверительному интервалу. * – достоверные различия мощности в левом полушарии между височной и лобной областью при попарном сравнении задач, апостериорный тест Тьюки, р < 0.05.
Fig. 5. Dynamics of the spectrum total power in different hemispheres and regions when observing and performing various types of motor movements – squeezing the hand, clapping and moving the cup. The vertical bars correspond to a 95% confidence interval. * – significant power differences in the left hemisphere between the temporal and frontal regions in pairwise comparison of tasks, a posteriori Tukey test, p < 0.05.
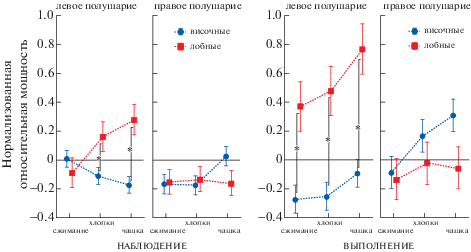
Таким образом, динамика мощности сигнала сильно различалась в разных полушариях и долях мозга в зависимости от пробы и типа движения.
Повышение мощности спектра происходило в левой лобной доле при реализации задач на активацию ЗСМ (наблюдение, представление и выполнение моторного акта), в большей степени при наиболее сложном типе движения – перемещении чашки.
Снижение мощности спектра сигнала происходило при просмотре движущегося шарика, причем более сильные изменения происходили в правой лобной доле.
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
Трактовка изменения мощности LFP-сигнала
Как уже было сказано во введении, в коре головного мозга сигнал LFP, по-видимому, в основном происходит от трансмембранных нейрональных токов, следующих за синаптическим входом. Было показано, что для LFP-сигнала характерно сильное затухание при пространственном распространении более высоких частот (более 60 Гц) по сравнению с низкими частотами (около 1 Гц) (Bédard et al., 2004; Michmizos et al., 2012). Так, низкочастотные компоненты LFP-сигнала могут простираться даже за пределы активной нейронной популяции из-за эффекта объемной проводимости (Łęski et al., 2013). Кроме того, пространственные профили LFP для таких популяций обычно охватывают всю вертикальную протяженность дендритов в популяции нейронов. В связи с этим мы можем наблюдать преобладание медленноволновой части спектра в регистрируемом LFP-сигнале и синхронное изменение амплитуды мощности спектра. Однако оценка численной совокупности нейронов, вносящих свой вклад в регистрируемый сигнал, или пространственная разрешающая способность LFP-сигнала, как и возможность сопоставления результатов с другими инвазивными исследованиями, осложняется тем, что на это будут влиять характеристики используемых электродов, значения сопротивления на них и локализация референтного электрода. Кроме того, положение инвазивных электродов в каждой подобной работе продиктовано в первую очередь клинической необходимостью и, несомненно, будет различаться в разных работах.
При трактовке изменений LFP-сигнала мы опирались на работы Денкер и соавт., которые экспериментально подтвердили гипотезу о том, что именно спайковая активность (причем именно синхронная спайковая активность большого количества нейронов) представляет собой основной темпорально и пространственно организованный компонент LFP-сигнала. В более ранней статье (Denker et al., 2007) были приведены доказательства в поддержку гипотезы о том, что увеличение амплитуды колебаний LFP-сигнала связано с большей степенью синхронизации между сигналами LFP и спайками. В следующей работе (Denker et al., 2011) было показано, что LFP-сигнал, являясь суммацией потенциалов, генерируемых близлежащими нейронами, отражает специфическую информацию о сетевых процессах. На мозге макак была продемонстрирована взаимосвязь между повышением амплитуды LFP и синхронизацией спайков нейронов, хотя существовала значительная доля одиночных спайков, не коррелирующая с амплитудой или фазой LFP. Таким образом, Денкер утверждает, что повышение амплитуды LFP-колебаний (в частности, в бета-диапазоне), является отражением активации нейронных сетей, распространяющих по своей сети синхронный спайковый залп.
Повышение мощности LFP-сигнала в левых лобных электродах
Самые значительные изменения мощности LFP-сигнала при реализации социально окрашенных задач (наблюдение, представление и выполнение различных жестов) были зарегистрированы в левых лобных электродах. Стоит напомнить, что все моторные задачи выполнялись правой рукой (кроме хлопков, которые выполняли обеими руками). Каждый левый лобный электрод погружался примерно на 6 см в глубину мозга, заводился через нижнюю лобную извилину в головку хвостатого ядра слева и, соответственно, проходил через вентролатеральную префронтальную кору, переднюю поясную кору, доходя до передних структур базальных ганглиев (головки хвостатого ядра). Рассмотрим каждую из структур, которая может давать вклад в изменение LFP-сигнала.
Нижняя лобная извилина уже достаточно давно рассматривается как часть зеркальной системы мозга человека. Еще Риццолатти в своем обзоре писал, что большое количество исследователей ЗСМ человека отмечают при наблюдении и выполнении активацию сложной сети, расположенной в ростральной части нижней теменной доли, нижней части прецентральной извилины и задней части нижней лобной извилины – эти области образуют ядро системы зеркальных нейронов человека (Rizzolatti, Craighero, 2004). Другие анатомические исследования на обезьянах показали, что премоторные области коры, в которых указывали наличие зеркальных нейронов, имеют множество других анатомических связей, которые могут расширить функциональные свойства данных областей (Borra et al., 2017; Rizzolatti et al., 2014). В частности, кортико-кортикальные связи F5 распространяются также на области префронтальной коры (Gerbella et al., 2013), где были описаны нейроны, связанные с движением (Simone et al., 2015). Эти данные свидетельствуют о том, что специфическая область вентролатеральной префронтальной коры (12, 44, 45, 47 поля Бродмана) участвует в контекстно-ориентированном контроле действий и в наблюдении за действиями (Simone et al., 2017). Последующие работы также подтверждают участие нижней лобной извилины в наблюдении за действием и его повторении (Iacoboni, 2005; Molnar-Szakacs et al., 2005), кроме того, инвазивные исследования на обезьянах показали, что вентролатеральная префронтальная кора также участвует в социальном взаимодействии у макак и имеет зеркальные свойства (Sliwa, Freiwald, 2017). Авторы работы указывают на то, что часть медиальной и вентролатеральной префронтальной коры, а также передней поясной коры головного мозга макак составляют сеть социального взаимодействия.
Известно, что области передней поясной коры связывают с выполнением когнитивных задач, таких как принятие решений, управление эмоциями (DiCarlo et al., 2012; Li et al., 2019b; Lockwood, 2016; Raine, 2019). За счет своих связей с дополнительной моторной областью передняя поясная кора участвует в планировании движений исходя из предполагаемых целей (Rolls, 2015). Многие работы, посвященные исследованию ЗСМ, также свидетельствуют о том, что поясная кора активируется во время работы ЗСМ (Karakale et al., 2019; Li et al., 2019a; Saito et al., 2018). В работе Р. Мукамеля, в которой были зарегистрированы ответы отдельных нейронов у пациентов с височной эпилепсией, было показано, что в этой области действительно находятся нейроны с зеркальными свойствами (Mukamel et al., 2010). В работе, описывающей различия активации ЗСМ при имитации жестов и мимики (Ferrari et al., 2017), приводятся данные о том, что область F5 у макак имеет обширные связи с передней поясной корой, передним островком и базолатеральной миндалиной. Нейровизуализационные исследования у людей также показали, что наблюдение и имитация мимических выражений лица активируют область передней поясной коры (Carr et al., 2003; Singer, 2004). Функционально проекции в переднюю поясную кору могут быть связаны с оценкой релевантности имитационного поведения и выявлением эмоциональной окрашенности наблюдаемых жестов (Cai, Padoa-Schioppa, 2012; Chang et al., 2013). Стоит отметить, что поясная кора головного мозга, связанная с зеркальной системой, также участвует в управлении моторикой рта и в голосовой коммуникации, как это было продемонстрировано в исследовании на обезьянах (West, Larson, 1995). Таким образом, исследования говорят о том, что передняя поясная кора может участвовать в эмоциональном распознавании и повторении мимических выражений лица во время социальных контактов, а также в процессах моторных эмоциональных реакций, таких как смех и улыбка (Caruana et al., 2017; Ferrari et al., 2017).
Еще одна важная функция передней поясной коры, которая может задействоваться в процессе восприятия и “отзеркаливания” действий – это контроль и выявление конфликтов между параллельными моторными процессами (Carter, van Veen, 2007; Campbell, Cunnington, 2017). В ситуации восприятия моторного движения оппонента зеркальная система, как предполагается, активирует моторную сеть по той же схеме проекций, как если бы данное движение делал сам индивид. Тем самым может возникнуть конфликт между собственными моторными программами и воспринимаемыми репрезентациями. Таким образом, в передней поясной коре может решаться задача одновременного удержания воспринимаемой моторной репрезентации и планирования собственного действия (Cross, Iacoboni, 2014).
Кончик лобного электрода располагался в головке хвостатого ядра – структуры, отвечающей не только за планирование выполнения движения, но также участвующей в процессах обучения, памяти, вознаграждения, мотивации, эмоциональных реакций (ten Donkelaar et al., 2020; Grahn et al., 2008). При этом хвостатое ядро получает от корковых структур (в основном из лобных долей) множество афферентных сигналов, содержащих интегральную информацию о внешнем мире, о положении тела, которая потом распространяется в другие структуры базальных ганглиев и таламус, причем в большинстве этих связей преобладает торможение, а не возбуждение. Головку хвостатого ядра можно условно назвать когнитивной и эмоциональной частью стриатума, так как она связана с латеральной и медиальной префронтальной корой и участвует в процессах рабочей памяти, исполнительном функционировании, возникновении эмоций (Graff-Radford et al., 2017). Ассоциативное обучение – еще одна важная функция хвостатого ядра, которое играет важную роль в соединении зрительных стимулов с двигательными реакциями, а также в обучении с подкреплением. Переднее хвостатое ядро кодирует одновременно пространственную информацию и информацию о вознаграждении и риске совместно с лобной корой, с которой оно имеет множество прочных связей (Yanike, Ferrera, 2014).
Система базальных ганглиев отвечает за сложные двигательные акты, безусловные и условные рефлексы, контроль двигательных программ, выражения эмоций, хранение двигательных навыков, требующих предварительного обучения (ten Donkelaar et al., 2020). Два недавних исследования показали, что базальные ганглии могут участвовать в процессе “отзеркаливания” во время социального взаимодействия: в исследованиях участвовали пациенты с болезнью Паркинсона, при этом схожие изменения активности в субталамическом ядре происходили как при наблюдении за движением, так и при выполнении этого движения, достоверно отличаясь от двух контрольных состояний (Alegre et al., 2010). С чем может быть связано вовлечение системы базальных ганглиев в процесс восприятия и “проецирования” социальных жестов и мимики? Во-первых, безусловно, с необходимостью затормаживать двигательную активность при наблюдении за собеседником. Ведь по определению зеркальных нейронов, данная функциональная сеть мозга должна “включать” моторную репрезентацию наблюдаемых действий для их восприятия и “отзеркаливания”, в этот самый момент должна подключаться затормаживающая система, чтобы каждое наблюдаемое действие не вызывало моторную реакцию повторения у собеседника. На эту роль лучше всего подходит система базальных ганглиев, но для того, чтобы это торможение произошло, система базальных ганглиев должна получить входящую информацию от системы зеркальных нейронов.
Функциональные связи ЗСМ
Марко Якобони, один из ветеранов исследования зеркальных нейронов, для описания их функциональных связей с другими системами предложил гипотезу “минимальной нейронной архитектуры подражания” (Iacoboni, 2005), согласно которой импульсы от сенсорных отделов коры приходят в верхнюю височную борозду, а затем – в задние теменные отделы в качестве соматосенсорной информации, необходимой для подражания. Из задних теменных отделов они поступают в нижнюю лобную извилину, где определяется мотив действия оппонента, затем импульсы приходят вновь в верхнюю височную борозду, где сравнивается полученное ранее представление о действии оппонента и принимается решение о необходимости подражания. Якобони также предполагал, что зеркальные нейроны находятся во всех вышеописанных областях, причем нейроны каждой зоны специфичны по своим функциям, традиционно приписываемым этим зонам (Косоногов, 2009).
Сейчас стало ясно, что процесс “отзеркаливания” при социальном взаимодействии – это не просто восприятие и отображение моторных реакций оппонента, но намного более сложный процесс, включающий стадии моторной репрезентации, контроля параллельных моторных программ, затормаживания повторения действий, определения эмоциональной окраски и мотива действия, принятия решения о собственном моторном ответе. Мы предполагаем, что на более поздних стадиях “отзеркаливания”, когда информация из соматосенсорных отделов переходит в нижнюю лобную извилину для определения мотиваций и принятия собственных решений, далее она распространяется через переднюю поясную кору и головку хвостатого ядра к системе базальных ганглиев. При этом в поясной коре может происходить эмоциональная оценка увиденного действия, мимические эмоциональные реакции, отслеживание конфликтов между собственными и воспринимаемыми моторными актами; головка хвостатого ядра может быть связана с процессами ассоциативного обучения и мотивации в ЗСМ; базальные ганглии в процессах “отзеркаливания” должны выполнять контроль собственных моторных актов и затормаживать воспринимаемые двигательные акты, которые активируют соответствующие области моторной коры через зеркальные нейроны. Мы полагаем, что повышение мощности LFP-сигнала вдоль всего левого лобного погружного электрода, описанное в нашей работе, может являться отражением активации пути ЗСМ “вентро-латеральная префронтальная кора – передняя поясная кора – головка хвостатого ядра” в доминантном полушарии. Безусловно, данная гипотеза нуждается в дальнейшей разработке и проверке, на которые и будут направлены наши последующие работы.
Изменения мощности LFP выше при представлении и выполнении действий
Несмотря на то что повышение мощности LFP-сигнала в левых лобных электродах происходило при наблюдении, представлении и выполнении моторных задач, более значительные изменения происходили при представлении и выполнении. В одной из инвазивных работ по изучению ЗСМ было показано, что у семи больных фармакорезистентной эпилепсией как сам захват предметов, так и наблюдение этих действий вызывали десинхронизацию субдуральных альфа- и бета-ритмов ЭКоГ в первичной соматосенсорно-моторной, латеральной премоторной и вентральной префронтальной областях (Babiloni et al., 2016). Кроме того, эти изменения были больше во время выполнения действия, чем во время его наблюдения. В следующей работе Вавилони и соавт. показывают, что запаздывающая линейная коннективность между первичной соматосенсорно-моторной, латеральной премоторной и вентральной префронтальной областями усилена во время выполнения действия по сравнению с наблюдением из-за большего потока визуальной и соматомоторной информации (Babiloni et al., 2017). Результаты нового анализа показали, что запаздывающая коннективность дельта-тета-ритмов между вентральной префронтальной и латеральной премоторной областями была больше во время выполнения действия, чем при его наблюдении. Кроме того, фаза медленных ритмов связана с амплитудой более быстрых альфа-бета-ритмов ЭКоГ. Вавилони и его коллеги предположили, что во время выполнения подобных транзитивных действий нейрофизиологические низкочастотные колебательные механизмы, работающие во фронтальной нейронной сети, сформированной BA6 и BA44/45, будут лежать в основе интеграции моторных команд и зрительно-соматосенсорной обратной связи, сопровождающей собственное выполнение.
В нашем случае более значительное повышение мощности сигнала левых лобных электродов, проходящих через переднюю поясную кору и головку хвостатого ядра, в задачах “представление” и “выполнение” может быть связано с тем, что этот путь связан с реальным или воображаемым осуществлением воспринятой моторной программы, для чего активируются связи с базальными ганглиями. В пробе “наблюдение” за движением руки перед человеком стояла задача восприятия и проецирования действия, при этом по нашей гипотезе активация путей, связывающих ЗСМ с базальными ганглиями, может задействоваться в меньшей степени, в отличие от задач представления и повторения действия.
Повышение мощности в правой височной доле при представлении и выполнении моторных задач
Повышение суммарной мощности при представлении и выполнении было наиболее выражено для правой височной области в заданиях “хлопки в ладоши” и “перемещение чашки”. Данное повышение мощности может быть связано с работой ЗСМ: вовлечение гиппокампа в работу ЗСМ было показано на мышах (Ushakov et al., 2013), а исследования у людей, проводимые с помощью фМРТ, указывают на то, что гиппокамп вносит вклад в деятельность ЗСМ человека (Ge et al., 2018; Krautheim et al., 2019). Также в работе Мукамеля, в которой были зарегистрированы ответы отдельных нейронов у пациентов с височной эпилепсией, было показано, что в гиппокампе, миндалевидном теле и энторинальной коре располагаются нейроны с зеркальными свойствами (Mukamel et al., 2010). Кроме того, возможно, активация гиппокампа связана с тем, что при выполнении этих заданий испытуемые обращаются к ассоциативным воспоминаниям, связанным с этими движениями, т.е. с декларативной памятью (Skelin et al., 2019; Solomon et al., 2019).
ЗАКЛЮЧЕНИЕ
В данной работе впервые проводили анализ изменений локальных потенциалов поля, зарегистрированных с погружных инвазивных электродов в лобных и височных подкорковых областях мозга у пациентов с эпилепсией в рамках исследования зеркальной системы мозга и ее связей с другими структурами мозга. Подводя итог, можно отметить, что мощность сигнала повышалась при наблюдении, представлении и выполнении моторных действий в левом лобном погружном электроде, кроме того, наибольшие изменения происходили во время представления и выполнения, а также при более сложных моторных задачах “хлопки в ладоши” и “перемещение чашки”. Причем повышение активности в левой лобной области происходило только при реализации социально окрашенных задач (пробы на “отзеркаливание”), в то время как наблюдение статической картины или движущегося небиологического объекта – шарика – не вызывало повышения активности ни в одной из рассматриваемых областей. Поскольку левый лобный электрод заводили через левую нижнюю лобную извилину к головке хвостатого ядра, повышение мощности LFP-сигнала вдоль этого электрода может отражать активацию нейрональных связей ЗСМ с системой базальных ганглиев через переднюю поясную кору и головку хвостатого ядра. Участие вентролатеральной префронтальной коры и передней поясной коры подтверждается другими работами и связано с определением эмоциональной оценки и мотива наблюдаемого движения, и принятием собственного решения об ответной реакции. Однако связь ЗСМ с системой базальных ганглиев может функционально обеспечивать контроль конфликтов параллельных моторных актов (воспринимаемого и собственного) и затормаживание нежелательного повтора воспринимаемого действия, а также принимать участие в процессе моторного ассоциативного обучения. Данное исследование ЗСМ с использованием инвазивных методик требует, однако, дальнейшего набора пациентов и увеличения числа выборки, что позволит повысить статистическую точность и приблизиться к пониманию механизмов работы зеркальных нейронов и их взаимосвязей с моторной, лимбической и другими системами мозга.
Работа выполнена в рамках государственного задания Министерства образования и науки Российской Федерации на 2018–2020 гг.
Список литературы
Косоногов В. Зеркальные нейроны: краткий научный обзор. Ростов-на-Дону: Антей, 2009. 22 с.
Alegre M. Rodríguez-Oroz M.C., Valencia M., Pérez-Alcázar M., Guridi J., Iriarte J., Obeso J.A., Artieda J. Changes in subthalamic activity during movement observation in Parkinson’s disease: Is the mirror system mirrored in the basal ganglia? Clinical Neurophysiology. 2010. 121 (3): 414–425. https://doi.org/10.1016/j.clinph.2009.11.013
Asano E., Juhasz C., Shah A., Muzik O., Chugani D.C., Shah J., Sood S., Chugani H.T. Origin and Propagation of Epileptic Spasms Delineated on Electrocorticography. Epilepsia. 2005. 46 (7): 1086–1097. https://doi.org/10.1111/j.1528-1167.2005.05205.x
Babiloni C., Del Percio C., Lopez S., Di Gennaro G., Quarato P.P., Pavone L., Morace R., Soricelli A., Noce G., Esposito V., Gallese V., Mirabella G. Frontal Functional Connectivity of Electrocorticographic Delta and Theta Rhythms during Action Execution Versus Action Observation in Humans. Frontiers in Behavioral Neuroscience. 2017. 11. https://doi.org/10.3389/fnbeh.2017.00020
Babiloni C., Del Percio C., Vecchio F., Sebastiano F., Di Gennaro G., Quarato P.P., Morace R., Pavone L., Soricelli A., Noce G., Esposito V., Rossini P.M., Gallese V., Mirabella G. Alpha, beta and gamma electrocorticographic rhythms in somatosensory, motor, premotor and prefrontal cortical areas differ in movement execution and observation in humans. Clinical Neurophysiology. 2016. 127 (1): 641–654. https://doi.org/10.1016/j.clinph.2015.04.068
Bédard C., Kröger H., Destexhe A. Modeling Extracellular Field Potentials and the Frequency-Filtering Properties of Extracellular Space. Biophysical Journal. 2004. 86 (3): 1829–1842. https://doi.org/10.1016/S0006-3495(04)74250-2
Bimbi M., Festante F., Coudé G., Vanderwert R.E., Fox N.A., Ferrari P.F. Simultaneous scalp recorded EEG and local field potentials from monkey ventral premotor cortex during action observation and execution reveals the contribution of mirror and motor neurons to the mu-rhythm. NeuroImage. 2018. 175: 22–31. https://doi.org/10.1016/j.neuroimage.2018.03.037
Borra E., Gerbella M., Rozzi S., Luppino G. The macaque lateral grasping network: A neural substrate for generating purposeful hand actions. Neuroscience & Biobehavioral Reviews. 2017. 75: 65–90. https://doi.org/10.1016/j.neubiorev.2017.01.017
Buzsáki G., Anastassiou C.A., Koch C. The origin of extracellular fields and currents – EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience. 2012. 13 (6): 407–420. https://doi.org/10.1038/nrn3241
Cai X., Padoa-Schioppa C. Neuronal Encoding of Subjective Value in Dorsal and Ventral Anterior Cingulate Cortex. Journal of Neuroscience. 2012. 32 (11): 3791–3808. https://doi.org/10.1523/JNEUROSCI.3864-11.2012
Campbell M.E.J., Cunnington R. More than an imitation game: Top-down modulation of the human mirror system. Neuroscience & Biobehavioral Reviews. 2017. 75: 195–202. https://doi.org/10.1016/j.neubiorev.2017.01.035
Carr L., Iacoboni M., Dubeau M.-C., Mazziotta J.C., Lenzi G.L. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences. 2003. 100 (9): 5497–5502. https://doi.org/10.1073/pnas.0935845100
Carter C.S., van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007. 7 (4): 367–379. https://doi.org/10.3758/CABN.7.4.367
Caruana F., Avanzini P., Gozzo F., Pelliccia V., Casaceli G., Rizzolatti G. A mirror mechanism for smiling in the anterior cingulate cortex. Emotion. 2017. 17 (2): 187–190. https://doi.org/10.1037/emo0000237
Caruana F., Sartori I., Lo Russo G., Avanzini P. Sequencing Biological and Physical Events Affects Specific Frequency Bands within the Human Premotor Cortex: An Intracerebral EEG Study. PLoS ONE. 2014. 9 (1): e86384. https://doi.org/10.1371/journal.pone.0086384
Chang L.J., Yarkoni T., Khaw M.W., Sanfey A.G. Decoding the Role of the Insula in Human Cognition: Functional Parcellation and Large-Scale Reverse Inference. Cerebral Cortex. 2013. 23 (3): 739–749. https://doi.org/10.1093/cercor/bhs065
Collinger J.L., Vinjamuri R., Degenhart A.D., Weber D.J., Sudre G.P., Boninger M.L., Tyler-Kabara E.C., Wang W. Motor-related brain activity during action observation: a neural substrate for electrocorticographic brain-computer interfaces after spinal cord injury. Frontiers in Integrative Neuroscience. 2014. 8. https://doi.org/10.3389/fnint.2014.00017
Cross K.A., Iacoboni M. Neural systems for preparatory control of imitation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014. 369 (1644): 20130176. https://doi.org/10.1098/rstb.2013.0176
Denker M., Roux S., Lindén H., Diesmann M., Riehle A., Grün S. The Local Field Potential Reflects Surplus Spike Synchrony. Cerebral Cortex. 2011. 21 (12): 2681–2695. https://doi.org/10.1093/cercor/bhr040
Denker M., Roux S., Timme M., Riehle A., Grün S. Phase synchronization between LFP and spiking activity in motor cortex during movement preparation. Neurocomputing. 2007. 70 (10–12): 2096–2101. https://doi.org/10.1016/j.neucom.2006.10.088
DiCarlo J.J., Zoccolan D., Rust N.C. How Does the Brain Solve Visual Object Recognition? Neuron. 2012. 73 (3): 415–434. https://doi.org/10.1016/j.neuron.2012.01.010
DiLorenzo D.J., Mangubat E.Z., Rossi M.A., Byrne R.W. Chronic unlimited recording electrocorticography–guided resective epilepsy surgery: technology-enabled enhanced fidelity in seizure focus localization with improved surgical efficacy. Journal of Neurosurgery. 2014. 120 (6): 1402–1414. https://doi.org/10.3171/2014.1.JNS131592
ten Donkelaar H.J., van de Warrenburg B., Willemsen M., Küsters B., Hashizume Y., Hori A. Basal Ganglia. Clinical Neuroanatomy. P. 591–667. Cham, Springer International Publishing. (also available at http://link.springer.com/10.1007/978-3-030-41878-6_11)
Ferrari P.F., Gerbella M., Coudé G., Rozzi S. Two different mirror neuron networks: The sensorimotor (hand) and limbic (face) pathways. Neuroscience. 2017. 358: 300–315. https://doi.org/10.1016/j.neuroscience.2017.06.052
Gallese V., Fadiga L., Fogassi L., Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996. 119 (2): 593–609. https://doi.org/10.1093/brain/119.2.593
Ge S., Liu H., Lin P., Gao J., Xiao C., Li Z. Neural Basis of Action Observation and Understanding From First- and Third-Person Perspectives: An fMRI Study. Frontiers in Behavioral Neuroscience. 2018. 12. https://doi.org/10.3389/fnbeh.2018.00283
Gerbella M., Borra E., Tonelli S., Rozzi S., Luppino G. Connectional Heterogeneity of the Ventral Part of the Macaque Area 46. Cerebral Cortex. 2013. 23 (4): 967–987. https://doi.org/10.1093/cercor/bhs096
Graff-Radford J., Williams L., Jones D.T., Benarroch E.E. Caudate nucleus as a component of networks controlling behavior. Neurology. 2017. 89 (21): 2192–2197. https://doi.org/10.1212/WNL.0000000000004680
Grafton S., Arbib M., Fadiga L., Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. Experimental Brain Research. 1996. 112 (1). https://doi.org/10.1007/BF00227183
Grahn J.A., Parkinson J.A., Owen A.M. The cognitive functions of the caudate nucleus. Progress in Neurobiology. 2008. 86 (3): 141–155. https://doi.org/10.1016/j.pneurobio.2008.09.004
Halje P., Seeck M., Blanke O., Ionta S. Inferior frontal oscillations reveal visuo-motor matching for actions and speech: evidence from human intracranial recordings. Neuropsychologia. 2015. 79: 206–214. https://doi.org/10.1016/j.neuropsychologia.2015.08.015
Hashiguchi K., Morioka T., Yoshida F., Miyagi Y., Nagata S., Sakata A., Sasaki T. Correlation between scalp-recorded electroencephalographic and electrocorticographic activities during ictal period. Seizure. 2007. 16 (3): 238–247. https://doi.org/10.1016/j.seizure.2006.12.010
Iacoboni M. Understanding others: imitation, language, empathy. Perspectives on Imitation: From Cognitive Neuroscience to Social Science. 2005.
Karakale O., Moore M.R., Kirk I.J. Mental Simulation of Facial Expressions: Mu Suppression to the Viewing of Dynamic Neutral Face Videos. Frontiers in Human Neuroscience. 2019. 13. https://doi.org/10.3389/fnhum.2019.00034
Krautheim J.T., Dannlowski U., Steines M., Neziroğlu G., Acosta H., Sommer J., Straube B., Kircher T. Intergroup empathy: Enhanced neural resonance for ingroup facial emotion in a shared neural production-perception network. NeuroImage. 2019. 194: 182–190. https://doi.org/10.1016/j.neuroimage.2019.03.048
Kuruvilla A., Flink R. Intraoperative electrocorticography in epilepsy surgery: useful or not? Seizure. 2003. 12 (8): 577–584. https://doi.org/10.1016/S1059-1311(03)00095-5
Łęski S., Lindén H., Tetzlaff T., Pettersen K.H., Einevoll G.T. Frequency Dependence of Signal Power and Spatial Reach of the Local Field Potential. PLoS Computational Biology. 2013. 9 (7): e1003137. https://doi.org/10.1371/journal.pcbi.1003137
Li Q., Becker B., Jiang X., Zhao Z., Zhang Q., Yao S., Kendrick K.M. Decreased interhemispheric functional connectivity rather than corpus callosum volume as a potential biomarker for autism spectrum disorder. Cortex. 2019a. 119: 258–266. https://doi.org/10.1016/j.cortex.2019.05.003
Li Y.S., Nassar M.R., Kable J.W., Gold J.I. Individual Neurons in the Cingulate Cortex Encode Action Monitoring, Not Selection, during Adaptive Decision-Making. The Journal of Neuroscience. 2019b. 39 (34): 6668–6683. https://doi.org/10.1523/JNEUROSCI.0159-19.2019
Lockwood P.L. The anatomy of empathy: Vicarious experience and disorders of social cognition. Behavioural Brain Research. 2016. 311: 255–266. https://doi.org/10.1016/j.bbr.2016.05.048
Michmizos K.P., Sakas D., Nikita K.S. Parameter identification for a local field potential driven model of the Parkinsonian subthalamic nucleus spike activity. Neural Networks. 2012. 36: 146–156. https://doi.org/10.1016/j.neunet.2012.10.003
Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiological Reviews. 1985. 65 (1): 37–100. https://doi.org/10.1152/physrev.1985.65.1.37
Molnar-Szakacs I., Iacoboni M., Koski L., Mazziotta J.C. Functional Segregation within Pars Opercularis of the Inferior Frontal Gyrus: Evidence from fMRI Studies of Imitation and Action Observation. Cerebral Cortex. 2005. 15 (7): 986–994. https://doi.org/10.1093/cercor/bhh199
Mukamel R., Ekstrom A.D., Kaplan J., Iacoboni M., Fried I. Single-Neuron Responses in Humans during Execution and Observation of Actions. Current Biology. 2010a. https://doi.org/10.1016/j.cub.2010.02.045
Mukamel R., Ekstrom A.D., Kaplan J., Iacoboni M., Fried I. Single-Neuron Responses in Humans during Execution and Observation of Actions. Current Biology. 2010b. 20 (8): 750–756. https://doi.org/10.1016/j.cub.2010.02.045
Nauhaus I., Busse L., Carandini M., Ringach D.L. Stimulus contrast modulates functional connectivity in visual cortex. Nature Neuroscience. 2009. 12(1): 70–76. https://doi.org/10.1038/nn.2232
Okun M., Naim A., Lampl I. The Subthreshold Relation between Cortical Local Field Potential and Neuronal Firing Unveiled by Intracellular Recordings in Awake Rats. Journal of Neuroscience. 2010. 30 (12): 4440–4448. https://doi.org/10.1523/JNEUROSCI.5062-09.2010
Perry A., Stiso J., Chang E.F., Lin J.J., Parvizi J., Knight R.T. Mirroring in the Human Brain: Deciphering the Spatial-Temporal Patterns of the Human Mirror Neuron System. Cerebral Cortex. 2018. 28 (3): 1039–1048. https://doi.org/10.1093/cercor/bhx013
Poulet J.F.A., Petersen C.C.H. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008. 454 (7206): 881–885. https://doi.org/10.1038/nature07150
Raine A. The neuromoral theory of antisocial, violent, and psychopathic behavior. Psychiatry Research. 2019. 277: 64–69. https://doi.org/10.1016/j.psychres.2018.11.025
Rizzolatti G., Cattaneo L., Fabbri-Destro M., Rozzi S. Cortical Mechanisms Underlying the Organization of Goal-Directed Actions and Mirror Neuron-Based Action Understanding. Physiological Reviews. 2014. 94 (2): 655–706. https://doi.org/10.1152/physrev.00009.2013
Rizzolatti G., Craighero L. THE MIRROR-NEURON SYSTEM. Annual Review of Neuroscience. 2004. 27 (1): 169–192. https://doi.org/10.1146/annurev.neuro.27.070203.144230
Rolls E.T. Limbic systems for emotion and for memory, but no single limbic system. Cortex. 2015. 62: 119–157. https://doi.org/10.1016/j.cortex.2013.12.005
Saito Y., Kubicki M., Koerte I., Otsuka T., Rathi Y., Pasternak O., Bouix S., Eckbo R., Kikinis Z., von Hohenberg C.C., Roppongi T., Del Re E., Asami T., Lee S.-H., Karmacharya S., Mesholam-Gately R.I., Seidman L.J., Levitt J., McCarley R.W., Shenton M.E., Niznikiewicz M.A. Impaired white matter connectivity between regions containing mirror neurons, and relationship to negative symptoms and social cognition, in patients with first-episode schizophrenia. Brain Imaging and Behavior. 2018. 12 (1): 229–237. https://doi.org/10.1007/s11682-017-9685-z
Simone L., Bimbi M., Rodà F., Fogassi L., Rozzi S. Action observation activates neurons of the monkey ventrolateral prefrontal cortex. Scientific Reports. 2017. 7 (1): 44378. https://doi.org/10.1038/srep44378
Simone L., Rozzi S., Bimbi M., Fogassi L. Movement-related activity during goal-directed hand actions in the monkey ventrolateral prefrontal cortex. European Journal of Neuroscience. 2015. 42 (11): 2882–2894. https://doi.org/10.1111/ejn.13040
Singer T. Empathy for Pain Involves the Affective but not Sensory Components of Pain. Science. 2004. 303 (5661): 1157–1162. https://doi.org/10.1126/science.1093535
Skelin I., Kilianski S., McNaughton B.L. Hippocampal coupling with cortical and subcortical structures in the context of memory consolidation. Neurobiology of Learning and Memory. 2019. 160: 21–31. https://doi.org/10.1016/j.nlm.2018.04.004
Sliwa J., Freiwald W.A. A dedicated network for social interaction processing in the primate brain. Science. 2017. 356 (6339): 745–749. https://doi.org/10.1126/science.aam6383
Solomon E.A., Stein J.M., Das S., Gorniak R., Sperling M.R., Worrell G., Inman C.S., Tan R.J., Jobst B.C., Rizzuto D.S., Kahana M.J. Dynamic Theta Networks in the Human Medial Temporal Lobe Support Episodic Memory. Current Biology. 2019. 29 (7): 1100–1111.e4. https://doi.org/10.1016/j.cub.2019.02.020
Ushakov V.L., Kartashov S.I., Zavyalova V. V., Bezverhiy D.D., Posichanyuk V.I., Terentev V.N., Anokhin K.V. Network activity of mirror neurons depends on experience. Journal of Integrative Neuroscience. 2013. 12(01): 35–46. https://doi.org/10.1142/S0219635213500040
Viswanathan A., Freeman R.D. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nature Neuroscience. 2007. 10 (10): 1308–1312. https://doi.org/10.1038/nn1977
Waldert S., Vigneswaran G., Philipp R., Lemon R.N., Kraskov A. Modulation of the Intracortical LFP during Action Execution and Observation. The Journal of Neuroscience. 2015. 35 (22): 8451–8461. https://doi.org/10.1523/JNEUROSCI.5137-14.2015
West R.A., Larson C.R. Neurons of the anterior mesial cortex related to faciovocal activity in the awake monkey. Journal of Neurophysiology. 1995. 74 (5): 1856–1869. https://doi.org/10.1152/jn.1995.74.5.1856
Yanike M., Ferrera V.P. Representation of Outcome Risk and Action in the Anterior Caudate Nucleus. Journal of Neuroscience. 2014. 34 (9): 3279–3290. https://doi.org/10.1523/JNEUROSCI.3818-13.2014
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


