Журнал высшей нервной деятельности им. И.П. Павлова, 2021, T. 71, № 4, стр. 453-467
BDNF и старческое угнетение когнитивных функций
Д. Г. Семенов 1, *, А. В. Беляков 1
1 Институт физиологии им. И.П. Павлова РАН
Санкт-Петербург, Россия
* E-mail: dsem50@rambler.ru
Поступила в редакцию 30.11.2020
После доработки 21.02.2021
Принята к публикации 02.03.2021
Аннотация
Возрастные нарушения способности воспринимать, хранить и пользоваться новой информацией интенсивно изучаются у различных видов животных и у человека. Когнитивный дефицит как при нормальном, так и при патологическом старении может быть результатом нарушенной регуляции транскрипции, трансляции, секреции, рецепции и сигналинга мозгового нейротрофического фактора (BDNF), являющегося ключевой молекулой, участвующей в процессах обучения и памяти, т.е. важных когнитивных компонентов, наиболее уязвимых при старении. В настоящем кратком обзоре рассматриваются современные представления об участии системы BDNF в формировании когнитивного статуса взрослого и стареющего мозга. Также описываются некоторые современные фармакологические и немедикаментозные подходы, стимулирующие экспрессию BDNF и/или воздействующие на соответствующие сигнальные каскады, которые апробированы на экспериментальных моделях и могут быть использованы или уже используются в когнитивной гериатрии.
СОКРАЩЕНИЯ
| ИУГГ | – интервальная умеренная гипобарическая гипоксия |
| Akt | – protein kinase B |
| BDNF | – brain-derived neurotrophic factor |
| CaMK | – Ca2+/сalmodulin-dependent protein kinase |
| CREB | – cAMP response element-binding protein |
| ERK 1/2 | – extracellular signal-regulated kinase 1/2 |
| FUS | – фокусированный ультразвук |
| MAPK | – mitogen-activated protein kinase |
| mTOR | – mechanistic target of rapamycin |
| PDPK1 | – 3-phosphoinositide-dependent protein kinase-1 |
| PI3K | – phosphoinositide 3-kinase |
| PKC | – protein kinase C |
| PRC | – perirhinal cortex |
| p75NTR | – p75 neurotrophin receptor |
| rTMS | – repetitive transcranial magnetic stimulation |
| STEP | – striatal-enriched phosphatase |
| tDCS | – transcranial direct current stimulation |
| TrkВ | – tyrosine receptor kinase B. TK+; TK– isoforms |
Процесс старения включает прогрессирующие нарушения гомеостатических механизмов в мозге, которые сопровождаются угнетением когнитивных функций. При нормальном старении наиболее уязвимой оказывается нейрональная пластичность гиппокампа и нескольких отделов коры. Психологическое тестирование пожилых людей с применением магнитно-резонансной визуализации показало, что признаки когнитивного ослабления (снижение характеристик долговременной эпизодической памяти, рабочей памяти, исполнительных функций и скорости реагирования) коррелируют с уменьшением объема гиппокампа, разветвленностью дендритов и количеством шипиков, что нарушает его функциональные взаимосвязи со структурами коры (O’Shea et al., 2016; Nyberg, 2017). Одним из звеньев системы регуляции работы мозга в целом и особенно его областей, реализующих когнитивные функции, выступает семейство нейротрофических факторов. Продукция нейротрофинов и активность сигнальных путей, в которых они задействованы, в значительной мере подвержены изменениям, происходящим как при нормальном старении, так и при нейродегенеративных заболеваниях, обычно сопровождающих старение. Молекулярные механизмы возрастных изменений синтеза, метаболизма, секреции, транспорта и рецепции нейротрофинов в различных отделах мозга пока недостаточно ясны (Гомазков, 2011). Одним из наиболее изученных членов этого семейства, отвечающего за формирование и поддержание когнитивных функций мозга, является мозговой нейротрофический фактор (brain-derived neurotrophic factor – BDNF). Многими исследователями отмечается корреляция между снижением синтеза, секреции и/или рецепции BDNF в мозге и развитием ряда психических дисфункций (Baranova et al., 2015; Cattaneo et al., 2016), в том числе старческого когнитивного ослабления (Barrientos et al., 2015; Budni, 2016; Miranda et al., 2019).
Цель настоящего обзора состоит в сопоставлении и анализе сведений о системе BDNF и ее возрастных изменениях, сопровождаемых нарушениями когнитивных функций, а также в освещении способов стимуляции этой системы, имеющих перспективы гериатрического применения.
1. ГЕНЫ BDNF И TrkB
Единственный ген BDNF человека расположен в 11-й хромосоме. Он содержит 9 функциональных промоторов, которые специфически активируются в различных тканях и областях мозга с использованием нескольких транскрипционных факторов (Esvald et al., 2020). Такая конструкция гена способна транскрибировать несколько сплайс-вариантов, отличающихся альтернативными, не транслируемыми участками в 5' концевой области (Cattaneo et al., 2016). Регуляция уровней молекул BDNF, синтезированных от различных транскриптов, осуществляется различными физиологическими стимулами, в числе которых наиболее часто обсуждается стресс (Fuchikami et al., 2009), уровень нейрональной активности (Hong et al., 2008), физические упражнения (Chieffi et al., 2017), уровень эстрогенов (Luine, Frankfurt, 2013), наличие антидепрессантов (Miranda et al., 2019). Одни изоформы специфичны для клеток периферической крови, другие для нейронов мозга, а часть – для обеих тканей (Timmusk et al., 1993). Последнее обстоятельство представляется практически важным, поскольку идентификация экспрессии таких “общих” изоформ в крови человека технически облегчает диагностику ряда психических расстройств, связанных с дефицитом BDNF в когнитивно значимых областях мозга (Cattaneo et al., 2016). Даже в пределах одного нервного образования – гиппокампа пирамидные нейроны областей СА1 и СА3 демонстрируют различное распределение BDNF, транслированного различными транскриптами. Более того, специфика экспрессии различных транскриптов мРНК BDNF обнаружена и на субклеточном уровне. На изолированных пирамидных нейронах гиппокампа мышей и в экспериментах in vivo было показано, что стимуляция формирования апикальных или базальных дендритов, разветвленности дендритного древа или образования шипиков осуществляется белками BDNF, синтезированными на основе различных транскриптов (Maynarda et al., 2017). Представления о нескольких сплайс-вариантах BDNF и данные, указывающие на клеточную и субклеточную специфику локализации соответствующих транскриптов, поддерживают гипотезу “пространственного кода”, которая утверждает, что экспрессия различных транскриптов мРНК BDNF обеспечивает пространственную, временную и стимул-специфичную продукцию BDNF (Tongiorgi, 2008).
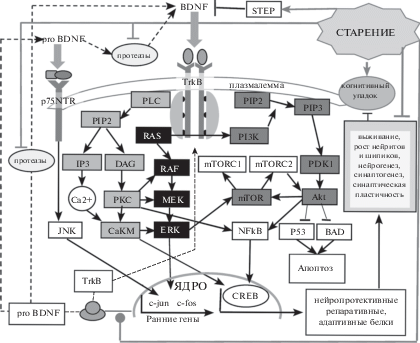
Внеклеточный BDNF, связываясь со своим плазмалеммным высокоафинным рецептором TrkВ (tyrosine receptor kinase B), может модулировать экспрессию собственной РНК через активацию сигналинга MAPK (mitogen-activated protein kinase), образуя, таким образом, транскрипционную положительную обратную связь (Nakajima et al., 2015). На культивируемых нейронах коры показано, что главным регулятором индукции всех основных транскриптов BDNF, зависящих от TrkB-сигналинга, является семейство транскрипционных факторов CREB (cAMP response element-binding protein) (Esvald et al., 2020). В мозге грызунов и людей наиболее активная экспрессия BDNF отмечается в гиппокампе (сильнее всего), амигдале, мозжечке и коре (Hofer et al., 1990). Рис. 1
Рис. 1.
Упрощенная схема сигнальных каскадов, инициируемых рецепторами BDNF в нейронах мозга.
Указание возможных мишеней старения.
Обозначения линий:
 активация;
активация;  инактивация;
инактивация;  модуляция;
модуляция;  транслокация.
транслокация.
Fig. 1. Simplified scheme of signal cascades initiated by BDNF receptors in brain neurons.
Indication of possible targets of aging.
Indication of lines:
 activation;
activation;  inhibition;
inhibition;  modulation;
modulation;  translocation.
translocation.
Оценивая индивидуальную специфичность роли BDNF в регуляции когнитивных функций, следует учитывать полиморфизм его гена у человека, который проявляется в двух вариантах 66-го кодона мРНК, обеспечивающих при трансляции альтернативный подбор аминокислот: либо Val, либо Met. Установлено, что носители аллели BDNFMet, в отличие от носителей гомозиготной аллельной пары BDNFVal/Val, проявляли существенно сниженную синаптическую пластичность, зависящую от NMDA- и ГАМК-трансмиссии в префронтальной коре, что коррелировало с когнитивным дефицитом (Pattwell et al., 2012).
Важную роль играют эпигенетические механизмы регуляции экспрессии BDNF в мозге человека, реализуемые через некоторые промоторы путем метилирования ДНК и/или гистонов. Отмечено, что у пациентов, хронически употреблявших антидепрессанты, подавляется метилирование гистона Н3К27, что способствовало повышению уровня BDNF (Chen et al., 2011). Установлено негативное влияние нескольких микроРНК на транскрипцию BDNF, приводящее к торможению нейрогенеза в зубчатой извилине гиппокампа и провоцирующее некоторые психические расстройства (Ruiz et al., 2014).
Помимо указанных источников вариабельности в экспрессии BDNF, определяющих его уровень в мозге, следует учитывать и вариабельность его рецептора. У человека белки TrkB кодируются геном NTRK2 (neurotrophic receptor tyrosine kinase, type 2), локализованном в 9-й хромосоме и транскрибирующим 3 сплайс-варианта. Три соответствующих вида рецептора TrkB располагаются на плазмалемме нейронов и глиальных клеток большинства млекопитающих. Белок TrkB-TK+ имеет полную аминокислотную последовательность и тиразинкиназную активность С-концевого домена (TK+). Два других сплайс-варианта не имеют полноценного внутриклеточного фрагмента (truncated forms, TrkB.Т1 и TrkB.Т2), лишены тирозинкиназной активности и обозначаются как TrkB-TK- (Sasi et al., 2017).
2. БЕЛКИ И СИГНАЛИНГ СИСТЕМЫ BDNF
Различные транскрипты гена BDNF содержат одну и ту же кодирующую последовательность, транслируемую в форме pro-BDNF (прекурсора BDNF). Этот полипептид поступает из эндоплазматического ретикулума в аппарат Гольджи и частично секретируется в неизмененном виде. Другая часть прекурсора подвергается протеолизу внутри везикул или в сети аппарата Гольджи различными протеазами путем отщепления N-концевого про-домена и образования зрелого BDNF (13 kDa), который также секретируется после транспортировки в пресинаптические окончания. Протеолиз pro-BDNF до зрелого BDNF может происходить и внеклеточно с помощью плазмина или матриксных металлопротеаз ММП-2 и ММП-9. (Pang et al., 2004).
Прекурсор pro-BDNF, как и прекурсоры других нейротрофинов (NGF, NT3, NT4), способен соединяться с рецептором p75 (p75NTR – p75 neurotrophin receptor). Взаимодействие p75NTR с корецептором сортилином и лигандом pro-BDNF приводят к активации JNK (c-Jun N-terminal kinase)-опосредованного сигналинга, запускающего нейрональный апоптоз (Lee et al., 2001; Friedman, 2010; Nykjaer, Willnow, 2012). Таким образом, динамический баланс BDNF и pro-BDNF, регулируемый внутри- и внеклеточным протеолизом прекурсора, может определять судьбу нейронов через поддержание их выживания или гибели и обеспечивать сбалансированную регуляцию синаптической пластичности путем облегчения LTP (long-term potentiation) или LTD (long-term depression) (Woo et al., 2005). Этот баланс зависит не только от функционального состояния нейрона, но и от его типа, и от стадии онтогенеза (Sasi et al., 2017).
Зрелый димер BDNF, связываясь с рецептором TrkB, инициирует его димеризацию и аутофосфорилирование. Полное фосфорилирование TrkB у человека происходит по трем тирозиновым остаткам: Tyr490, Tyr515 и Tyr816. В зависимости от комбинации фосфорилирования по этим сайтам и активности соответствующих адаптерных белков комплекс BDNF/TrkB способен инициировать несколько сигнальных каскадов, регулирующих и координирующих процессы выживания, дифференцировки, синаптической пластичности, необходимые для реализации и развития когнитивных функций. Наиболее изученными представляются три каскада: PI3K/Akt, Ras/MAPK и PLCγ (Gupta et al., 2013; Kowianski et al., 2018).
Активированная Trk-рецептором PI3K (phosphoinositide 3-kinase) обеспечивает условия для примембранного фосфорилирования Akt (protein kinase B). Akt известна своей антиапоптозной функцией, реализуемой через активирующее или ингибирующее фосфорилирование множества белков, вовлеченных в механизмы гибели и выживания. Akt-сигналинг также модулирует NMDA-зависимую синаптическую пластичность и стимулирует развитие цитоскелета и рост и ветвление дендритов (Ruiz et al., 2014). Одним из следствий запуска PI3K/Akt -сигнального каскада является активация mTOR (mechanistic target of rapamycine) – каталитического центра двух сложных и различных по составу киназных комплексов mTORC1 и mTORC2, которые регулируют различные клеточные функции (Parkhitko et al., 2014; Lipton, Sahin, 2014). Комплекс mTORC1 через множество механизмов и соответствующих посредников активирует процессы трансляции и негативно регулирует аутофагию. В контексте воздействия на когнитивные функции особое значение имеет комплекс mTORC2. Он фосфорилирует PKC и совместно с PDPK1 (3-phosphoinositide-dependent protein kinase-1) обеспечивает полное фосфорилирование Akt. Таким образом замыкается положительная обратная связь между Akt и mTOR.
Важно отметить, что PI3K для активации Akt может использовать фосфоинозитиды не только плазматической мембраны, но и внутренних мембранных образований, в том числе эндосом, и локально запускать Akt-сигналинг в тех местах, куда они транспортируются (Jethwa et al., 2015). Механизмы эндосомальной интернализации и внутринейрональной транспортировки активированных Trk-рецепторов играют принципиально важную роль в регуляции сигнальных путей, повышая ее эффективность в определенных внутриклеточных компартментах (Bucci et al., 2014). В частности, взаимодействие BDNF с TrkB в дендритах стимулирует интернализацию и эндосомальный транспорт этого лиганд-рецепторного комплекса к ядру (Moya-Alvarado et al., 2018). В плане локальной активации PI3K/Akt-сигналинга в нейронах гиппокампа большой интерес представляют недавние исследования, доказавшие участие в этом процессе p75NTR, который после фосфорилирования TrkB формирует с ним комплекс, активирующий свою интернализацию первичной эндосомой, и последующий внутриклеточный транспорт (Zanin et al., 2019).
Второй важный сигнальный путь, запускаемый BDNF/TrkB-комплексом, представляет собой цепь киназ: MEKK (MAPK/ERK kinase kinase), MEK (MAPK/ERK kinase) и ERK 1/2 (extracellular signal-regulated kinase 1/2). Фосфорилированная ERK 1/2 диффундирует в цитоплазму, а затем в ядро, где она индуцирует транскрипцию ранних генов, продукты которых являются транскрипционными факторами поздних генов, а их продукты, в частности, регулируют дифференциацию прогениторов и выживание нейритов в области зубчатой извилины (Wang, Mao, 2019).
Сигнальный путь, опосредованный активацией PLCγ (phospholipase Cγ), начинается с гидролиза PIP2 (phosphatidylinositol 4,5-bisphosphate) и образования двух вторичных посредников: DAG (1,2-diacylglycerol) и IP3 (inositol-3-phosphate). Они, соответственно, активируют PKC (protein kinase-C) и высвобождение Ca2+ из внутриклеточных депо, что сопровождается активацией CaMK (Ca2+/сalmodulin-dependent protein kinase). Одно из следствий этих двух киназных сигналов состоит в формировании LTP глутаматергической возбуждающей трансмиссии в гиппокампе, что является важнейшим клеточным элементом в механизмах памяти (Kowianski et al., 2018). Следует отметить, что указанные каскады при определенных условиях способны взаимодействовать друг с другом.
Таким образом, TrkB-опосредованные сигнальные пути в конечном итоге, через повышение синаптической пластичности, рост и ветвление дендритов, развитие цитоскелета, антиапоптозную активность и прочее, поддерживают ряд когнитивных функций, выполняемых нейронными структурами гиппокампа и коры (Tyler et al., 2002). Считается, что любые факторы, нарушающие экспрессию в мозге BDNF и/или TrkB, через нарушение внутриклеточной сигнализации приводят к структурным и функциональным повреждениям нейронных сетей, что инициирует психические расстройства (Hempstead, 2015; Cattaneo et al., 2016), в том числе старческое когнитивное ослабление (Budni, 2016). На компилятивной схеме в упрощенной форме представлены указанные сигнальные каскады, их эффекторы и некоторые из известных негативных влияний старения.
3. ИЗМЕНЕНИЯ СИСТЕМЫ BDNF ПРИ СТАРЕНИИ
В основе возрастных когнитивных дисфункций лежат несколько общепризнанных процессов старения: повреждение когнитивно-значимых областей мозга, замедление нейрогенеза в гиппокампе и субвентрикулярной области, оксидативный стресс, нейровоспалительные процессы, сокращение теломеров, экспрессия теломеразной обратной транскриптазы и др. (Levada, Troyan, 2020). Большинство из них могут быть либо следствием, либо причиной нарушения нейротрофического обеспечения в мозге. Вместе с тем представления о конкретных механизмах, нарушающих это обеспечение в старости, пока не полны и иногда противоречивы. Нет единого мнения относительно того, является ли снижение уровня BDNF характерным для всех областей стареющего мозга и является ли оно вообще атрибутом старческого когнитивного ослабления.
Гиппокамп привлекает наибольшее внимание исследователей когнитивных функций, особенно механизмов формирования рабочей и долговременной памяти, обеспечиваемых нейротрофинами. Тем не менее в гиппокампе пожилых людей не обнаружено снижения уровня мРНК BDNF, хотя такое снижение наблюдалось у них в теменной коре. При этом в обоих отделах был понижен уровень мРНК рецептора TrkB (Webster et al., 2006).
В нейронах нижневисочной доли префронтальной коры старых макак также показано снижение уровня мРНК BDNF, но уровни TrkB и зрелого BDNF оставались стабильными. При этом сниженным оказался уровень прекурсора. Этот смещенный баланс двух форм нейротрофина в условиях сниженной транскрипции мРНК BDNF, по мнению авторов, отражает компенсаторный ответ, препятствующий pro-BDNF-опосредуемой деградации аксонов и шипиков (Robinson et al., 2018).
Периринальная кора (PRC), передающая сенсорную информацию в гиппокамп через латеральную энторинальную кору, поддерживает объектную (не пространственную) информацию (Knierim et al., 2006). Считается, что путь от PRC к гиппокампу является наиболее уязвимым при старении из-за деградации синапсов в перфорантном пути, соединяющем энторинальную кору с GD и СА3 гиппокампа. (Leal, Yassa, 2015). Восприятие новых объектов связано с повышением экспрессии BDNF в PRC (Romero-Granados et al., 2010). Это подтверждается тем, что подавление трансляции BDNF антисмысловыми олигонуклеотидами в PRC крыс снижает их способность разделять не четко различимые признаки зрительных стимулов. Эти и другие данные позволяют предположить, что характерное для пожилых людей нарушение консолидаци различительной объектной памяти может быть исправлено стимуляцией BDNF-системы (Miranda et al., 2019).
При геномном исследовании большой группы испытуемых (16–96 лет) были идентифицированы 200 генов, экспрессия которых в префронтальной коре положительно коррелирует с BDNF. Уровни мРНК BDNF, NTRK2 и генов, коэкспрессируемых с BDNF, у пожилых испытуемых были значительно снижены, причем около половины этих генов связаны с синаптической функцией. Продолжение исследования на мышах позволило авторам сделать вывод, что возрастное снижение сигналинга BDNF может нарушать синаптические функции, преимущественно угнетая ГАМК-ергическую систему (Oh et al., 2016).
Важную роль в возрастных изменениях экспрессии BDNF играет эпигенетический фактор. Причину старческого снижения концентрации BDNF в префронтальной коре людей усматривают в активации метилирования ДНК в определенных промоторных областях гена BDNF (Keleshian et al., 2013). В гиппокампе крыс с возрастом возникает дефицит синаптической пластичности и потеря дендритных шипиков, что связано со снижением ацетилирования гистонов Н3 и Н4 в нескольких промоторных областях гена BDNF, вызванным повышением активности гистондеацетилазы и снижением экспрессии гистонацетилтрансферазы. В результате в старом гиппокампе экспрессия BDNF была значительно снижена и, соответственно, нарушены его сигнальные пути. Эти нарушения исправлялись подавлением гистондеацетилазы и активацией TrkB с помощью его агониста 7,8-дигидроксифлавона (Zeng et al., 2011).
Противоречивость мнений о направленности изменений уровня BDNF в стареющем мозге, вероятно, отражает специфичность экспрессии нейротрофина для различных регионов мозга и ее сложную динамику, включающую как угнетение, так и компенсаторную активацию.
Одним из вероятных механизмов старческого снижения экспрессии BDNF выступает накопление STEP (striatal-enriched phosphatase), наблюдаемое в гиппокампе грызунов, приматов и человека в старости. STEP способна дефосфорилировать BDNF, снижая общий уровень его активности. Уровень этой фосфатазы в норме ограничивается убиквитин-протеосомной системой, но в старости этот контроль ослабевает (Castonguay et al., 2018). Известной моделью “успешного” старения является инбредная линия крыс Lou/C/Jall, которая отличается от крыс Wistar отсутствием повышения уровня STEP с возрастом и при этом демонстрирует высокий уровень BDNF, долголетие, отсутствие ожирения в старости, повышенную чувствительность к инсулину и высокую активность PI3K/Акт-сигнального пути. Отмечено также, что у этих животных при старении сохраняются высокие показатели рабочей и долговременной памяти (Silhol et al., 2008; Kollen et al., 2010).
Определенное значение в динамике старческого угасания когнитивных функций и развития деменции имеют индивидуальные генетические особенности. Исследования на людях, показывающие возрастное ослабление декларативной памяти, установили, что наиболее ярко этот дефицит проявляется у пожилых носителей аллели BDNFMet по сравнению с носителями гомозиготной пары BDNFVal/Val (Sambataro et al., 2010; Brown et al., 2020).
Многими исследователями поддерживается мнение, что естественное старение мозга, как и большинство нейродегенеративных заболеваний, у грызунов и у людей определяется не столько нарушениями выработки BDNF, сколько изменениями его рецепции. Показано, что в гиппокампе крыс уровень TrkB-TK+ значительно снижается с возрастом (Silhol et al., 2005). У человека отмечено снижение уровня мРНК TrkB-TK+, характерное не только для гиппокампа, но и для ряда других когнитивно-значимых структур стареющего мозга (Webster et al., 2006). При этом обнаружено некоторое гомогенное увеличение с возрастом уровня мРНК TrkB-TK- в большинстве областей (Romanczuk et al., 2002). Вероятно, именно дефицит полномерных рецепторов (ТК+) и соответствующая пониженная активность TrkB-опосредованных сигналов роста, дифференциации, синаптогенеза, развития цитоскелета и пр. приводят к типичной для стареющего мозга морфологической деградации межнейронных коммуникаций, включая нарушения структуры шипиков, что коррелирует со снижением когнитивных способностей (Vecchio et al., 2018).
Помимо вариабельности в экспрессии сплайс-вариантов белка TrkB, важную роль в развитии старческих когнитивных дисфункций играет баланс в экспрессии и активности рецепторов TrkB-TK+ и p75NTR. Недавно было проведено исследование преждевременно стареющих крыс линии OXYS, которые также существенно отличались от контрольных животных (Wistar) по транскриптому генов, вовлеченных в сигналинг нейротрофинов (Рудницкая и др., 2017). Было установлено, что к 18 месяцам, несмотря на одинаковый уровень белка TrkB в префронтальной коре у обеих групп, у крыс OXYS был снижен уровень его фосфорилированной формы по сайту Tyr817, обеспечивающему запуск PLCγ-опосредованного сигнального каскада (He et al., 2010). Кроме того, у этих животных отмечена усиленная иммунореактивность proBDNF и p75NTR и их колокализация. Эти данные позволяют авторам предположить, что ускоренное старение и склонность к спорадической форме болезни Альцгеймера крыс OXYS являются результатом усиления проапоптозного сигналинга proBDNF/p75NTR и ослабления сигнального пути BDNF/TrkB/PLCγ, регулирующего синаптическую пластичность (Рудницкая и др., 2017).
Еще одной важной причиной расстройств гиппокамп-зависимых когнитивных процессов может выступать нарушение нейрогенеза в зубчатой извилине, который в норме поддерживается системой BDNF. У старых макак иммуногистохимические методы показали значительное сокращение генерации молодых нейронов в субгранулярной зоне и увеличение периода их созревания. При этом снижение нейрогенеза совпадало с ухудшением освоения когнитивных задач и с ослаблением системы рабочей памяти (Ngwenya et al., 2015). Оценка уровня прогениторов и молодых нейронов в гиппокампе взрослого и, особенно, пожилого человека показала крайне слабую активность нейрогенеза (Sorrells et al., 2018). В отличие от данных о нейротрофиновой стимуляции нейрогенеза у старых грызунов, мы не нашли прямых указаний на то, что этот путь эффективен в отношении старых приматов и человека.
4. СПОСОБЫ СТИМУЛЯЦИИ BDNF/TrkB-СИСТЕМЫ
4.1 . Прямое введение BDNF
Внутривенное введение BDNF не практикуется из-за мизерного проникновения нейротрофина через гематоэнцефалический барьер, высокой скорости его метаболизирования различными компонентами крови и значительных побочных влияний на дыхательную и сердечно-сосудистую систему. Более эффективным путем доставки экзогенных нейротрофинов в мозг представляется интраназальное введение. При таком подходе препарат диффундирует вдоль тройничного нерва, попадает в периваскулярные пространства и механизмом “периваскулярной помпы” (пульсации) перемещается в различные области мозга вдоль сосудов. Одна из работ по технологии интраназальной доставки ряда нейротрофических факторов в мозг крыс показала, что при таком введении 70 мкг меченого BDNF его концентрация в паренхиме мозга за 25 мин достигает 1 нМ, чего было достаточно для активации PI3K/Akt-сигнального пути (Alcalá-Barraza et al., 2010). Вместе с тем этот метод не всегда дает достаточно узкий охват области-мишени. “Прицельность” интраназальной доставки BDNF существенно повышается при использовании фокусированного ультразвука (FUS). Благодаря физическому эффекту FUS на малом участке ткани (до миллиметра) удается создать пульсирование микропузырьков межклеточной среды, что облегчает проникновение препарата в облучаемую область. На мышах был испытан вариант интраназальной доставки BDNF с FUS, который показал более локальную область доставки и более высокую концентрацию в ней эндогенного препарата по сравнению с интраназальным введением без FUS. При этом никаких побочных эффектов не отмечено (Chen et al., 2016). На мышах, моделирующих болезнь Паркинсона, введение BDNF этим методом в область нигро-стриатного пути существенно сократило признаки нейродегенерации в ипсилатеральных структурах, в то время как интраназальное введение без FUS не дало такого результата (Ji et al., 2019).
Для доставки BDNF в поврежденные участки мозга применяют в качестве транспортера гидрогель гиалуроновой кислоты. На мышах и приматах с экспериментальным инсультом было показано, что после введения препарата в очаг поражения в периинфарктной зоне усилился рост аксонов и повысились миграция и вживление незрелых нейронов (Cook et al., 2017).
В последнее время развивается сложный, но и более эффективный подход к терапевтическому повышению уровня нейротрофинов, состоящий в вирус-векторной инъекции генов в определенные локусы мозга под контролем МРТ. В частности, была разработана методика инъекции раствора, содержащего меченый вектор AAV2-BDNF в медиальную энторинальную кору макак на глубину 1 мм. Последующий иммуногистохимический анализ показал успешное антероградное проникновение и распределение вектора в зубчатой извилине гиппокампа даже при минимальной порции в 15 мкл (Nagahara et al., 2018).
4.2. Фармакологическая стимуляция BDNF/TrkB-системы
В профилактике или лечении когнитивных расстройств путем активации TrkB-зависимых сигнальных путей существует множество фармакологических подходов, не требующих прямого введения нейротрофина. Один из них базируется на применении натуральных или синтетических BDNF-миметиков, стимулирующих либо продукцию BDNF, либо непосредственно активирующих TrkB-рецептор (Fletcher, Hughes, 2006; Numakawa, 2014). В частности, деоксигедунин – натуральный терпеноид, изолированный из индийской сирени (Azadirachta indica), является мощным агонистом TrkB, не влияющим на синтез BDNF. При пероральном введении мышам он показал сильный нейропротективный, антидепрессивный и прокогнитивный эффект (Jang et al., 2010). Представитель психопластогенов флавоноид спорыньи 7,8-дигидроксифлавон и его синтетические дериваты проявили себя в модельных экспериментах как мощные перорально вводимые агонисты TrkB, обладающие быстрым и стойким антидепрессивным действием, исправляющие дефицит памяти в экспериментальных моделях болезни Альцгеймера, стимулирующие нейрогенез в зубчатой извилине и облегчающие синаптическую пластичность при старении. Впрочем, несмотря на внушительные результаты, полученные на мышах и обезьянах, пока нет данных о применении препаратов этой группы на людях (He et al., 2016; Benko et al., 2020). Особую группу отечественных синтетических лигандов TrkB представляют димерные дипептидные миметики BDNF, сохраняющие минорную часть нативной молекулы нейротрофина, очевидно наиболее существенную для взаимодействия с рецептором. Препараты могут вводиться внутрибрюшинно и перорально и проникают через гематоэнцефалический барьер. Установлена их высокая агонистическая активность в отношении TrkB с последующей активацией PI3K/AKT- и MAPK/ERK-сигналинга. В экспериментах in vitro установлены их нейропротективная активность и стимуляция синаптогенеза. В доклинических исследованиях показана их антидепрессивная, антидиабетическая и анальгетическая активность (Gudasheva et al., 2017, 2019).
Известный позитивный эффект ингибиторов ацетилхолинэстеразы, как протекторов когнитивных функций при старении и нейродегенеративных заболеваниях, очевидно также включает механизм стимуляции BDNF/TrkB-сигналинга. В частности, для гиппокампа мышей этот механизм доказан в отношении донепезила и галантамина (Autio et al., 2011). На пожилых пациентах с развивающейся старческой деменцией тоже отмечен позитивный эффект донепезила, вероятно, связанный с активацией именно TrkB, поскольку он не сопровождался повышением уровня BDNF в плазме (Diniz et al., 2014).
Ряд работ указывает на прокогнитивный нейротрофин-опосредованный эффект синтетического гептапептида Семакс как для молодых, так и пожилых людей. Препарат усиливал селективное внимание и консолидацию кратковременной памяти. Однократное интраназальное введение Семакса крысам в дозе 50 мкг/кг с высокой скоростью и на длительный период повышало экспрессию генов NGF и BDNF в гиппокампе и лобной коре (Agapova et al., 2008).
Особое направление терапевтических исследований посвящено системе mTOR, входящей в состав сигнального пути PI3K/Akt. На множестве биологических моделей от нематоды до приматов показано подавление активности комплекса mTORC1 белками семейства сестринов или бактериальным токсином рапамицином, что способствовало увеличению продолжительности жизни. В современной литературе обсуждаются возможности и ограничения “рапамициновой терапии” при нормальном старении и старческой нейродегенерации мозга человека (Balasubramanian et al., 2017).
4.3. Tранскраниальная электро(магнито)стимуляция
В контексте коррекции старческого ослабления механизмов памяти определенный интерес вызывает неинвазивное воздействие в виде транскраниальной стимуляции постоянным током (transcranial direct current stimulation – tDCS). В поведенческих тестах на пожилых людях tDCS, направленная в область дорзо-латеральной префронтальной коры, временно улучшала реконсолидацию долговременной семантической памяти в задачах с подбором слов до показателей, свойственных молодым (Meinzer et al., 2013). Механизмы прокогнитивных эффектов tDCS не вполне ясны. Однако эксперименты на животных позволяют предположить, что один из них состоит в стимуляции транскрипции BDNF и TrkB (Cocco et al., 2018). Применение многодневной процедуры высокочастотной прерывистой транскраниальной магнитной стимуляции (repetitive transcranial magnate stimulation – rTMS) на крысах с нарушенными когнитивными функциями вследствие пренатального стресса исправило дефицит памяти и показало улучшение структурно-функциональных и нейрохимических характеристик гиппокампа. Сделан вывод о возможном BDNF/TrkB-опосредованном механизме благотворного эффекта rTMS, поскольку он блокировался инъекцией ингибитора TrkB (Shang et al., 2019).
4.4. Интервальная умеренная гипобарическая гипоксия
Множество работ, в основном посвященных горной болезни, доказывает патогенный эффект длительной и/или тяжелой гипобарической гипоксии на ряд систем организма, включая и высшие функции мозга. Отмечается и угнетение системы BDNF. Однако щадящая мера этого воздействия, а именно варианты интервальной умеренной гипобарической гипоксии (ИУГГ), не только проявляет адаптивный прокогнитивный эффект в эксперименте, но и применяется в медицинских центрах гипобаропрофилактики и гипобаротерапии (Юпатов и др., 2013). В модельных экспериментах на взрослых животных исследуются различные уровни неспецифического нейропротективного механизма ИУГГ (Рыбникова, Самойлов, 2016). Вместе с тем лишь небольшое число исследований доказывает вовлечение системы BDNF в этот механизм. На грызунах успешно применялась степень барокамерной гипоксии, соответствующая разрежению воздуха на высоте около 5000 м над уровнем моря, хотя в разных работах при данной степени разрежения существенно варьируют длительность экспозиции и число ежедневных сеансов ИУГГ. На мышах 28 сеансов ИУГГ с 6-часовой ежедневной экспозицией повышали экспрессию BDNF и уровень фосфорилирования TrkB, ERK1/2 и CREB. Это воздействие в режиме прекондиционирования предотвращало гибель нейронов в СА1-области гиппокампа и угнетение когнитивных функций, что развивалось бы в результате экспериментальной ишемии/реперфузии без прекондиционирующей ИУГГ (Wang et al., 2017). Доза “14 сеансов, 4 ч, ежедневно”, помимо известного антидепрессивного эффекта, вызывала в гиппокампе крыс активацию BDNF/TrkB-системы и нейрогенеза (Zhu et al., 2010). Вероятно, доза ИУГГ, сохраняющая эффективную стимуляцию когнитивных функций и компонентов BDNF/TrkB-сигналинга в гиппокампе и нео-кортексе крыс, может быть снижена до 3 сеансов по 2 ч (Samoilov et al., 2014; Churilova, Samoilov, 2015). Этот режим ИУГГ, примененный в качестве прекондиционирования за сутки перед тяжелой гипобарической гипоксией, предотвращал анксиогенный и амнестический эффекты последней, причем нейропротекция была опосредована активацией PI3K/Akt-сигнального пути (Belyakov, Semenov, 2017; 2019). Существуют данные о благотворном прокогнитивном влиянии ИУГГ на пожилых приматов, хотя участие BDNF при этом не анализировалось (Belyakov, Semenov, 2019). Нам пока не удалось обнаружить прямых данных о BDNF-опосредованных эффектах ИУГГ именно в геропротекторном когнитивном аспекте.
4.5. Физические упражнения
Сниженная физическая активность характерна для образа жизни пожилых людей. Однако известно, что люди старше 65 лет, получающие регулярную умеренную физическую нагрузку, показывают высокие результаты при тестировании памяти и имеют сниженный риск деменции и старческих нарушений экзекутивных функций. Прокогнитивные эффекты таких нагрузок проявляются не только на относительно здоровых пожилых людях, но и на тех, для кого уже диагностированы умеренные когнитивные расстройства или деменция (Bherer et al., 2013). В молекулярно-клеточных исследованиях на грызунах ведется поиск механизмов благотворных эффектов физических нагрузок. В их числе – укрепление гематоэнцефалического барьера, сдерживание накопления бета-амилоида в гиппокампе и коре, активация синтеза нейротрансмиттеров, вовлеченных в когнитивную деятельность. Особое внимание уделяется прокогнитивному воздействию физических нагрузок, которое реализуется через стимуляцию системы BDNF/TrkB и соответствующих сигнальных каскадов (Fletcher, Hughes, 2006). В гиппокампе людей и грызунов наблюдается возрастное подавление нейрогенеза, коррелирующее со снижением активности BDNF-сигналинга (Spalding et al., 2013; Sorrells et al., 2018). Несколько исследований на крысах убедительно показали стимулирующий эффект периодических беговых тренировок и пребывания в обогащенной среде на BDNF-опосредованный нейрогенез в зубчатой извилине гиппокампа (Voss et al., 2013). Один из предполагаемых механизмов заключается в стимуляции транскрипции BDNF миокином CTSB (catepsine B), секретируемым работающими скелетными мышцами. Эксперименты in vitro и in vivo установили эту связь для нейронов гиппокампа мышей и ее корреляцию с усилением нейрогенеза в зубчатой извилине и улучшением показателей пространственной памяти (Moon et al., 2016).
4.6. Ограничения в стратегии стимуляции BDNF/TrkB-системы
Описанные выше пути стимуляции BDNF/TrkB-сигналинга направлены на предохранение мозга млекопитающего от когнитивного угасания при нормальном или патологическом старении. Однако такое терапевтическое вторжение в сложнейшую и тонко отрегулированную систему экспрессии различных форм и транскриптов BDNF и TrkB или экзогенная стимуляция того или иного TrkB-опосредованного киназного каскада должна проводиться с определенной осторожностью. Известно, например, что избыточная TrkB-опосредованная активация MAPK- и PI3K/Akt-путей способствует выживанию раковых клеток при множественной миеломе (Pearse et al., 2005), неконтролируемая активация PLCγ-сигналинга приводит к лимбическому эпилептогенезу у мышей (He et al., 2010), нарушения механизмов негативной регуляции TrkB ведут к ряду легочных гиперплазий (Avcuoglu et al., 2011). Хотя эта сторона BDNF/TrkB-опосредованных процессов не является предметом настоящего обзора, следует отметить, что существует множество исследований, направленных на поддержание и/или стимуляцию именно негативной регуляции BDNF/TrkB-сигналинга (Cazorla et al., 2010; Ho et al., 2011; Gupta et al., 2013; Meng et al., 2019).
ЗАКЛЮЧЕНИЕ
Постоянно растущее число экспериментальных статей и обзоров, посвященных BDNF, демонстрирует его вовлечение во множество психофизиологических процессов. BDNF признается ключевым регулятором функциональной и структурной пластичности в мозге, а также важным “игроком” в запуске нейропротективных сигнальных каскадов. На жи-вотных моделях исследованы нарушения динамичных и тонко отрегулированных процессов его транскрипции, трансляции, транспорта, секреции, рецепции и дальнейшего внутриклеточного сигналинга, возникающие при старении и/или сопутствующих нейродегенеративных заболеваниях, которые непосредственно влияют на эффективность когнитивных процессов. Однако в медицине из-за ряда технологических ограничений связь нарушений BDNF/TrkB-системы с возрастными когнитивными дисфункциями пока отмечается лишь на коррелятивном уровне. Вместе с тем важная физиологическая роль системы BDNF является объектом возрастающего внимания разработчиков различных терапевтических стратегий, направленных на оптимальную стимуляцию экспрессии BDNF-системы, способную снизить или предотвратить когнитивные нарушения, вызванные естественным или патологическим старением мозга.
Работа выполнена в рамках программы ПФНИ ГАН ГП-4 (направление 63).
Список литературы
Гомазков О.А. Старение мозга и нейротрофическая терапия. М. ИКАР. 2011. 41–68.
Рудницкая Е.А., Колосова Н.Г., Стефанова Н.А. Анализ вклада изменения нейротрофического обеспечения в развитие признаков болезни Альцгеймера у крыс OXYS. Биохимия. 2017. 82 (3): 460–469.
Рыбникова Е.А., Самойлов М.О. Современные представления о церебральных механизмах гипоксического пре- и посткондиционирования. Успехи физиологических наук. 2016. 4: 3–17.
Юпатов Г.И., Доценко Э.А., Юпатов Ю.Г. Применение технологий гипобароадаптации в клинике внутренних болезней (обзор литературы). Вестник ВГМУ. 2013. 12 (4): 7–18.
Agapova T.Y., Agniulin Y.V., Silachev D.N., Shadrina M.I., Slominskii P.A., Shram S.I., Limborskaya S.A., Myasoedov N.F. Time course of the expression of genes of brain-derived neurotrophic factor and nerve growth factor in the hippocampus and frontal cortex induced by semax in rats. Mol. Gen., Microbiol. and Virol. 2008. 23 (3): 142–146.
Alcalá-Barraza S.R., Lee M.S., Hanson L.R., McDonald A.A., Frey 2nd W.H., McLoon L.K. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J. Drug Target. 2010. 18 (3): 179–190.
Autio H., Matlik K., Rantamaki T., Lindemann L., Hoener M.C., Chao M., Arumäe U., Castrén E. Acetylcholinesterase inhibitors rapidly activate Trk neurotrophin receptors in the mouse hippocampus. Neuropharmacology. 2011. 61: 1291–1296.
Avcuoglu S., Wygrecka M., Marsh L.M., Günther A., Seeger W., Weissmann N., Fink L., Morty R.E., Kwapiszewska G. Neurotrophic tyrosine kinase receptor B/neurotrophin 4 signaling axis is perturbed in clinical and experimental pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011. 45: 768–780.
Balasubramanian P., Mattison J.A., Anderson R.M. Nutrition, metabolism, and targeting aging in nonhuman primates. Ageing Res. Rev. 2017. 39: 29–35.
Baranova K.A., Rybnikova E.A., Samoilov M.O. The neurotrophin BDNF is involved in the development and prevention of stress-induced psychopathologies. Neurochem. J. 2015. 9: 108–115.
Barrientos R.M., Kitt M.M., Watkins L.R., Maier S.F. Neuroinflammation in the normal aging hippocampus. Neurosci. 2015. 309: 84–99.
Belyakov A.V., Semenov D.G. The PI3K/Akt system is involved in the neuroprotective preconditioning of rats with moderate hypobaric hypoxia. Neurochem. J. 2017. 11: 213–220.
Belyakov A.V., Semenov D.G. Stimulation of cognitive abilities in aged macaques via moderate hypobaric hypoxia. Advances in Gerontology. 2019. 9(2): 190–196.
Benko J., Vranková S. Natural psychoplastogens as antidepressant agents. Molecules. 2020. 25 (5): 1172.
Bherer L., Erickson K.I., Liu-Ambrose T. A Review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J. of Aging Research. 2013. 2013: ID 657508.
Brown D.T., Vickers J.C., Stuart K.E., Cechova K., Wardet D.D. The BDNF Val66Met polymorphism modulates resilience of neurological functioning to brain ageing and dementia: a narrative review. Brain Sci. 2020. 10 (4): 195–211.
Budni J. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging and diseases. 2016. 6 (5): 331–341.
Bucci C., Alifano P., Cogli L. The role of rab proteins in neuronal cells and in the trafficking of neurotrophin receptors. Membranes. 2014. 4: 642–677.
Castonguay D., Dufort-Gervais J., Ménard C., Chatterjee M., Quirion R., Bontempi B., Schneider J.S., Arnsten A.F.T., Nairn A.C., Norris Ch.M., Ferland G., Bézard E., Gaudreau P., Lombroso P.J., Brouillette J. The tyrosine phosphatase STEP is involved in age-related memory decline. Curr. Biol. 2018. 28 (7): 1079–1089.
Cattaneo A., Cattane N., Begni V., Pariante C.M., Riva M.A. The human BDNF gene: peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Translational Psychiatry. 2016. 6 (11): e958.
Cazorla M., Jouvenceau A., Rose C., Guilloux J.P., Pilon C., Dranovsky A., Premont J. Cyclotraxin-B, the first highly potent and selective TrkB inhibitor, has anxiolytic properties in mice. PLoS One. 2010. 5: e9777.
Chen E.S., Ernst C., Turecki G. The epigenetic effects of antidepressant treatment on human prefrontal cortex BDNF expression. Int. J. Neuropsychopharmacol. 2011. 14: 427–429.
Chen H., Xin G.Z.Y., Getachew H., Acosta Sierra C.S., Konofagou E.E., Ji R., Smith M., Niimi Y. Focused ultrasound-enhanced intranasal brain delivery of brain-derived neurotrophic factor. Scientific Reports. 2016. 6: 28599.
Chieffi S., Messina G., Villano I., Messina A., Valenzano A., Moscatelli F., Salerno M., Sullo A., Avola R., Monda V., Cibelli G., Monda M. Neuroprotective effects of physical activity: evidence from human and animal studies. Front. Neurol. 2017. 8:188.
Churilova A., Samoilov M. The effect of different modes of hypobaric hypoxia on the expression of transcription factor pCREB and pro-survival proteins BDNF and BCL-2 in rat neocortex and hippocampus. Springerplus. 2015. 4 (Suppl. 1): L27.
Cocco S., Podda M.V., Grassi C. Role of BDNF signaling in memory enhancement induced by transcranial direct current stimulation. Front. Neurosci. 2018. 12: 427.
Cook D.J., Nguyen C., Chun H.N., Llorente I.L., Chiu A.S., Machnicki M., Zarembinski T.I., Carmichael S.T. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow Metab. 2017. 37 (3):1030–1045.
Diniz B.S., Reynolds C.F. 3rd, Begley A., Dew M.A., Anderson S.J., Lotrich F., Erickson K.I., Lopez O., Aizenstein H., Sibille E.L., Butters M.A. Brain-derived neurotrophic factor levels in late-life depression and comorbid mild cognitive impairment: a longitudinal study. J. Psychiatr. Res. 2014. 49: 96–101.
Esvald E.E., Tuvikene J., Sirp A., XPatil S., Bramham C.R., Timmusk T. CREB family transcription factors are major mediators of BDNF transcriptional autoregulation in cortical neurons. J. Neurosci. 2020. 40 (7): 1405–1426.
Fletcher J.M., Hughes R.A. Novel monocyclic and bicyclic loop mimetics of brain-derived neurotrophic factor. J. Pept. Sci. 2006. 12: 515–524.
Friedman W.J. Proneurotrophins, seizures, and neuronal apoptosis. Neurosci. 2010. 16: 244–252.
Fuchikami M., Morinobu S., Kurata A., Yamamoto S., Yamawaki S. Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus. Int. J. Neuropsychopharmacol. 2009. 12 (1): 73–82.
Gudasheva T.A., Tarasiuk A.V., Sazonova N.M., Povarnina P.Yu., Antipova T.A., Seredenin S.B. A novel dimeric dipeptide mimetic of the BDNF selectively activates the MAPK-Erk signaling pathway. Dokl. Biochem. Biophys. 2017. 476: 291–295.
Gudasheva T.A., Konstantinopolsky M.A., Tarasiuk A.V., Kolik L.G., Seredenin S.B. Dipeptide mimetic of the BDNF loop 4 possesses analgetic activity. Dokl. Biochem. Biophys. 2019. 485: 123–125.
Gupta V.K., You Y., Gupta V.B., Klistorner A., Graham S.L. TrkB receptor signaling: implications in neurodegenerative, psychiatric and proliferative disorders. Int. J. Mol. Sci. 2013. 14 (5): 10122–10142.
He X.P., Pan E., Sciarretta C., Minichiello L., McNamara J.O. Disruption of TrkB-Mediated Phospholipase Cγ Signaling Inhibits Limbic Epileptogenesis. J. Neurosci. 2010. 30 (18): 6188–6196.
He J., Xiang Z., Zhu X., Ai Z., Shen J., Huang T., Liu L., Ji W., Li T. Neuroprotective effects of 7, 8-dihydroxyflavone on midbrain dopaminergic neurons in MPP+-treated monkeys. Sci. Rep. 2016. 12 (6): 34339.
Hempstead B.L. Brain-derived neurotrophic factor: three ligands, many actions. Trans. Am. Clin. Assoc. 2015. 126: 9–19.
Ho R., Minturn J.E., Simpson A.M., Iyer R., Light J.E., Evans A.E., Brodeur G.M. The effect of P75 on Trk receptors in neuroblastomas. Cancer Lett. 305: 76–85. 2011.
Hofer M., Pagliusi S.R., Hohn A., Leibrock J., Barde Y.A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990. 9: 2459–2464.
Hong E.J., McCord A.E., Greenberg M.E. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008. 60: 610–624.
Jang S.W., Liu X., Chan C.B., France S.A., Sayeed I., Tang W., Lin X., Xiao G., Andero R., Chang O., Ressler K.J., Ye K. Deoxygedunin, a natural product with potent neurotrophic activity in mice. PLoS One. 2010. 5 (7): e11528.
Jethwa N., Chung G.H., Lete M.G., Alonso A., Byrne R.D., Calleja V., Larijani B. Endomembrane PtdIns(3,4,5)P3 activates the PI3K-Akt pathway. J. Cell Sci. 2015. 128 (18): 3456–3465.
Ji R., Smith M., Niimi Y., Karakatsani M.E., Murillo M.F., Jackson-Lewis V., Przedborski S., Konofagou E.E. Focused ultrasound enhanced intranasal delivery of brain derived neurotrophic factor produces neurorestorative effects in a Parkinson’s disease mouse model. Sci. Rep. 2019. 9 (1): 19402.
Keleshian V.L., Modi H.R., Rapoport S.I., Rao J.S. Aging is associated with altered inflammatory, arachidonic acid cascade, and synaptic markers, influenced by epigenetic modifications, in the human frontal cortex. J. Neurochem. 2013. 125: 63–73.
Knierim J.J., Lee I., Hargreaves E.L. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006. 16 (9): 755–764.
Kollen M., Stéphan A., Faivre-Bauman A., Loudes C., Sinet P.M., Alliot J., Billard J.M., Epelbaum J., Dutar P., Jouvenceau A. Preserved memory capacities in aged Lou/C/Jall rats. Neurobiol. Aging. 2010. 31(1): 129–142.
Kowianski P., Lietzau G., Czuba E., Wa’skow M., Steliga A., Mory’s J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol. Neurobiol. 2018. 38: 579–593.
Leal S.L., Yassa M.A. Neurocognitive Aging and the Hippocampus Across Species. Trends Neurosci. 2015. 38(12): 800–812.
Lee R., Kermani P., Teng K.K., Hempstead B.L. Regulation of cell survival by secreted proneurotrophins. Science. 2001. 294: 1945–1948.
Levada O.A., Troyan A.S. Major depressive disorder and accelerated aging from a peripheral IGF-1 overexpression perspective. Med. Hypotheses. 2020. 138:109610.
Lipton J.O., Sahin M. The Neurology of mTOR. Neuron. 2014. 84 (2): 275–291.
Luine V., Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neurosci. 2013. 239: 34–45.
Maynarda K.R., Hobbsa J.W., Sukumara M., Kardiana A.S., Jimeneza D.V., Schloesserb R.J., Martinowich K. Bdnf mRNA splice variants differentially impact CA1 and CA3 dendrite complexity and spine morphology in the hippocampus. Brain Struc.t Funct. 2017. 222 (7): 3295–3307.
Meinzer M., Lindenberg R., Antonenko D., Flaisch T., Flöel A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J. Neurosci. 2013. 33 (30): 12470–12478.
Meng L., Liu B., Ji R., Jiang X., Yan X., Xin Y. Targeting the BDNF/TrkB pathway for the treatment of tumors (Review). Oncol. Lett. 2019. 17: 2031–2039.
Miranda M., Morici J.F., Zanoni M.B., Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain front cell. Neurosci. 2019. 13: 363.
Moon H.Y., Becke A., Berron D., Becker B., Sah N., Benoni G., Janke E., Lubejko S.T., Greig N.H., Mattison J.A., Duzel E., van Praag H. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 2016. 24 (2): 332–340.
Moya-Alvarado G., Gonzalez A., Stuardo N., Bronfman F.C. Brain-Derived Neurotrophic Factor (BDNF) Regulates Rab5-Positive Early Endosomes in Hippocampal Neurons to Induce Dendritic Branching. Front. Cell Neurosci. 2018. 12: 493.
Nakajima S., Numakawa T., Adachi N., OoshimaY., Odaka H., Yoshimura A., Kunugi H. Self-amplified BDNF transcription is a regulatory system for synaptic maturation in cultured cortical neurons. Neurochem. Int. 2015. 91: 55–61.
Nagahara A.H., Wilson B.R., Ivasyk I., Kovacs I. MR-guided delivery of AAV2-BDNF into the entorhinal cortex of nonhuman primates. Gene Ther. 2018. 25 (2): 104–114.
Ngwenya L.B., Heyworth N.C., Shwe Y., Moore T.L., Rosene D.L. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front. Syst. Neurosci. 2015. 9: 102.
Numakawa T. Possible protective action of neurotrophic factors and natural compounds against common neurodegenerative diseases. Neural. Regen. Res. 2014. 9 (16): 1506–1508.
Nyberg L. Functional brain imaging of episodic memory decline in ageing. J. Intern. Med. 2017. 281 (1): 65–74.
Nykjaer A., Willnow T.E. Sortilin: a receptor to regulate neuronal viability and function. Trends Neurosci. 2012. 35 (4): 261–270.
Oh H., Lewis D.A., Sibille E. The Role of BDNF in Age-Dependent Changes of Excitatory and Inhibitory Synaptic Markers in the Human Prefrontal Cortex. Neuropsychopharmacology. 2016. 41 (13): 3080–3091.
O’Shea A., Cohen R.A., Porges E.C., Nissim N.R., Woods A.J. Cognitive Aging and the Hippocampus in Older Adults. Front. Aging Neurosci. 2016. 8: 298.
Pang P.T., Teng H.K., Zaitsev E., Woo N.T., Sakata K., Zhen Sh., Teng K.K., Yung W.-H., Hempstead B.L., Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004. 306: 487–491.
Parkhitko A.A., Favorova O.O., Khabibullin D.I., Anisimov V.N., Henske E.P. Kinase mTOR: regulation and role in maintenance of cellular homeostasis, tumor development, and aging. Biochemistry (Moscow). 2014. 79: 88–101.
Pattwell S.S., Bath K.G., Perez-Castro R., Lee F.S., Chao M.V., Ninan I. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J. Neurosci. 2012. 32 (7): 2410–2421.
Pearse R.N., Swendeman S.L., Li Y., Rafii D., Hempstead B.L. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood. 2005. 105: 4429–4436.
Robinson A.A., Abraham C.R., Rosene D.L. Candidate molecular pathways of white matter vulnerability in the brain of normal aging rhesus monkeys. Geroscience. 2018. 40 (1): 31–47.
Romanczyk T.B., Weickert C.S., Webster M.J., Herman M.M., Akil M., Kleinman J.E. Alterations in trkB mRNA in the human prefrontal cortex throughout the lifespan. Eur. J. Neurosci. 2002. 15 (2): 269–80.
Romero-Granados R., Fontán-Lozano A., Delgado-García J.M., Carrión A.M. From learning to forgetting: behavioral, circuitry, and molecular properties define the different functional states of the recognition memory trace. Hippocampus. 2010. 20 (5): 584–595.
Ruiz C.R., Shi J., Meffert M.K. Transcript specificity in BDNF-regulated protein synthesis. Neuropharmacology. 2014. 76: Pt C (0 0). 657–663.
Sambataro F., Murty V.P., Lemaitre H.S., Reed J.D., Das S., Goldberg T.E., Callicott J.H., Weinberger D.R., Mattay V.S. BDNF modulates normal human hippocampal ageing. Mol. Psychiatry. 2010. 15 (2): 116–118.
Samoilov M., Churilova A., Gluschenko T., Rybnikova E. Neocortical pCREB and BDNF expression under different modes of hypobaric hypoxia: role in brain hypoxic tolerance in rats. Acta Histochem. 2014. 116 (5): 949–957.
Sasi M., Vignoli B., Canossa M., Blum R. Neurobiology of local and intercellular BDNF signaling. Pflugers Arch. 2017. 469 (5–6): 593–610.
Shang Y., Wang X., Li F., Yin T., Zhang J., Zhang T. rTMS ameliorates prenatal stress-induced cognitive deficits in male-offspring rats associated with BDNF/TrkB signaling pathway. Neurorehabil. Neural Repair. 2019. 33 (4): 271–283.
Silhol M., Bonnichon V., Rage F., Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neurosci. 2005. 132 (3): 613–624.
Silhol M., Arancibia S., Perrin D., Maurice T., Alliot J., Tapia-Arancibia L. Effect of aging on brain-derived neurotrophic factor, proBDNF, and their receptors in the hippocampus of Lou/C rats. Rejuvenation Res. 2008. 11 (6): 1031–1040.
Sorrells S.F., Paredes M.F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K.W., James D., Mayer S., Chang J., Auguste K.I., Chang E.F., Gutierrez A.J., Kriegstein A.R., Mathern G.W., Oldham M.C., Huang E.J., Garcia-Verdugo J.M., Yang Z., Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018. 555 (7696): 377–381.
Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Boström E., Westerlund I., Vial C., Buchholz B.A., Possnert G., Mash D.C., Druid H., Frisén J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013. 153 (6): 1219–1227.
Timmusk T., Palm K., Metsis M., Reintam T., Paalme V., Saarma M., Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993. 10: 475–489.
Tongiorgi E. Activity-dependent expression of brain-derived neurotrophic factor in dendrites: facts and open questions. Neurosciю Res. 2008. 61: 335–346.
Tyler W.J., Alonso M., Bramham C.R., Pozzo-Miller L.D. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem. 2002. 9 (5): 224–237.
Vecchio L.M., Meng Y., Xhima K., Lipsman N., Hamani C. The neuroprotective effects of exercise: maintaining a healthy brain throughout aging. Brain. Plast. 2018. 4 (1): 17–52.
Voss M.W., Vivar C., Kramer A.F., van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013. 17 (10): H.525–244.
Wang J., Zhang S., Ma H. Yang S., Liu Z., Wu X., Wang S., Zhang Y., Liu Y. Chronic intermittent hypobaric hypoxia pretreatment ameliorates ischemia-induced cognitive dysfunction through activation of ERK1/2-CREB-BDNF pathway in anesthetized mice. Neurochem. Res. 2017. 42 (2): 501–512.
Wang J.Q., Mao L. The ERK pathway: molecular mechanisms and treatment of depression. Mol. Neurobiol. 2019. 56 (9): 6197–6205.
Webster M.J., Herman M.M., Kleinman J.E., Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr. Patterns. 2006. 6 (8): 941–951.
Woo N.H., Teng H.K., Siao C.J., Chiaruttini C., Pang P.T., Milner T.A., Hempstead B.L., Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005. 8: 1069–1077.
Zanin J.P., Montroull L.E., Volosin M., Friedman W.J. The p75 Neurotrophin Receptor Facilitates TrkB Signaling and Function in Rat Hippocampal Neurons. Front. Cell. Neurosci. 2019. 13: 485–495.
Zeng Y., Tan M., Kohyama J., Sneddon M., Watson J.B., Sun Yi.E., Xie C.-W. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging J. Neurosci. 2011. 31 (49): 17800–17810.
Zhu X.H., Yan H.C., Zhang J., Qu H.D., Qiu X.S., Chen L., Li S.J., Cao X., Bean J.C., Chen L.H., Qin X.H., Liu J.H., Bai X.C., Mei L., Gao T.M. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J. Neurosci. 2010. 30 (38): 12653–12663.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


