Журнал высшей нервной деятельности им. И.П. Павлова, 2021, T. 71, № 3, стр. 306-320
Серотониновая система в оогенезе млекопитающих
Ю. Б. Шмуклер 1, *, Н. М. Алешина 1, Л. А. Мальченко 1, Д. А. Никишин 1
1 ФГБУН Институт биологии развития им. Н.К. Кольцова РАН
Москва, Россия
* E-mail: yurishmukler@yahoo.com
Поступила в редакцию 15.12.2020
После доработки 16.02.2021
Принята к публикации 02.03.2021
Аннотация
Трансмиттеры, в частности серотонин, наряду с классической функцией передачи нервного импульса участвуют в целом ряде регуляторных процессов на протяжении всего онтогенеза, в том числе реализующихся задолго до формирования нервной системы. Рассмотрены структура и функции серотониновой системы в оогенезе млекопитающих, источники и механизмы аккумулирования трансмиттера в ооцитах, а также экспрессия компонентов серотониновой системы – рецепторов, ферментов синтеза и деградации, а также мембранного и везикулярного транспортеров в клетках женской репродуктивной системы млекопитающих. Приведены данные о влиянии блокаторов обратного захвата серотонина (SERT) на оогенез и эмбриогенез.
СЕРОТОНИН В ОНТОГЕНЕЗЕ
Опыт Отто Леви (Loewi, 1921), поставленный столетие назад, открыл собой эпоху исследований химических механизмов передачи межклеточных сигналов. В силу огромного теоретического и практического значения эти работы невероятно размножились и привели не только к расцвету теории химической передачи нервных импульсов, но и к бесчисленным прикладным результатам в области нейрофизиологии. Тот факт, что функции многих трансмиттеров не ограничиваются нервной системой взрослых животных (см. Amireault et al., 2013), привел в начале 60-х годов ХХ столетия и к первым предположениям о смене функций этих веществ в ходе индивидуального развития, в том числе – в эмбриогенезе (Бузников, Манухин, 1960; Коштоянц, 1963; Бузников, 1967, 2007).
Идея об эмбриональных функциях трансмиттеров проделала эволюцию от смелого предположения, встретившего у многих физиологов непонимание и отторжение, к представлению о совершенно специфическом механизме, радикально отличающемся от имеющего место во взрослом организме, и, наконец, к постепенному пониманию принципиального совпадения основных звеньев эмбрионального и дефинитивного трансмиттерных процессов.
К настоящему времени накоплены данные, свидетельствующие о том, что моноаминергические трансмиттеры являются универсальными сигнальными молекулами, контролирующими многообразные процессы развития. Серотонин выполняет наибольшее число таких функций в течение всего онтогенеза – как на эмбриональных стадиях, так и вне нервной системы взрослых организмов.
В раннем развитии это, прежде всего, различные аспекты контроля серотониновой системой клеточного цикла в период делений дробления (термин “серотониновая” использован для отличия этой эмбриональной системы от серотонинергического процесса в нервных клетках взрослых организмов) – запуск клеточного цикла (Бузников, 1987; Buznikov et al., 2005) и контроль состояния цитоскелета (Григорьев, 1988). Далее, серотонинергические препараты влияют на ряд процессов межбластомерных взаимодействий – адгезию бластомеров (Бузников, Шмуклер, 1978) и собственно обмен между бластомерами химическими сигналами (Шмуклер, 1981; Shmukler, Nikishin, 2012). На основе этих данных было впервые выдвинуто представление о “протосинапсе” – механизме межбластомерного взаимодействия, представляющего собой симметричную структуру, в которой каждая из двух взаимодействующих эмбриональных клеток является как источником, так и акцептором химического сигнала (Shmukler, 1993; Shmukler, Nikishin, 2012). Эта концепция дает представление о возможной эволюции механизма межклеточных взаимодействий в развитии, поскольку демонстрирует первое в онтогенезе взаимодействие клеток с участием трансмиттеров, которое с дальнейшей специализацией клеток может преобразовываться в синаптическое.
В дальнейшем развитии трансмиттеры контролируют формирование ресничной моторики (Katow et al., 2007), морфогенетические процессы (Мартынова, 1981; Shuey et al., 1993), динамику развития и формирование программ поведения потомства (Voronezhskaya et al., 2004; Ivashkin et al., 2015), установление лево-правой асимметрии тела (Levin et al., 2006; Beyer et al., 2012), а также дифференцировку и пролиферацию клеток (Azmitia, 2001), вплоть до органо- (Lauder et al., 1994) и онкогенеза (Peters et al., 2014; Sarrouilhe, Mesnil, 2019). Функция же передачи нервного сигнала оказывается лишь одной из множества, возникающей к тому же в развитии далеко не первой.
Эмбриофизиологические исследования на методическом уровне 70–80-х годов ХХ столетия (без современных молекулярно-биологических данных об экспрессии трансмиттерных рецепторов и сведений о специфичности нейрофармакологических препаратов) показали, что эффекты трансмиттеров в раннем эмбриогенезе реализуются, вероятнее всего, путем их взаимодействия с соответствующими рецепторами или, как осторожно выражался основатель исследований эмбриональных функций трансмиттеров профессор Бузников (Бузников, 1987, 2007), их функциональными аналогами. Это, в свою очередь, приводит к активации цепей передачи внутриклеточных сигналов с участием аденилатциклазы и протеинкиназы С, подобно тому, как это происходит в клетках зрелого организма (Шмуклер и др., 1984; Ростомян и др., 1985; Capasso et al., 1988; Buznikov et al., 1998; Shmukler et al., 1999).
В последние десятилетия прогресс в изучении трансмиттерных механизмов наиболее ранних стадий онтогенеза – оогенеза и раннего эмбриогенеза – связан с новыми возможностями, которые дают молекулярно-биологические методы исследования. Это привело к переходу от подхода, основанного на аналогиях фармакологических эффектов на разных объектах, к исследованию экспрессии трансмиттерных механизмов в развитии. В частности, была исчерпывающим образом исследована экспрессия компонентов серотониновой системы в раннем эмбриональном развитии амфибий (Nikishin et al., 2012; Никишин и др., 2012; Tan et al., 2013; Collart et al., 2014; Session et al., 2016; Owens et al., 1016) и птиц (Stępińska et al., 2015). Вопрос о наличии на этом этапе серотониновых рецепторов, идентичных таковым взрослых организмов, решен однозначно и положительно, по крайней мере, на уровне экспрессии соответствующих мРНК. Более того, результаты этих исследований дают основание предполагать, что у дробящихся зародышей могут одновременно экспрессироваться сразу несколько типов серотониновых рецепторов, а также, наряду с ними, соответствующие ферменты синтеза и деградации, а также транспортеры, в том числе SERT. Сходные данные об экспрессии набора компонентов серотониновой системы, в том числе рецепторов, были получены и на доимплантационных стадиях развития млекопитающих (Vesela et al., 2003; Iľkova et al., 2004; Amireault, Dube, 2005a, b; Hinckley et al., 2005; Basu et al., 2008; Никишин и др., 2018б). Впрочем, большая часть этих данных ожидает подтверждения экспрессии в раннем эмбриогенезе соответствующих белков, а также подтверждения их функциональной активности.
СЕРОТОНИН И ЕГО СИНТЕЗ В ООГЕНЕЗЕ МЛЕКОПИТАЮЩИХ
Многие факторы, определяющие паттерн и параметры течения раннего развития большинства животных, закладываются в ходе предшествующего процесса оогенеза. Поэтому отдельным принципиально важным вопросом является то, насколько трансмиттерные системы активны в ходе созревания яйцеклеток и как это влияет на последующее развитие. Данные об участии серотониновой системы в оогенезе менее обильны и более разрозненны, чем об их функции в раннем эмбриогенезе. Тем не менее на целом ряде таксонов было показано, в основном – с помощью фармакологических воздействий, участие различающихся между собой у разных видов серотониновых механизмов в созревании ооцитов у амфибий (Никитина и др., 1988,1993; Buznikov et al., 1993; Sheng et al., 2005) и иглокожих (Бузников и др., 1990; Buznikov et al., 1993; Chaiyamoon et al., 2018). Затем сходные данные были получены на двустворчатых (Krantic et al., 1991; Masseau et al., 2002; Wang, He, 2014) и головоногих моллюсках (Zatylny et al., 2000), ракообразных (Tinikul et al., 2008), немертинах (Stricker, Smythe, 2001), рыбах (Cerdà et al., 1998; Lister et al., 2009) и млекопитающих (Terranova et al., 1990; Tanaka et al., 1993; Sheng et al., 2005). Кроме того, серотонин влияет на функциональную активность фолликулярных клеток, которые не только играют важную роль в процессе созревания яйцеклеток, но также являются основным источником эстрогена в теле самки (Terranova et al., 1990; Tanaka et al., 1993; Koppan et al., 2004; Graveleau et al., 2000). На различных моделях показано, что серотонин обладает стимулирующим действием на выброс половых продуктов (Masseau et al., 2002; Garnerot et al., 2006). Такая универсальность серотониновой регуляции процессов, связанных с созреванием яйцеклеток, у многочисленных и эволюционно далеко отстоящих друг от друга таксонов свидетельствует о ее фундаментальном значении для оогенеза.
Особая сложность анализа серотониновой системы в оогенезе млекопитающих обусловлена многокомпонентностью клеточного комплекса, обеспечивающего формирование фолликула, содержащего ооцит, а также динамичностью происходящих в нем процессов. Основным соматическим компонентом, опосредующим взаимодействие материнского организма с созревающим ооцитом, является гранулеза. Ее клеточная субпопуляция – кумулюс – непосредственно окружает ооцит, в том числе в ходе овуляции и при оплодотворении. Клетки кумулюса непосредственно взаимодействуют с ооцитом через щелевые контакты (Lawrence et al., 1978) и передают стимулы, обеспечивающие поддержание (Aktas et al., 1995) или устранение (Batta, Knudsen, 1980) блока мейоза. От них также зависит метаболизм глюкозы и пирувата в ооците (Gardner et al., 1996; Preis et al., 2005). На месте овулировавшего фолликула образуется желтое тело, которое является временной железой внутренней секреции. Конкретные особенности серотониновых механизмов этих компонентов системы оогенеза различаются.
Серотонин в физиологических концентрациях выявлен в ооцит-кумулюсных комплексах самок млекопитающих (Amireault, Dubé, 2005a,b; Dubé, Amireault, 2007). Концентрация серотонина в яичнике крысы меняется в ходе репродуктивного цикла (Clausell, Soliman, 1978), он также присутствует в фолликулярной жидкости человека (Bòdis et al., 1992, 1993), зрелых ооцитах и клетках кумулюса мышей (Il’kova et al., 2004; Amireault and Dubé, 2005a; Dubé, Amireault, 2007).
Недавно иммуногистохимическим окрашиванием криосрезов яичника мыши антителами против серотонина было показано, что этот трансмиттер локализуется в овариальных фолликулах – как в ооцитах, так и в окружающих его клетках гранулезы и теки. Предварительное введение серотонина приводит к увеличению степени иммунореактивности, но при сохранении локализации реакции неизменной (Никишин и др., 2017а).
Вопрос о происхождении выявляемого в яичнике серотонина некоторое время был дискуссионным, тем более что он очень важен для понимания механизмов серотониновой регуляции репродуктивной функции. Первоначально предполагалось, что в яичнике может присутствовать собственная система синтеза серотонина, независимая в этом плане от остального организма. Действительно, в клетках кумулюса была показана экспрессия мРНК фермента биосинтеза серотонина – триптофангидроксилазы Tph1 (Dube, Amireault, 2007), а в зрелых ооцитах, как и в доимплантационных эмбрионах (Basu et al., 2008), – Tph2. Таким образом, TPH2, которая долгое время считалась нейтральной формой фермента, может служить маркером половой линии в яичнике, так как не экспрессируется в окружающих ооцит фолликулярных клетках. Наличие мРНК триптофангидроксилаз в клетках гранулезы и ооцитах мыши подтверждается нашими данными (Nikishin et al., 2019).
Первая стадия биосинтеза серотонина – гидроксилирование – лимитирует скорость процесса (Jequier et al., 1969), поэтому наличие триптофангидроксилазы может ошибочно рассматриваться как достаточное условие синтеза серотонина в ткани (Dubé, Amireault, 2007), так как второй фермент синтеза – декарбоксилаза ароматических L-аминокислот, DDC, часто считается вездесущим и экспрессирующимся постоянно. Тем не менее DDC экпрессируется не во всех типах клеток и, таким образом, лимитирующим звеном в системе синтеза серотонина может быть именно этот фермент (Никишин и др., 2018б).
В компонентах стромы яичника и в ооцитах примордиальных фолликулов DDC выявляется иммуногистохимически. В ходе постнатального развития ферменты синтеза серотонина в яичниках демонстрируют тенденцию к снижению уровня экспрессии. Максимальный уровень экспрессии DDC наблюдается у новорожденных мышат, когда завершается формирование пула примордиальных фолликулов (Niu et al., 2016), а минимальный – в возрасте 14 дней, когда происходит первая волна фолликулогенеза (Nikishin et al., 2019).
Согласно данным количественной ПЦР DDC экспрессируется в овариальных фолликулах на очень низком уровне, а эксперимент по инкубации фрагментов яичника с предшественником серотонина гидрокситриптофаном не выявил выраженной активности этого фермента в примордиальных или растущих овариальных фолликулах. Соответственно, можно заключить, что синтез не может быть существенным источником серотонина в клетках гранулезы и зрелых ооцитах млекопитающих в постнатальном яичнике (Nikishin et al., 2019). К аналогичному выводу об экзогенном происхождении серотонина, накапливающегося в яйцеклетках и функционально активного на ранних стадиях эмбрионального развития, пришли и другие авторы (Côté et al., 2007).
Исследование дальнейшей динамики экспрессии мРНК ферментов синтеза серотонина в ооцитах и доимплантационных эмбрионах показало, что триптофангидроксилаза Tph2, экспрессирующаяся в ооцитах, исчезает к стадии делений дробления, тогда как декарбоксилаза Ddc появляется в эмбриогенезе только на стадии бластоцисты. В желтом теле, в отличие от других компонентов яичника, синтез серотонина возможен, поскольку здесь выявляется экспрессия и Tph1, и Ddc, однако прямых данных, подтверждающих это, нет (Никишин и др., 2018б).
Поскольку возможность локального синтеза серотонина в яичнике предельно ограничена, напрашивается вывод, что он может аккумулироваться здесь из внешних источников, которыми могут служить тромбоциты кровяного русла, тучные клетки, локализующиеся в строме яичника, а также немногочисленные нервные волокна, сопровождающие крупные медуллярные сосуды (Amenta et al., 1992). При этом накопление серотонина в ооцитах и окружающих его фолликулярных клетках, вероятнее всего, происходит за счет мембранного транспорта трансмиттера из внеклеточной среды.
РЕЦЕПТОРЫ К СЕРОТОНИНУ В ООГЕНЕЗЕ
Ключевое звено любого процесса с участием серотонина – это его рецепторы, которых насчитывается 7 типов, подразделенных на 15 подтипов (см., напр., Hoyer et al., 1994; Frazer, Hensler, 1999). За исключением 5НТ3- рецептора (канального), остальные являются семидоменными метаботропными рецепторами, связанными либо с аденилатциклазным, либо фосфатидил-инозитольным каскадом внутриклеточной сигнализации. Как уже говорилось выше, само по себе присутствие рецепторов к трансмиттерам в ооцитах и ранних эмбрионах (на донервных стадиях развития) было предметом больших сомнений и дискуссий, поскольку фармакологические эксперименты, в том числе по связыванию меченых лигандов рецепторов, давали результаты, существенно отличающиеся от получаемых на классических объектах (Shmukler et al., 1986; Шмуклер, 1992; см. Бузников, 1987).
Ситуация резко изменилась с применением в этой области молекулярно-биологических методов, которые, начиная с конца ХХ столетия, стали приносить сведения об экспрессии различных трансмиттерных рецепторов в ранних эмбрионах многих видов животных, в том числе млекопитающих. Со временем количество таких данных только нарастало, они систематизировались и ныне на многих видах дают исчерпывающую картину экспрессии рецепторных компонентов различных трансмиттеров, в частности – серотонина у птиц (Stępińska et al., 2015) и земноводных (Nikishin et al., 2012; см. также обзор Шмуклер, Никишин, 2018). Подробные исследования проведены и в оогенезе, и на ранних эмбрионах млекопитающих (Никишин и др., 2018б) (табл. 1).
Таблица 1.
Первые публикации об экспрессии рецепторов к трансмиттерам в эмбрионах на стадиях делений дробления (по Nikishin et al., 2012 с исправлениями) Table 1. Initial publications on the expression of transmitters’ receptors in cleaving embryos (after Nikishin et al., 2012 with corrections)
| Вид | Тип рецептора | Источник |
|---|---|---|
| Мышь Mus musculus | HTR1D | Vesela et al., 2003 |
| HTR5 | Hinckley et al., 2005 | |
| HTR7 | Amireault, Dube, 2005b | |
| β-AdR | Čikoš et al., 2005 | |
| Нематода Caenorhabditis elegans | HTR2C | Hamdan et al., 1999 |
| Брюхоногий моллюск Lymnaea stagnalis | HTR2 | Ivashkin et al., 2015 |
| Рыба Danio rerio | HTR1A | Никишин и др., 2009 |
| Морской еж Paracentrotus lividus | HTR4 | Никишин и др., 2012 |
| nAChR α6-субъединица | Aluigi et al., 2012 | |
| nAChR α10-субъединица | ||
| nAChR α7-субъединица | ||
| Шпорцевая лягушка Xenopus laevis | HTR2C | Nikishin et al., 2012 |
| HTR7 | ||
| β-AdR | Devic et al., 1997 |
Первые данные в этой области были получены десятилетием ранее с помощью ОТ-ПЦР: было показано, что 5-HT7-рецептор экспрессируется не только в 4-клеточных эмбрионах, но и в клетках кумулюса и ооцитах, а мРНК 5-HT2A- и 5-HT2B-рецепторов – только в клетках кумулюса, тогда как экспрессия 5-HT2C-, 5-HT4- и 5-HT6-рецепторов отсутствовала во всех этих объектах (Amireault, Dubé, 2005a). Нами было проведено более подробное исследование экспрессии всех основных компонентов серотониновой сигнальной системы в фолликулярных клетках, полученных на разных стадиях фолликулогенеза (гранулеза стенки антрального фолликула, клетки пост-овуляторного кумулюса и желтое тело), а также в зрелых MII-ооцитах мыши (Никишин и др., 2018б). Было показано, что в ооцитах выявляется экспрессия мРНК трех серотониновых рецепторов, причем Htr7 и канальный Htr3a исчезают к дроблению, тогда как Htr5b экспрессируется в течение всего доимплантационного развития. Стоит отметить совпадение времени транзиторной экспрессии редко встречающегося в оогенезе и раннем эмбриогенезе рецептора серотонина 3-го типа в ооцитах и перепелки (Stępińska et al., 2015), и мыши, постоянная экспрессия которого у обоих видов затем наблюдается значительно позже. Такое явление иллюстрирует наблюдающуюся в доимплантационном развитии млекопитающих временную избыточность и стохастическую экспрессию разных генов в клетках раннего эмбриона (Dietrich, Hiiragi, 2007). Оговоримся здесь, что выявление экспрессии мРНК еще не означает присутствие соответствующего белка, и вопрос о функциональной активности этих рецепторов требует специального исследования.
Профили экспрессии ряда генов в клетках стенки фолликула и кумулюсе различаются (Burnik-Papler et al., 2015), но это не касается рецепторов к серотонину, за исключением рецептора Htr7, мРНК которого присутствует в клетках кумулюса, а затем в ооците (Никишин и др., 2018б). От ооцитов клетки гранулезы стенки антрального фолликула, клетки кумулюса и желтого тела отличаются наличием экспрессии мРНК рецепторов Htr1b, Htr1d и Htr2a. В желтом теле также экспрессируется рецептор Hhtr2b (Никишин и др., 2018б).
Важным показателем функциональной активности гена является динамика его экспрессии. Выраженные изменения уровня экспрессии гена могут свидетельствовать о его функциональной активности на той или иной стадии. Часть генов, такие как Htr1d, Htr2a, Htr2b, в ходе фолликулярного роста выраженной динамики не проявляют. В то же время относительное количество мРНК генов Htr1b и Htr7 уменьшается в ходе фолликулогенеза. В желтых телах, по сравнению с клетками гранулезы преовуляторного фолликула, для гена Htr1b наблюдается увеличение относительной экспрессии, как и в случае классических маркеров лютеинизации. Количество мРНК генов Htr7 при лютеинизации уменьшается (Никишин и др., 2017б).
Данные об одновременной экспрессии мРНК серотониновых рецепторов нескольких типов в фолликулогенезе и раннем эмбриогенезе млекопитающих и аналогичные данные на зародышах амфибий и птиц (Nikishin et al., 2012; Stępińska et al., 2015) возвращают к высказанной нами ранее идее о том, что к одному и тому же трансмиттеру в единичной эмбриональной клетке или ооците присутствуют рецепторы более чем одного типа, способные одновременно активировать разные сигнальные пути и, таким образом, реализовывать различные функции (Шмуклер, Никишин, 2018).
СЕРОТОНИНОВЫЕ СИГНАЛЬНЫЕ ПУТИ В ООГЕНЕЗЕ
Роль вторичных мессенджеров (прежде всего, цАМФ и внутриклеточного Ca2+) в росте фолликула и мейотическом созревании ооцита известна и исследуется достаточно давно (Cho et al., 1974; Dekel, Beers, 1978; Darszon et al., 1999; Stricker, 1999; см. также обзор Silvestre et al., 2011). Активация систем вторичных мессенджеров трансмиттерами и конкретно серотонином в оогенезе млекопитающих была впервые исследована на ооцитах и окружающих клетках кумулюса мыши. Экзогенный серотонин в физиологических концентрациях не вызывал изменений уровня внутриклеточного цАМФ или Ca2+ в изолированных ооцитах в метафазе II, тогда как такое воздействие вызывало дозозависимое повышение уровня цАМФ и Ca2+ в клетках кумулюса. Предполагалось, что этот эффект обусловлен активацией 5-HT7-рецептора. С другой стороны, при добавлении серотонина в клетках кумулюса наблюдалось дозозависимое повышение Ca2+, что могло быть связано с активацией 5-HT2A- и 5-HT2B-рецепторов, экспрессия мРНК которых была выявлена в клетках кумулюса (Amireault, Dubé, 2005a).
Воздействуя через такие стандартные цепи внутриклеточной передачи сигнала, серотонин способен вызывать ряд специфических эффектов в клетках женской репродуктивной системы. На клетках гранулезы было выявлено три гена, которые изменяют уровень экспрессии в клетках гранулезы в ответ на серотонин: Has2, Ihh и Ccnd1 (Никишин и др., 2018в). Has2 является ферментом синтеза гиалуроновой кислоты, характерного для клеток гранулезы (Salustri et al., 1992). Экспрессия этого гена коррелирует с синдромом поликистозного яичника (Kaur et al., 2012). Ihh является сигнальной молекулой Hedgehog-сигнального пути, экспрессия которой характерна для клеток гранулезы овариальных фолликулов на ранних стадиях фолликулогенеза (Liu et al., 2015). Третьим геном, экспрессия которого усиливается в ответ на серотонин, является один из регуляторов клеточного цикла циклин D1 Ccnd1, экспрессия которого запускает переход от G1 к S-фазе (Yang et al., 2006). По всей вероятности, серотонин через воздействие на экспрессию циклина D1 модулирует эффекты других факторов, активирующих пролиферацию клеток гранулезы, и способствует поддержанию активно пролиферирующего состояния. В культуре изолированных овариальных фолликулов наблюдается более выраженный эффект серотонина, что, по всей вероятности, объясняется большей восприимчивостью клеток гранулезы к воздействию серотонина в условиях, более приближенных к нативному состоянию. Ооциты теснейшим образом взаимосвязаны с фолликулярными клетками и регулируют функциональное состояние друг друга (Kidder, Vanderhyden, 2010; Никишин и др., 2021). Логично предполагать, что усиление влияния серотонина на функциональную активность клеток гранулезы связано с дополнительным воздействием ооцитов. В пользу этого предположения говорит подавление наблюдаемых эффектов при ингибировании обратного захвата серотонина, который активен в ооцитах, но не в клетках гранулезы (Никишин и др., 2021).
Наконец, на преовуляторных фолликулах крысы, культивируемых in vitro, было показано, что серотонин стимулирует синтез эстрадиола, а специфические антагонисты серотониновых рецепторов 2-го типа устраняют этот эффект (Tanaka et al., 1993). Стимулирующая активность серотониновых рецепторов 1-го типа на этот процесс была продемонстрирована на хомячках (Terranova et al., 1990), а в аналогичных экспериментах стимулирующий эффект серотонина наблюдается на клетках гранулезы человека (Koppan et al., 2004; Graveleau et al., 2000). При этом в наших экспериментах не было обнаружено воздействия серотонина на экспрессию ферментов и регуляторов стероидогенеза в клетках гранулезы. Вероятно это, как и отсутствие прямого эффекта серотонина на пролиферацию, обусловлено тем, что эффекты серотонина на клетки гранулезы носят лишь модуляторный, а не триггерный характер, и обусловлены не прямым влиянием этого трансмиттера, а опять-таки опосредованы другими клеточными компонентами яичника, вероятнее всего, ооцитом (Никишин и др., 2018в). Можно предположить, что ооцит способен экскретировать накопленный серотонин и создавать вокруг себя локальную область его повышенной концентрации, усиливая таким образом его воздействие на клетки гранулезы (Никишин и др., 2018б, 2021).
МЕМБРАННЫЙ ТРАНСПОРТ (ЗАХВАТ) СЕРОТОНИНА В ООГЕНЕЗЕ
Мембранный транспорт серотонина обнаружили и стали изучать с конца 60-х годов ХХ столетия на тромбоцитах (Sneddon, 1969; Rudnick, Nelson, 1978), которые также предположили, что в нейронах имеет место идентичный процесс, что и подтвердилось впоследствии (Giannaccini et al., 2010; Hohmann et al., 2011; см. также обзор Rudnick, Sandtner, 2019).
В индивидуальном развитии мыши выраженный пик экспрессии мРНК SERT в яичнике выявляется на 14-й день после рождения, а иммуногистохимически SERT детектируется во всех клеточных компартментах яичника с максимальным уровнем иммуноокрашивания в ооцитах растущих овариальных фолликулов (Nikishin et al., 2019). На стадии первичного многослойного фолликула, наступающей после выхода примордиального фолликула из состояния покоя (блока мейоза), во время которой происходит реактивация оогенеза, подготовка к делениям созревания и активный рост яйцеклетки и всего фолликула, мРНК Sert экспрессируется и в фолликулярных клетках, и в ооцитах.
Однако при инкубации овариальных фолликулов с серотонином его накопление наблюдалось только в ооцитах. Следовательно, в клетках гранулезы отсутствует не только синтез, но и специфический захват серотонина. Было сделано предположение, что связанная с мембранным транспортом регуляция серотонином функциональной активности фолликулярных клеток может происходить опосредованно через ооцит (Никишин и др., 2018а). То есть серотонин может модулировать синтез и выделение ооцитом факторов, влияющих на окружающие фолликулярные клетки, что образует обратную связь с кумулюсом, который, как указывалось выше, контролирует ооцит (Никишин и др., 2021) (рис. 1).
Рис. 1.
Схема прямого (А) и опосредованного ооцитом (Б) влияния серотонина (5HT) на функциональную активность клеток гранулезы. (А) – Серотонин повышает экспрессию мРНК циклинов Ccnd1, Ccnd2 и Ccne1 в первичной культуре клеток гранулезы. Эффект не отменяется флуоксетином и, предположительно, опосредован рецепторами серотонина, экспрессирующимися в клетках гранулезы (HTR1B, HTR1D, HTR2A, HTR5 или HTR7 – согласно Никишин и др., 2018). (Б) – В культуре изолированных овариальных фолликулов серотонин повышает экспрессию мРНК генов Has2, Ptgs2, Ptgfr, Ihh и Igfbp4 в клетках гранулезы. Эффект отменяется флуоксетином. Предполагается, что эффект опосредован ооцитарными факторами роста, так как серотонин накапливается в ооцитах и повышает в них экспрессию мРНК Gdf9 (Никишин и др., 2021).
Fig. 1. The scheme of direct (a) and oocyte mediated (б) serotonin influence on the functional activity of granulose cells. (a) – Serotonin enhances the expression of Ccnd1, Ccnd2 and Ccne1 cyclins’ mRNAs in the primary granulose cells culture. Fluoxetine do not eliminate this effect that is presumably mediated by serotonin receptors expressed in granulose cells (HTR1B, HTR1D, HTR2A, HTR5 or HTR7 – after Nikishin et al., 2018). (б) – Serotonin enhances the expression of mRNAs of Has2, Ptgs2, Ptgfr, Ihh, and Igfbp4 in the granulose cells in the culture of isolated ovatial follicles. Fluoxetine eliminates this effect. It is suggested that serotonin is accumulated in the oocytes and it evokes the enhance of Gdf9 mRNA expression there.
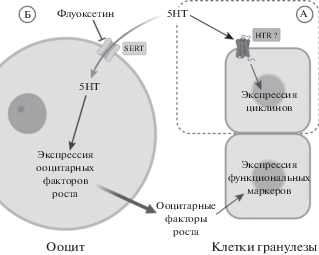
Экспрессия и активность мембранного транспортера серотонина SERT далее продолжаются практически в ходе всего оогенеза и раннего эмбриогенеза – в ооцитах и на всех стадиях доимплантационного развития эмбрионов, но исчезают к стадии бластоцисты (Никишин и др., 2018б). На этих стадиях развития выявлен и белок SERT, а также его функциональная активность (Amireault, Dubé, 2005b; Basu et al., 2008). Как и в ооцитах, экспрессия и активность Sert показаны и в клетках кумулюса (Dubé, Amireault, 2007). Согласно нашим данным, мРНК гена Sert экспрессировалась также и в клетках гранулезы (Никишин и др., 2018а). Кроме того, в ней выявляется мРНК везикулярного транспортера Vmat2, а в желтом теле экспрессируются и Sert, и оба типа везикулярных транспортеров моноаминов, что может быть связано с интенсивной секреторной активностью лютеоцитов.
Таким образом, в отсутствие локального синтеза серотонина его захват специфическим мембранным транспортером SERT, вероятно, является основным механизмом аккумулирования серотонина в яичниках мыши.
БЛОКАТОРЫ ОБРАТНОГО ЗАХВАТА СЕРОТОНИНА – ЭФФЕКТЫ В ЭМБРИОГЕНЕЗЕ И ООГЕНЕЗЕ
Из изложенного выше ясно, что процесс обратного захвата серотонина, осуществляющийся транспортером SERT, критически важен для взаимодействия ооцита и окружающих его соматических клеток. Этот факт приобрел в последние десятилетия особое значение в связи с тем, что блокаторы этого белка-транспортера оказались эффективными средствами в терапии депрессивных и тревожных состояний, в частности, у беременных, которые, как выяснилось, могут подавляться специфическими блокаторами этого транспортера (Misri, Kendrick, 2007; Ram and Gandotra, 2015; Muzik, Hamilton, 2016).
Вопрос о потенциальных тератогенных эффектах трициклических антидепрессантов у человека впервые возник во времена известной “талидомидной трагедии”. В конце 50-х – начале 60-х годовХХ столетия произошла многотысячная вспышка рождений младенцев с разной степенью недоразвития конечностей и другими дефектами. Быстро выяснилось, что все их матери в период беременности получали седативно-снотворный трициклический препарат талидомид, прошедший принятые в те времена процедуры проверки безопасности. Это послужило причиной для введения более углубленных испытаний новых препаратов, в том числе – расширения круга модельных животных, в частности хомячков (Homburger et al., 1965). Также при действии талидомида драматические нарушения эмбрионального скелета, отсутствовавшие на стандартных для того времени тест-объектах, были обнаружены на стадиях призмы и плутеуса в развитии морских ежей (Hagström, Lönning, 1973), что выгодно подчеркнуло пригодность этого объекта для первичного выявления потенциально тератогенных препаратов.
Тератогенные эффекты трициклического антидепрессанта имипрамина были на “талидомидной” волне таких исследований обнаружены у кроликов (Robson, Sullivan, 1964). Впоследствии и на зародышах морских ежей была обнаружена способность мелипрамина (русское название имипрамина) блокировать деления дробления (Бузников, 1967; Nikishin et al., 2016) и эффективно подавлять межбластомерные взаимодействия (Шмуклер, 1981; Shmukler, Nikishin, 2012). Однако в то время и позднее на основании разрозненных данных был сделан вывод, что достаточные основания считать трициклические антидепрессанты тератогенами отсутствуют. В дальнейшем исследования, сравнивавшие эффекты трициклических антидепрессантов с новейшими блокаторами обратного захвата серотонина, не выявляли повышенной тератогенности, а обнаруживали лишь кратковременные преходящие симптомы у новорожденных (Misri et al., 2006). Однако позднее отмечалось, что при применении некоторых препаратов этой категории в ходе беременности выявлялся повышенный риск неблагоприятных последствий для человека (см. обзор Creeley and Denton, 2019).
Одно из немногочисленных исследований воздействия наиболее назначаемого препарата этой категории – флуоксетина – непосредственно на эмбрион человека показало отсутствие его влияния в малых дозах на развитие. При этом действие 0.5 мкМ флуоксетина приводило к появлению 45 белков, уникально экспрессирующихся в таких эмбрионах. Эти белки участвуют в клеточном росте, выживании, пролиферации и воспалительном ответе. Культивирование с 0.5 мкМ флуоксетина, кроме того, вызывает существенное увеличение плазминоген-активатора урокиназного типа, а также небольшие эффекты на временные характеристики стадий развития (Kaihola et al., 2016).
Употребление ингибитора обратного захвата серотонина флуоксетина беременными самками крыс приводило к нарушениям репродуктивного цикла, изменениям фолликулярного пула, в том числе увеличению овариального апоптоза, а также к количественным изменениям экспрессии генов, регулирующих серотониновую сигнализацию и биологические ритмы у их потомства (Moore et al., 2015). На мышах использование клинических доз флуоксетина резко увеличивало смертность потомства. Большинство плодов погибало постнатально от тяжелых патологий сердца, вызванных дозозависимой дилятационной кардиомиопатией (Noorlander et al., 2008).
Исследование влияния флуоксетина на транскрипцию генов, связанных с транспортерами серотонина, дофамина и норадреналина и рецепторами на ранних стадиях развития на костистой рыбе Danio rerio показало, что уровень транскрипции транспортеров серотонина и дофамина (sert и dat), а также vmat снижался при адекватных возможным в среде концентрациях. Уровни мРНК рецепторов 5-ht2c, drd2b adra2b и adra2c также снижались после воздействия флуоксетина (Cunha et al., 2017). У самок Danio rerio флуоксетин приводил также к уменьшению количества яйцеклеток, содержания эстрадиола в яичниках, а также к снижению уровня экспрессии мРНК овариальной ароматазы и рецепторов к гонадотропным гормонам (Lister et al., 2009).
Данные о воздействии флуоксетина на собственно оогенез ограничиваются показанным нами устранением этим блокатором SERT стимулирующего эффекта серотонина на экспрессию ряда генов в клетках гранулезы (Nikishin et al., 2019).
Пароксетин, еще один блокатор обратного захвата серотонина, обратил на себя внимание большим канадским эпидемиологическим исследованием, сообщившим о пугающем увеличении на 620 процентов заболеваемости раком груди у женщин, которые его получали в течение четырех лет. Хотя затем эта величина была сочтена избыточной, другие исследования показали многочисленные побочные эффекты пароксетина, не обнаруженные при клинических испытаниях препарата, среди которых и дефекты у новорожденных (Nevels et al., 2016).
Другой ингибитор – сертралин – на мышах вызывал даже в умеренных дозах увеличение процента плодов с висцеральными и скелетными нарушениями. Кроме того, у получивших препарат животных наблюдалось неполное окостенение, у ряда плодов наблюдалось расщепление неба (Cabrera et al., 2020). На Drosophila melanogaster, использованной в качестве модельной системы для определения эффектов сертралина на развитие, это вещество не влияло на фертильность самок и эмбриогенез потомства, но у личинок, получивших сертралин, наблюдались задержка развития и уменьшенная выживаемость на всех стадиях развития (Jajoo et al., 2020).
Таким образом, блокаторы обратного захвата серотонина в течение всего периода оогенеза и эмбриогенеза в большей или меньшей степени представляют собой опасность различных патологий развития, помимо куда более изученных эффектов на психику взрослых. Талидомидная трагедия, казалось бы, многому научила исследователей, работающих в этой области, но, как можно видеть из нашего обзора, далеко не всегда заставляет проявлять достаточную бдительность, особенно когда речь идет о ходовом, хорошо продающемся препарате.
ЗАКЛЮЧЕНИЕ
Развитие исследований роли трансмиттеров в донервный период еще больше, чем на классических объектах, зависит не только от возникающих у исследователей идей, но в немалой степени и от появления и доступности тех или иных новых технологий. Начавшись во вполне традиционном духе эмбриологических и физиологических экспериментов 60-х годов ХХ века, они прошли стадию применения биохимических, цитологических и электрофизиологических методик, которые радикально сменились молекулярно-биологическими подходами, пока более ограничивающимися исследованиями экспрессии мРНК различных компонентов трансмиттерной системы. Очевидно, что предстоит переход к уточнению вопроса об экспрессии и функциональной активности соответствующих белков, а тогда – на основе этих знаний – и возвращение на новом уровне собственно физиологического подхода к изучению функций трансмиттеров – от ооцита до нейрона.
Список литературы
Бузников Г.А. Низкомолекулярные регуляторы зародышевого развития. 1967. Москва, Наука. 265 с.
Бузников Г.А. Нейротрансмиттеры в эмбриогенезе. 1987. М. Наука
Бузников Г.А. Донервные трансмиттеры как регуляторы эмбриогенеза. Современное состояние проблемы. Онтогенез. 2007. 38 (4): 262–270.
Бузников Г.А., Манухин Б.Н. Влияние серотонина на эмбриональную моторику голожаберных моллюсков. Ж. общ. биол. 1960. 21 (5): 347–352.
Бузников Г.А., Шмуклер Ю.Б. Влияние препаратов-антимедиаторов на межклеточные связи у ранних зародышей морских ежей. Онтогенез. 1978. 9 (2): 173–178.
Бузников Г.А., Мальченко Л.А., Никитина Л.А., Галанов А.Ю., Еманов В.С. Эффект нейротрансмиттеров и их антагонистов на созревание ооцитов. 1. Эффект серотонина и его антагонистов на чувствительность ооцитов морской звезды к 1-метиладенину. Онтогенез. 1990. 21: 375–380.
Григорьев Н.Г. Кортикальный слой цитоплазмы – возможное место действия донервных трансмиттеров. Ж. эвол. биохим. физиол. 1988. 24 (5): 625–629.
Коштоянц Х.С. Проблемы энзимохимии возбуждения и торможения и эволюции функций нервной системы. Изд. АН СССР. 1963. Москва.
Мартынова Л.Е. Гаструляция у морского ежа Strongylocentrotus droebachiensis в норме и при обработке различными веществами. Онтогенез. 1981. 12: 310–315.
Никитина Л.А., Мальченко Л.А., Теплиц Н.А., Бузников Г.А. Эффект серотонина и его аналогов на созревание ооцитов in vitro. Онтогенез. 1988. 19: 336–343.
Никитина Л.А., Трубникова О.Б., Бузников Г.А. Эффекты нейротрансмиттеров и их антагонистов на созревание ооцитов. Эффект антагонистов серотонина на созревание in vitro ооцитов амфибий. Онтогенез. 1993. 24: 229–236.
Никишин Д.А., Семенова М.Н., Шмуклер Ю.Б. Экспрессия генов трансмиттерных рецепторов в раннем развитии морского ежа Paracentrotus lividus. Онтогенез. 2012. 43 (3): 212–216.
Никишин Д.А., Алешина Н.М., Семенова М.Л., Шмуклер Ю.Б. Локализация серотонина и его мембранного транспортера в яичнике мыши. Современная наука: актуальные проблемы теории и практики. Серия “Естественные и технические науки”. 2017а. № 11: 22–25.
Никишин Д.А., Алешина Н.М., Семенова М.Л., Шмуклер Ю.Б. Динамика экспрессии компонентов серотонинергической системы в клетках гранулезы развивающегося овариального фолликула и при лютеинизации. Гены и клетки. 2017б. 12 (4): 37–42.
Никишин Д.А., Алешина Н.М., Шмуклер Ю.Б. Синтез и мембранный транспорт серотонина в развивающемся овариальном фолликуле мыши. Доклады Академии наук. 2018а. 478 (1): 103–106.
Никишин Д.А., Храмова Ю.В., Багаева Т.С., Семенова М.Л., Шмуклер Ю.Б. Экспрессия компонентов серотонинергической системы в фолликулогенезе и доимплантационном развитии мыши. Онтогенез. 2018б. 49 (3): 208–216.
Никишин Д.А., Алешина Н.М., Семенова М.Л., Шмуклер Ю.Б. Влияние серотонина на экспрессию маркеров функционального состояния клеток гранулезы в культуре in vitro. Фундаментальные аспекты психического здоровья, 2018в. № 4: 13–17.
Никишин Д.А., Храмова Ю.В., Алешина Н.М., Мальченко Л.А., Шмуклер Ю.Б. Опосредованное ооцитом влияние серотонина на функциональный статус клеток гранулезы. Онтогенез, 2021. 52 (2): 000–000 в печати.
Ростомян М.A., Абрамян К.С., Бузников Г.А., Гусарева Э.В. Электронно-цитохимическое выявление аденилатциклазы у ранних эмбрионов морского ежа. Цитология. 1985. 27: 877–881.
Шмуклер Ю.Б. Межклеточные взаимодействия у ранних зародышей морских ежей. III. Влияние нейрофармакологических препаратов на тип дробления половинных зародышей Scaphechinus mirabilis. Онтогенез. 1981. 12 (4): 404–409.
Шмуклер Ю.Б. Специфическое связывание [H3]8-OH-DPAT ранними зародышами морского ежа Strongylocentrotus intermedius. Биол. Мембр. 1992. 9 (10–11): 1167–1169.
Шмуклер Ю.Б., Никишин Д.А. Трансмиттерные системы в эмбриогенезе – современное состояние проблемы. Успехи физиологических наук. 2018. 49 (4): 81–92.
Шмуклер Ю.Б., Бузников Г.А., Григорьев Н.Г., Maльченко Л.A. Влияние циклических нуклеотидов на чувствительность ранних зародышей морских ежей к цитотоксическим нейрофармакологическим препаратам. Бюлл. эксп. биол. мед. 1984. 97 (3): 354–355.
Aktas H., Wheeler M.B., First N.L., Leibfried-Rutledge M.L. Maintenance of meiotic arrest by increasing [cAMP]i may have physiological relevance in bovine oocytes. J. Reprod. Fertil. 1995. 105: 237–245.
Aluigi M.G., Diaspro A., Ramoino P., Russo P., Falugi C. The sea urchin, Paracentrotus lividus, as a model to investigate the onset of molecules immunologically related to the α-7 subunit of nicotinic receptors during embryonic and larval development. Curr. Drug Targets. 2012. 13 (5): 587–593.
Amenta F., Vega J.A., Ricci A., Collier W.L. Localization of 5-hydroxytryptamine-like immunoreactive cells and nerve fibers in the rat female reproductive system. Anat. Rec. 1992. 233. (3): 478–484.
Amireault P., Dubé F. Intracellular cAMP and calcium signaling by serotonin in mouse cumulus-oocyte complexes. Mol. Pharmacol. 2005a. 68 (6): 1678–1687.
Amireault P., Dubé F. Serotonin and its antidepressant-sensitive transport in mouse cumulus-oocyte complexes and early embryos. Biol. Reprod. 2005б. 73 (2): 358–365.
Amireault P., Sibon D., Côté F. Life without peripheral serotonin: insights from tryptophan hydroxylase 1 knockout mice reveal the existence of paracrine/autocrine serotonergic networks. ACS Chem. Neurosci. 2013. 4 (1): 64–71.
Azmitia E.C. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res. Bull. 2001. 56 (5): 413–424.
Basu B., Desai R., Balaji J., Chaerkady R., Sriram V., Maiti S., Panicker. Serotonin in pre-implantation mouse embryos is localized to the mitochondria and can modulate mitochondrial potential.MM. Reproduction. 2008. 135 (5): 657–669.
Batta S.K., Knudsen J.F. Calcium concentration in cumulus enclosed oocytes of rats after treatment with pregnant mares serum. Biol. Reprod. 1980. 22: 243–246.
Beyer T., Danilchik M., Thumberger T., Vick P., Tisler M., Schneider I., Bogusch S., Andre P., Ulmer B., Walentek P., Niesler B., Blum M., Schweickert A. 2012. Serotonin signaling is required for Wnt-dependent GRP specification and leftward flow in Xenopus. Curr. Biol. 22, 33–39.
Bòdis J., Bognàr Z., Hartmann G., Török A., Csaba I.F. Measurement of noradrenaline, dopamine and serotonin contents in follicular fluid of human graafian follicles after superovulation treatment. Gynecol. Obstet. Invest. 1992. 33 (3): 165–167.
Bòdis J., Török A., Tinneberg H.R., Hanf V., Papenfuss F., Schwarz H. Serotonin induces progesterone release from human granulosa cells in a superfused granulosa cell system. Archives of Gynecology and Obstetrics. 1993. 253 (2): 59–64.
Burnik-Papler T., Vrtacnik-Bokal E., Maver A., Kopitar A.N., Lovrečić L. Transcriptomic analysis and meta-analysis of human granulosa and cumulus cells. PloS One. 2015. 10 (8): e0136473.
Buznikov G.A., Marshak T.L., Malchenko L.A., Nikitina L.A., Shmukler Yu.B., Buznikov A.G., Rakic Lj., Whitaker M.J. Serotonin and acetylcholine modulate the sensitivity of early sea urchin embryos to protein kinase C activators. Comp. Biochem. Physiol. 1998. 120A (2): 457–462.
Buznikov G.A., Nikitina L.A., Galanov A.Y., Malchenko L.A., Trubnikova O.B. The control of oocyte maturation in the starfish and amphibians by serotonin and its antagonists. Int. J. Dev. Biol. 1993. 37: 363–364.
Buznikov G.A., Peterson R.E., Nikitina L.A., Bezuglov V.V., Lauder J.M. The pre-nervous serotonergic system of developing sea urchin embryos and larvae: pharmacologic and immunocytochemical evidence. Neurochem. Res. 2005. 30 (6–7): 825–837.
Cabrera R.M., Lin Y.-L., Law E., Kim J., Wlodarczyk B.J. The teratogenic effects of sertraline in mice. Birth Defects Res. 2020. 112 (13): 1014–1024.
Capasso A., Creti P., De Petrocellis B., De Prisco P., Parisi E. Role of dopamine and indolamine derivatives in the regulation of sea urchin adenylate cyclase. Biochem. Biophys. Res. Comm. 1988. 154: 758–764.
Cerdà J., Subhedar N., Reich G., Wallace R.A., Selman K. Oocyte sensitivity to serotonergic regulation during the follicular cycle of the teleost Fundulus heteroclitus. Biol. Reprod. 1998. 59 (1): 53–61.
Chaiyamoon A., Tinikul R., Chaichotranunt S., Poomthong T., Suphamungmee W., Sobhon P., Tinikul Y. Distribution and dynamic expression of serotonin and dopamine in the nervous system and ovary of Holothuria scabra during ovarian maturation. Journal of Comparative Physiology A. 2018. 204: 391–407.
Cho W.K., Stern S., Biggers J.D. Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J. Exp. Zool. 1974. 187: 383–386.
Čikoš Š., Veselá J., Il’kova G., Rehák P., Czikková S., Koppel J. Expression of beta adrenergic receptors in mouse oocytes and preimplantation embryos. Mol. Reprod. Dev. 2005. 71: 145–153.
Clausell D.E., Soliman K.F. Ovarian serotonin content in relation to ovulation. Experientia. 1978. 34 (3): 410–411.
Collart C., Owens N.D.L., Bhaw-Rosun L., Cooper B., De Domenico E., Patrushev I., Sesay A.K., Smith J.N., Smith J.C., Gilchrist M.J. High-resolution analysis of gene activity during the Xenopus mid-blastula transition. Development, 2014. 141 (9): 1927–1939.
Noorlander C.W., Ververs F.F.T., Nikkels P.G.J., van Echteld C.J.A., Visser G.H.A., Smidt M.P. Modulation of serotonin transporter function during fetal development causes dilated heart cardiomyopathy and lifelong behavioral abnormalities. PLoS One. 2008. 3 (7): e2782.
Côté F., Fligny C., Bayard E., Launay J.-M., Gershon M.D., Mallet J., Vodjdani G. Maternal serotonin is crucial for murine embryonic development. Proc. Natl Acad. Sci. U S A. 2007. 104 (1): 329–334.
Creeley C.E.,Denton L.K. Use of Prescribed Psychotropics during Pregnancy: A Systematic Review of Pregnancy, Neonatal, and Childhood Outcomes. Brain Sci. 2019. 9 (9): 235.
Cunha V., Rodrigues P., Santos M.M., Moradas-Ferreira P., Ferreira M. Fluoxetine modulates the transcription of genes involved in serotonin, dopamine and adrenergic signalling in zebrafish embryos, Chemosphere. 2017.
Darszon A., Labarca P., Nishigaki T., Espinosa F. 1999. Ion channels in sperm physiology. Physiol Rev 79: 481–510.
Dekel N., Beers W.H. 1978. Rat oocyte maturation in vitro: Relief of cyclic AMP inhibition by gonadotropins. Proc. Natl Acad. Sci. USA 75: 4369–4373.
Devic E., Paquereau L., Steinberg R., Caput D., Audigier Y. Early expression of a beta1-adrenergic receptor and catecholamines in Xenopus oocytes and embryos. FEBS Lett. 1997. 417: 184–190.
Dietrich J.E., Hiiragi T. Stochastic patterning in the mouse preimplantation embryo. Development. 2007. 134 (23): 4219–4231.
Dubé F., Amireault P. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life Sciences. 2007. 81: 1627–1637.
Frazer A., Hensler J.G. Serotonin Receptors. In: Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. (Siegel G.J., Agranoff B.W., Albers R.W., eds). Philadelphia: Lippincott-Raven; 1999.
Gardner D.K., Lane M., Calderon I., Leeton J. Environment of the preimplantation human embryo in vivo: Metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells. Fertil. Steril. 1996. 65: 349–353.
Garnerot F., Pellerin J., Blaise C., Mathieu M. Immunohistochemical localization of serotonin (5-hydroxytryptamine) in the gonad and digestive gland of Mya arenaria (Mollusca: Bivalvia). Gen. Comp. Endocrinol. 2006. 149 (3): 278–284.
Giannaccini G., Betti L., Palego L., Schmid L., Fabbrini L., Pelosini C., Gargini C., Da Valle Y., Lanza M., Marsili A., Maffei M., Santini F., Vitti P., Pinchera A., Lucacchini A. Human serotonin transporter expression during megakaryocytic differentiation of MEG-01 cells. Neurochem. Res. 2010; 35 (4): 628–635.
Graveleau C., Paust H.J., Schmidt-Grimminger D., Mukhopadhyay A.K. Presence of a 5-HT7 receptor positively coupled to adenylate cyclase activation in human granulosa-lutein cells. Journal of Clinical Endocrinology and Metabolism. 2000. 85 (3): 1277–1286.
Hagström B.E., Lönning S. The sea urchin egg as a testing object in toxicology. Acta Pharmacol Toxicol (Copenh). 1973. 1: 3–49.
Hamdan F.F., Ungrin M.D., Abramovitz M., Ribeiro P. Characterization of a novel serotonin receptor from Caenorhabditis elegans: cloning and expression of two splice variants. J Neurochem. 1999. 72: 1372–1383.
Hinckley M., Vaccari S., Horner K., Chen R., Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev. Biol. 2005.287: 249–261.
Hohmann S., Schweinfurth N., Lau T., Deuschle M., Lederbogen F., Banaschewski T., Schloss P. Differential expression of neuronal dopamine and serotonin transporters DAT and SERT in megakaryocytes and platelets generated from human MEG-01 megakaryoblasts. Cell Tissue Res. 2011. 346 (2): 151–161.
Homburger F., Chaube S., Eppenberger M., Bogdonoff P.D., Nixon C.W. Susceptibility of certain inbred strains of hamsters to teratogenic effects of thalidomide. Toxicol. Appl. Pharmacol. 1965. 7 (5): 686–693.
Hoyer D., Clarke D.E., Fozard J.R., Hartig P.R., Martin G.R., Mylecharane E.J., Saxena P.R., Humphrey P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994. 46 (2): 157–203.
Iľkova G., Rehak P., Vesela J., Čikoš Š., Fabian D., Czikkova S., Koppel J. Serotonin localization and its functional significance during mouse preimplantation embryo development. Zygote. 2004. 12 (3): 205–213.
Ivashkin E., Khabarova M.Yu., Melnikova V., Nezlin L.P., Kharchenko O., Voronezhskaya E.E., Adameyko I. Serotonin Mediates Maternal Effects and Directs Developmental and Behavioral Changes in the Progeny of Snails. Cell Rep. 2015 Aug 18; 12 (7): 1144–58. https://doi.org/10.1016/j.celrep.2015.07.022
Jequier E., Robinson D.S., Lovenberg W., Sjoerdsma A. Further studies on tryptophan hydroxylase in rat brainstem and beef pineal. Biochem. Pharmacol. 1969. 18: 1071–1081.
Jajoo A., Donlon C., Shnayder S., Levin M., McVey M. Sertraline induces DNA damage and cellular toxicity in Drosophila that can be ameliorated by antioxidants Sci. Rep. 2020. 10 (1): 4512.
Kaihola H., Yaldir F.G., Hreinsson J., Hörnaeus K., Bergquist J., Olivier J.D.A., Åkerud H., Sundström-Poromaa I. Effects of Fluoxetine on Human Embryo Development Front Cell Neurosci. 2016. 10: 160.
Katow H., Yaguchi S., Kyozuka K. Serotonin stimulates [Ca2+]i elevation in ciliary ectodermal cells of echinoplutei through a serotonin receptor cell network in the blastocoel. J. Exp. Biol. 2007. 210 (Pt 3): 403–412.
Kaur S., Archer K.J., Devi M.G., Kriplani A., Strauss J.F., Singh R. Differential gene expression in granulosa cells from polycystic ovary syndrome patients with and without insulin resistance: identification of susceptibility gene sets through network analysis. The Journal of Clinical Endocrinology and Metabolism. 2012. 97 (10): E2016–2021.
Kidder G.M., Vanderhyden B.C. Bidirectional communication between oocytes and follicle cells: Ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 2010. 88 (4): 399–413.
Koppan M., Bodis J., Verzar Z. Tinneberg H.-R., Török A. Serotonin may alter the pattern of gonadotropin-induced progesterone release of human granulosa cells in superfusion system. Endocrine. 2004. 24 (2): 155–159.
Krantic S., Dube F., Querion R. and Guerrier P. Pharmacology of the serotonin induced meiosis reinitiation of Spisula oocytes. Develop.Biol. 1991. 146: 491–497.
Lauder J.M., Moiseiwitsch J., Liu J., Wilkie M.B. Serotonin in development and pathophysiology. In: Brain Lesions in the Newborn (Lou H.C., Griesen G., Larsen J., Falck, eds.). 1994. Munksgaard, Copenhagen. P. 60–72.
Lawrence T.S., Beers W.H., Gilula N.B. Transmission of hormonal stimulation by cell-to-cell communication. Nature. 1978. 272: 501–506.
Levin M., Buznikov G.A., Lauder J.M. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci. 2006. 28 (3): 171–185.
Lister A., Regan C., Van Zwol J., Van Der Kraak G. Inhibition of egg production in zebrafish by fluoxetine and municipal effluents: a mechanistic evaluation. Aquat. Toxicol. 2009. 95 (4): 320–329. Aquat. Toxicol. 2009. 95 (4): 320–329.
Liu C., Peng J., Matzuk M.M., Yao H.H.-C. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nature Communications. 2015. 6: 6934.
Loewi O. Über humorale übertragbarkeit der Herznervenwirkund. I: Mittellung. Pflügers Arch. 1921. 189 (3): 239–242.
Masseau I., Bannon P., Anctil M., Dubé F. Localization and quantification of gonad serotonin during gametogenesis of the surf clam, Spisula solidissima. Biol. Bull. 2002. 202 (1): 23–33.
Misri S., Kendrick K. Treatment of perinatal mood and anxiety disorders: a review. Can J Psychiatry. 2007. 52 (8): 489–98.
Misri S., Reebye P., Kendrick K., Carter D., Ryan D., Grunau R.E., Oberlander T.F. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am. J. Psychiatry. 2006. 163: 1026–1032.
Moore C.J., DeLong N.E., Chan K.A., Holloway A.C., Petrik J.J., Sloboda D.M. Perinatal administration of a selective serotonin reuptake inhibitor induces impairments in reproductive function and follicular dynamics in female rat offspring. Reprod. Sci. 2015. 22 (10): 1297–1311.
Muzik M., Hamilton S.E. Use of Antidepressants During Pregnancy? What to Consider when Weighing Treatment with Antidepressants Against Untreated Depression. Matern. Child Health J. 2016. 20 (11): 2268–2279.
Nevels R.M., Gontkovsky S.T., Williams B.E. Paroxetine – The Antidepressant from Hell? Probably Not, But Caution Required. Psychopharmacol. Bull. 2016. 46 (1): 77–104.
Nikishin D.A., Ivashkin E.G., Mikaelyan A.S., Shmukler Y.B. Expression of serotonin receptors during early embryogenesis. Simpler Nervous Systems, IX East European Conference of the International Society for Invertebrate Neurobiology. 2009. P. 70 (Abstr).
Nikishin D.A., Kremnyov S.V., Konduktorova V.V., Shmukler Yu.B. Expression of serotonergic system components during early Xenopus embryogenesis. Int. J. Developm. Biol. 2012. 56: 385–391.
Nikishin D.A., Milošević I., Gojković M., Rakić Lj., Bezuglov V.V., Shmukler Yu.B. Expression and functional activity of neurotransmitter system components in sea urchins' early development. Zygote. 2016. 24 (2): 206–218.
Nikishin D.A., Alyoshina N.M., Semenova M.L., Shmukler Yu.B. Analysis of Expression and Functional Activity of Aromatic L-Amino Acid Decarboxylase (DDC) and Serotonin Transporter (SERT) as Potential Sources of Serotonin in Mouse Ovary. Int. J. Mol. Sci. 2019. 20: 3070.
Niu W., Wang Y., Wang Z.; Xin Q., Wang Y., Feng L., Zhao L., Wen J., Zhang H., Wang C., Xia G. JNK signaling regulates E-cadherin junctions in germline cysts and determines primordial follicle formation in mice. Development. 2016. 143, 1778–1787.
Owens N.D., Blitz I.L., Lane M.A., Patrushev I., Overton J.D., Gilchrist M.J., Cho K.W., Khokha M.K. Measuring Absolute RNA Copy Numbers at High Temporal Resolution Reveals Transcriptome Kinetics in Development. Cell Rep. 2016. 14 (3): 632–647.
Peters M.A., Walenkamp A.M., Kema I.P., Meijer C., de Vries E.G., Oosting S.F. Dopamine and serotonin regulate tumor behavior by affecting angiogenesis. Drug Resist Updat. 2014. 17 (4–6): 96–104.
Preis K.A., Seidel G.Jr., Gardner D.K. Metabolic markers of developmental competence for in vitro-matured mouse oocytes. Reproduction. 2005. 130: 475–483.
Ram D., Gandotra S. Antidepressants, anxiolytics, and hypnotics in pregnancy and lactation. Indian J Psychiatry. 2015. 57 (Suppl. 2): S354–S371.
Robson J.M., Sullivan F.M. Serotonin as a Teratogen. BMJ. 1964. 5379: 370.
Rudnick G., Nelson P.J. Platelet 5-hydroxytryptamine transport, an electroneutral mechanism coupled to potassium. Biochemistry. 1978. 17 (22): 4739–42.
Rudnick G., Sandtner W. Serotonin transport in the 21st century. J. Gen. Physiol. 2019. 151 (11): 1248–1264.
Salustri A., Yanagishita M., Underhill C.B., Laurent T.C., Hascall V.C. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev. Biol. 1992. 151 (2): 541–551.
Sarrouilhe D., Mesnil M. Serotonin and human cancer: A critical view. Biochimie. 2019. 161: 46–50.
Session A.M., Uno Y., Kwon T., Chapman J.A., Toyoda A., Takahashi S., Fukui A., Hikosaka A., Suzuki A., Kondo M., van Heeringen S.J., Quigley I., Heinz S., Ogino H., Ochi H., Hellsten U., Lyons J.B., Simakov O., Putnam N., Stites J., Kuroki Y., Tanaka T., Michiue T., Watanabe M., Bogdanovic O., Lister R., Georgiou G., Paranjpe S.S., van Kruijsbergen I., Shu S., Carlson J., Kinoshita T., Ohta Y., Mawaribuchi S., Jenkins J., Grimwood J., Schmutz J., Mitros T., Mozaffari S.V., Suzuki Y., Haramoto Y., Yamamoto T.S., Takagi C., Heald R., Miller K., Haudenschild C., Kitzman J., Nakayama T., Izutsu Y., Robert J., Fortriede J., Burns K., Lotay V., Karimi K., Yasuoka Y., Dichmann D.S., Flajnik M.F., Houston D.W., Shendure J., DuPasquier L., Vize P.D., Zorn A.M., Ito M., Marcotte E.M., Wallingford J.B., Ito Y., Asashima M., Ueno N., Matsuda Y., Veenstra G.J., Fujiyama A., Harland R.M., Taira M., Rokhsar D.S. Genome evolution in the allotetraploid frog Xenopus laevis. Nature. 2016. 538 (7625): 336–343.
Sheng Y., Wang L., Liu X.S.J.S., Montplaisir V., Tiberi M., Baltz J.M., Liu X.J. A serotonin receptor antagonist induces oocyte maturation in both frogs and mice: evidence that the same G protein-coupled receptor is responsible for maintaining meiosis arrest in both species. J. Cell Physiol. 2005. 202: 777–786.
Shmukler Yu.B. On the possibility of membrane reception of neurotransmitter in sea urchin early embryos. Comp. Biochem. Physiol. 1993. 106C (1): 269–273.
Shmukler Y., Nikishin D. Transmitters in Blastomere Interactions. In: Cell Interactions (ed. S.Gowder), InTech, 2012, Ch. 2. P. 31–65.
Shmukler Yu.B., Buznikov G.A., Whitaker M.J. Action of serotonin antagonists on cytoplasmic calcium level in early embryos of sea urchin Lytechinus pictus. Int. J. Dev. Biol. 1999. 42 (3): 179–182.
Shmukler Yu.B., Grigoriev N.G., Buznikov G.A., Turpaev T.M. Regulation of cleavage divisions: participation of “prenervous” neurotransmitters coupled with second messengers. Comp. Biochem. Physiol. 1986. 83C (2): 423–427.
Shuey D.L., Sadler T.W., Tamir H., Lauder J.M. Serotonin and morphogenesis. Transient expression of serotonin uptake and binding protein during craniofacial morphogenesis in the mouse. Anat. Embryol. (Berl). 1993. 187 (1): 75–85.
Silvestre F., Boni R., Fissore R.A., and Tosti E. Ca2+ Signaling During Maturation of Cumulus–Oocyte Complex in Mammals. Molecular Reproduction & Development. 2011. 78: 744–756.
Sneddon J.M. Sodium-dependent accumulation of 5-hydroxytryptamine by rat blood platelets. Br. J. Pharmacol. 1969. 37 (3): 680–688.
Stępińska U., Kuwana T., Olszańska B. Serotonin receptors are selectively expressed in the avian germ cells and early embryos. Zygote. 2015. 23 (3): 394–405.
Stricker S.A. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 1999. 211: 157–176.
Stricker S.A., Smythe T.L. 5-HT causes an increase in cAMP that stimulates, rather than inhibits, oocyte maturation in marine nemertean worms. Development. 2001. 128 (8): 1415–1427.
Tan M.H., Au K.F., Yablonovitch A.L., Wills A.E., Chuang J., Baker J.C., Wong W.H., Li J.B. RNA sequencing reveals a diverse and dynamic repertoire of the Xenopus tropicalis transcriptome over development. Genome Res. 2013. 23 (1): 201–216.
Tanaka E., Baba N., Toshida K., Suzuki K. Serotonin stimulates steroidogenesis in rat preovulatory follicles: involvement of 5-HT2 receptor. Life Sci. 1993. 53: 563–570.
Tinikul Y., Joffre Mercier A., Soonklang N., Sobhon P. Changes in the levels of serotonin and dopamine in the central nervous system and ovary, and their possible roles in the ovarian development in the giant freshwater prawn, Macrobrachium rosenbergii. Gen. Comp. Endocrinol. 2008. 158 (3): 250–258.
Terranova P.F., Uilenbroek J.T., Saville L., Horst D., Nakamura Y. Serotonin enhances oestradiol production by hamster preovulatory follicles in vitro: effects of experimentally induced atresia. J. Endocrinol. 1990 125: 433–438.
Vesela J., Rehak P., Mihalik J., Czikkova S., Pokorny J., Koppel J. Expression of serotonin receptors in mouse oocytes and preimplantation embryos. Physiol. Res. 2003. 52: 223–228.
Voronezhskaya E.E., Khabarova M.Yu., Nezlin L.P. Apical sensory neurones mediate developmental retardation induced by conspecific environmental stimuli in freshwater pulmonate snails. Development. 2004. 131 (15): 3671–80. https://doi.org/10.1242/dev.01237
Wang Q., He M. Molecular characterization and analysis of a putative 5-HT receptor involved in reproduction process of the pearl oyster Pinctada fucata. Gen. Comp. Endocrinol. 2014. 204: 71–79.
Yang K., Hitomi M., Stacey D.W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Division. 2006. 1: 32.
Zatylny C., Durantou F., Boucaud-Camou E., Henry J. Evidence of 5-hydroxytryptamine synthesis in the follicles of Sepia officinalis and direct involvement in the control of egglaying. Mol. Reprod. Dev. 2000. 55 (2): 182–188.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


