Журнал высшей нервной деятельности им. И.П. Павлова, 2021, T. 71, № 3, стр. 295-305
Моноамины как адаптивные регуляторы развития: феномен и механизмы действия
Е. Е. Воронежская 1, *, В. И. Мельникова 1, Е. Г. Ивашкин 1, 2, 3
1 ФГБУН Институт биологии развития им. Н.К. Кольцова РАН
Москва, Россия
2 ФГБУН Институт проблем экологии и эволюции им. А.Н. Северцова РАН
Москва, Россия
3 Marine Biological Laboratory
Woods Hole, USA
* E-mail: elena.voronezhskaya@idbras.ru
Поступила в редакцию 10.12.2020
После доработки 17.02.2021
Принята к публикации 02.03.2021
Аннотация
Значительная часть интегративных функций в организме осуществляется через моноаминергические системы: комплекс из низкомолекулярного медиатора – биогенного амина (серотонина и дофамина), ферментов его метаболизма и рецепторов. Это делает моноамины важнейшим компонентом нервной и эндокринной системы в организме, определяющим адаптационные способности организма в непрерывно меняющихся условиях окружающей среды. На примере собственного экспериментального материала и существующей литературы дается представление о регуляторной роли моноаминов в процессе развития, начиная со стадии яйцеклетки и раннего дробления и до формирования нейронных сетей, лежащих в основе поведения. Рассмотрены классический лиганд-рецепторный механизм и механизм неканонической модификации внутриклеточных белков (моноаминилирование) и их вклад в адаптивную регуляцию в разные периоды развития. Показана роль моноаминов и моноаминилирования как консервативного фактора, связывающего сигналы окружающей среды и физиологию развивающегося организма.
С момента открытия нейрональных моноаминов – серотонина и дофамина – в середине ХХ века ученые нашли огромное количество функций, в которых участвуют эти вещества. Пожалуй, трудно найти такой физиологический процесс во взрослом организме, в котором серотонин и дофамин не были бы задействованы в той или иной степени. У человека это такие жизненно важные функции, как регуляция сна и бодрствования, агрессия и тревожность, пищеварение, воспаление и многое другое. Исследованию механизмов, обеспечивающих многогранные регуляторные функции моноаминов, посвящено огромное количество работ. На сегодняшний день изучены на молекулярном уровне процессы синтеза и деградации серотонина и дофамина, мембранный транспорт, упаковка и выброс из везикул, особенности работы широкого спектра рецепторов. В последние десятилетия было показано, что регулярные механизмы действия моноаминов также включают в себя сложные многокомпонентные системы, в которых важную роль играет баланс моноаминов внутри и снаружи клеток (Walther et al., 2003; Duerschmied, Bode, 2009). Такие системы могут существовать как в пределах единичных клеток, так и быть разнесены на тканевом, органном и даже организменном уровне (Paulmann et al., 2009), позволяя говорить о моноаминах как о системных интегративных модуляторах (Sakharov, 1990), в частности, для нейральных процессов (Сахаров, 1990; Сахаров, 2012). В данной работе мы не будем затрагивать собственно нейрональные адаптации (изменения на уровне отдельных нервных клеток или нервных сетей), в которых участвуют моноамины. Этой теме посвящено огромное количество работ. Мы же представляем моноамины как одно из звеньев адаптаций на уровне формирующегося организма, позволяющее увеличить разнообразие физиологических реакций отдельных особей, происходящее на базе одной и той же генетической программы (без изменения базовой генетической основы). Увеличение пластичности в процессе развития приводит к расширению (или направленному сдвигу) границ реакции в пределах всей популяции и таким образом повышает устойчивость ее существования при меняющихся внешних условиях. Именно в контексте значения для формирования экологической приспособленности и будет рассмотрена роль моноаминов в период раннего развития эмбриона или зародыша.
В представленной работе будет дано представление о присутствии и роли моноаминов в раннем развитии, возможных механизмах долговременных моноамин-зависимых регуляций, являющихся основой адаптаций на уровне всего организма, рассмотрены перспективы подхода к моноаминам с точки зрения их отложенного действия при формировании паттернов развития и поведения.
ПРЕДПОСЫЛКИ УЧАСТИЯ МОНОАМИНОВ В РЕГУЛЯЦИЯХ НА РАННИХ СТАДИЯХ РАЗВИТИЯ
Еще в конце 50-х – начале 60-х годов ХХ века было впервые обнаружено, что некоторые моноамины и другие низкомолекулярные вещества, являющиеся во взрослом организме нейромедиаторами (нейротрансмиттерами), присутствуют на стадиях раннего развития задолго до появления нервной системы (Numanoi, 1955; Бузников, Манухин, 1961). В дальнейшем было показано, что серотонин содержится в ооцитах и дробящихся эмбрионах животных самой разной систематической принадлежности: полихет (Emanuelsson, 1974), немертин, (Buznikov et al., 1964), голожаберных моллюсков (Buznikov et al., 2003), головохордовых (Candiani et al., 2001), иглокожих, костистых рыб (Buznikov et al., 1964), амфибий (Fukumoto et al., 2005; Beyer et al., 2012; Nikishin et al., 2012), птиц (Emanuelsson et al., 1988) и млекопитающих (Basu et al., 2008). В экспериментальных работах было продемонстрировано, что моноамины вовлечены в регуляцию широкого спектра процессов на донервных стадиях развития у самых разных животных (см. обзор Buznikov et al., 2001; Бузников, 2007). Показано, что на ранних стадиях развития серотонин и дофамин действуют как нейрогормоны, воспринимаемые клетками-мишенями зародыша (Ugrumov, 1997).
Больше всего данных в литературе имеется о ранней моноаминергической системе млекопитающих (Burden, Lawrence, 1973; Neilson et al., 2000; Il’kova et al., 2004; Amireault, Dubé, 2005a,b; Hinckley et al., 2005; Basu et al., 2008; Никишин и др., 2018b). Известно, что сами ооциты не имеют фермента синтеза серотонина (триптофангидроксилазы), в то время как обкладочные клетки и клетки Граафова пузырька способны синтезировать и выделять серотонин, обеспечивая им расположенные вблизи ооциты и даже ранние эмбрионы (Amireault, Dubé, 2005a; Dubé, Amireault, 2007; Никишин и др., 2018a; Nikishin et al., 2019). Было показано наличие мРНК рецепторов к серотонину нескольких типов в развивающихся бластомерах, а также экспрессия практически всех компонентов серотонинергической системы в различных частях формирующегося зародыша (Amireault, Dube 2005a; Никишин и др., 2018b). Так, мембранный транспортер серотонина (SERT) присутствует в клетках фолликула мыши, а также в ооцитах и ранних эмбрионах. У беспозвоночных животных (моллюсков, морских ежей и полихет) в дробящихся бластомерах присутствуют и функционально активны ферменты синтеза серотонина и мембранные транспортеры (Buznikov et al., 2003; Ivashkin et al., 2012, 2015; Nikishin et al., 2016; Никишин и др., 2018b). Наличие рецепторов к моноаминам и мембранных транспортеров создает возможность для локальной регуляции процессов, происходящих в клетках зародыша во время развития. Причем такая система способна чутко реагировать на изменение уровня моноаминов как в организме родителя (непосредственно во время прохождения или нахождения зародыша в репродуктивных путях), так и во внешней среде (в случае развития зародыша вне материнского организма, например, в отложенном яйце). В свою очередь, уровень моноаминов в материнском организме отражает изменения в физиологии и функциональном состоянии конкретной особи. Так, уровень серотонина, например, зависит от сезона (Ivashkin et al., 2015), существенно меняется при состоянии сытости-голода, после активной физической нагрузки (Дьяконова, 2012; Aonuma et al., 2020), при определенных стрессовых воздействиях (Fossat et al., 2014).
Таким образом, наличие полноценно функционирующих компонентов моноаминергической системы (наиболее полно изученной для серотонина), начиная с самых ранних стадий эмбрионального развития, динамическое изменение уровня моноаминов вокруг зародыша в процессе развития в ответ на модуляцию уровня моноаминов в материнском организме при воздействии внешних и внутренних стимулов, создает все предпосылки для участия серотонина и дофамина в долговременных регуляторных процессах в течение всего процесса развития, начиная с самых ранних донервных стадий.
РЕЦЕПТОРНЫЙ МЕХАНИЗМ АДАПТАЦИОННЫХ МОНОАМИНЕРГИЧЕСКИХ РЕГУЛЯЦИЙ
Одним из примеров регуляции эмбрионального развития через моноаминергическую систему является модуляция темпов эмбрионального развития и локомоторных программ зародыша у пресноводных брюхоногих моллюсков. У большинства гастропод оплодотворение происходит внутри материнского организма, после оплодотворения яйцо проходит достаточно длинный путь по каналам репродуктивной системы, где покрывается несколькими оболочками и упаковывается в яйцевой кокон, который моллюск и откладывает в виде кладки (Мещеряков, 1975; Koene, 2010). Однако, несмотря на кажущуюся простоту процесса размножения моллюсков, он включает в себя и систему регуляций, работающих после откладки яиц. В ответ на специфический фактор, выделяемый взрослыми в случае неблагоприятных условий (например, недостатка пищи или скученности в популяции), развивающиеся зародыши могут отвечать изменением темпов развития. При этом один и тот же выделяемый взрослыми животными фактор действует разнонаправленно на эмбрионы разного возраста: зародыши, находящиеся на ранних стадиях развития, замедляются, вплоть до полной остановки, в то время как особи, вступившие в метаморфоз, наоборот, ускоряют свое развитие (Воронежская, Хабарова, 2003; Voronezhskaya et al., 2004). Сигнальный фактор от взрослых действует не сам по себе, а через активацию моноаминергических хемосенсорных нейронов, которые, в свою очередь, начинают активно синтезировать и выделять во внутреннюю среду зародыша серотонин (у зародышей катушки Helisoma trivolvis) или дофамин (у зародышей прудовика Lymnaea stagnalis).
В основе скоординированной реакции всего организма, который при этом еще и находится в процессе непрерывного развития, лежит баланс экспрессии рецепторов нейромедиаторов и связанных с ними G-белков (Voronezhskaya et al., 2012; Glebov et al., 2014). У Helisoma в процессе развития в клетках эмбриона экспрессированы как минимум четыре типа серотониновых рецепторов (5-НТ1, 5-НТ4, 5-НТ5, 5-НТ7). На ранних стадиях преобладают рецепторы 5-НТ4/7, в то время как на поздних 5-НТ1/5 (и связанные с этими типами рецепторов G-белки: Gαs и Gαi). Таким образом, направление ответа определяет пропорциональное изменение темпорального (зависимого от стадии развития) паттерна экспрессии рецепторов, активируемых одним и тем же лигандом – серотонином, выделяемым сенсорными нейронами зародыша в ответ на химический сигнал от взрослых особей. В результате преобладающей активации рецепторов 5-НТ4/7 и внутриклеточных сигнальных каскадов, опосредованных через Gαs белки, происходит замедление темпов развития на ранних стадиях, в то время как активация 5-НТ1/5 и Gαi-опосредованных путей вызывает ускорения развития на стадиях после метаморфоза (Glebov et al., 2014).
Одновременно с темпами развития меняются и такие важнейшие поведенческие паттерны эмбриона, как локомоторная и жевательная активность (обеспечивающие прогрызание яйцевых оболочек и выход из яйца), а также ряд физиологических процессов, таких как частота сердечных сокращений (Воронежская и др., 2007; Khabarova, Voronezhskaya, 2012). То есть, через серотонинергическую систему, происходит интеграция скоординированного ответа развивающегося (находящегося в яйце) зародыша на внешний стимул, которая позволяет пластически подстроиться к имеющимся на момент выхода из яйца условиям. Такой механизм позволяет переждать неблагоприятные условия зародышам ранних стадий развития и поскорее завершить развитие зародышам поздних стадий, направленно сдвигая стандартную норму реакции (в случае моллюсков модулируя средний темп развития).
Моноаминергические нейроны, продуцирующие серотонин, дофамин и гистамин, являются одними из самых ранних нервных клеток, дифференцирующихся в зародышах и личинках многих беспозвоночных животных. В дальнейшем моноамины регулируют различные поведенческие паттерны личинки, участвуют в таких важных для жизненного цикла регуляторных процессах, как, например, метаморфоз (Schmidt-Rhaesa et al., 2016), или индивидуальная приспособленность в различные фазы жизненного цикла (Issa et al., 2020). Известны и примеры, когда нарушение в формировании определенных нейронов приводит к сбоям адаптивных регуляций, не позволяя, например, личинкам нематоды Caenorhabditis elegans перейти в ждущую “дауэровскую” форму (Sze et al., 2000).
Таким образом, регуляторные механизмы, в основе которых лежит баланс активности рецепторов разного типа, потенциально не ограничиваются моллюсками и темпами эмбрионального развития. Во многих случаях они обеспечивают индивидуальную пластичность в процессе развития, лежащую в основе разнообразия нормы реакции отдельных особей в популяции.
МОНОАМИНИЛИРОВАНИЕ КАК РЕГУЛЯТОРНЫЙ МЕХАНИЗМ ПРОЦЕССОВ РАЗВИТИЯ
Для серотонина, как и других моноаминов, характерно широкое вовлечение в гуморальные регуляторные механизмы. Однако разнообразие наблюдающихся при этом физиологических эффектов явно не укладывается в то, что мы наблюдаем при действии моноаминов как нейромедиаторов, осуществляющих передачу сигнала между клетками и меняющих ответ нейронов или работу нервных сетей (нейрональная адаптивность или пластичность). Ученые, работающие с данными системами, давно предполагали, что помимо лиганд-рецепторного регуляторного действия моноаминов, как правило, вовлеченного в быстрые по времени протекания процессы, существуют и иные механизмы, более подходящие для растянутых во времени (отложенных и долговременных) вариантов ответа.
Еще в работах академика Х.С. Коштоянца были получены данные, указывающие на то, что моноамины действуют не только через мембранные рецепторы, но и имеют эффекты, основанные на их вовлечении во внутриклеточные процессы. К настоящему моменту накоплен большой пул данных, подтверждающих, что различные лиганды, задействующие G-белки (GPCR-coupled), связаны с внутриклеточными мембранами, где они активируют сигнальные системы, отличающиеся или даже уникальные по сравнению с действием тех же лигандов на рецепторы, находящиеся на поверхностной мембране клетки (Jong et al., 2018). Получены свидетельства и о присутствии таких явлений в развитии (Шмуклер, Никишин, 2018). Не так давно к каноническому рецепторному механизму, при котором связывание моноамина с рецептором является триггером разнообразных клеточных процессов, добавился неканонический механизм действия моноаминов – через посттрансляционную модификацию внутриклеточных белков (Watt et al., 2009; Bader, 2020).
Регуляции физиологических процессов, основанные на ковалентном связывании моноаминов с внутриклеточными белками посредством трансглутаминаз, широко распространены у млекопитающих и человека (Walther et al., 2011; Muma, Mi, 2015). В большинстве случаев в активацию таких процессов также вовлечены и классические рецепторные механизмы. Однако в качестве основного сигнального события внутри клетки выступают клеточный метаболизм и кинетика транспорта моноамина через клеточную мембрану (Pavone, Norris, 2013; Tanaka et al., 2014). Попадая внутрь клетки и накапливаясь в достаточном количестве, серотонин, дофамин, а также другие моноамины выступают как биохимический субстрат для реакции трансамидации. С помощью специфического фермента – трансглутаминазы – происходит ковалентное присоединение моноамина по остаткам глутамина в белке. В итоге происходит прямая модуляция эффекторных функций белков: изменяется связывание с другими белками, продолжительность их жизни в клетках, возможность к полимеризации и многое другое. Подобный процесс получил название моноаминилирования в целом. И, соответственно, серотонилирования, дофаминилирования, гистаминилирования в зависимости от конкретного вовлеченного моноамина (Muma, Mi, 2015). Такой механизм действия, с одной стороны, происходит медленнее, чем активация внутриклеточных путей, опосредованных G-белками, а с другой стороны, приводит к появлению модифицированных белковых молекул, действие которых сохраняется до момента их утилизации в клетке. Многие авторы высказывают предположение, что именно такой механизм включается в тех случаях, когда наблюдается долговременная и отсроченная регуляция какого-либо физиологического процесса со стороны моноаминов. Таким образом, например, происходит активация белков семейства малых ГТФаз (Walther et al., 2003; Mercado et al., 2011), модуляция агрегации белков внеклеточного матрикса, включая фибриноген и некоторые белки глиальных клеток (Hummerich et al., 2010; 2012; 2015), реорганизация цитоскелета и изменение матрикса клеток сердечных клапанов вследствие серотонилирования филамина А (Pavone, Norris, 2013). Есть данные, что такая модификация, как серотонилирование, способствует убиквитин-зависимой утилизации белков (Guilluy et al., 2007), определяя время жизни и функциональной активности белка в клетке.
Наконец, было обнаружено, что моноаминилирование не является прерогативой млекопитающих и не ограничено физиологией клеток на финальных стадиях дифференцировки. В раннем развитии животных серотонилирование впервые было описано на модели развития брюхоногих моллюсков. Было обнаружено, что повышение уровня серотонина в дробящихся бластомерах приводит к отложенным и долговременным изменениям в развитии эмбрионов и поведении молодых особей (Ivashkin et al., 2015). В ответ на повышение серотонина как непосредственно в дробящихся бластомерах, так и в организме матери (на самых ранних, донервных стадиях развития), у зародышей прудовиков меняется динамика темпа развития – изменяется картина роста по стадиям. Позднее эффект проявляется и в поведении животных. Существенно повышается скорость вращения в яйце. Вылупившиеся ювенильные моллюски стремятся выползти из воды, начинают преимущественно использовать не ресничный, а мышечный тип локомоции. По достижении половой зрелости такие улитки демонстрируют повышенную яйценоскость. Все эти признаки являются значимыми компонентами программы расселения. В норме аналогичные комплексы поведения можно наблюдать у моллюсков, родившихся в весенне-летний период, в который происходит интенсивное расселение этого вида. С другой стороны, именно весной у половозрелых животных наблюдается максимальный уровень серотонина в определенных участках женской части репродуктивной системы. Оказалось, что именно в чувствительный к уровню серотонина период на ранних стадиях развития у зародышей активно происходит серотонилирование и выявляются характерные модифицированные белки, а ингибирование трансглутаминаз полностью снимает все эффекты повышения уровня серотонина у раннего зародыша. Таким образом, серотонилирование белков в клетках формирующегося зародыша выступает как звено, связывающее сезонные изменения уровня серотонина в материнском организме и подстройку поведенческих паттернов и физиологических параметров потомков к конкретным условиям среды. В данном случае моноаминилирование выступает как механизм, интегрирующий физиологические и экологические аспекты жизни животного.
В приведенном выше случае серотонин выступает как медиатор целого ряда материнских эффектов – адаптационных изменений, носящих долговременный характер и проявляющихся в разнообразии фенотипа потомков при одном и том же исходном генотипе. Множество материнских эффектов было описано у самых разных животных (Mousseau, Fox, 1998; Räsänen, Kruuk, 2007). В этих явлениях участвуют как ряд прямых сигнальных механизмов (St-Pierre et al., 2016), так и эпигенетические процессы на основе метилирования–деметилирования ДНК (Cooney et al., 2002). Факторы внешней среды, такие, как разные уровни материнской заботы (Weaver et al., 2004), диета взрослой самки (Сооney et al., 2002; Waterland, Jirtle, 2003), травмирующие переживания у самцов, сочетанные с запахом (Dias, Ressler, 2014), вызывают комплексный ответ, определяющий фенотип потомков, в котором эпигенетические процессы являются конечным интегратором. Многообразие материнских и отцовских эффектов задействует множество связей между разными механизмами их реализации. Одним из таких недавно описанных механизмов является связь между метилированием, серотонилированием и дифференцировкой нейронов (Farrelli et al., 2019). В серотонинергических нейронах cеротонилирование гистона H3 способствует связыванию транскрипционного фактора IID (TFIID) с соответствующими участками в молекуле ДНК, определяя уровень экспрессии TFIID-зависимых генов регуляторной сети. Блокада серотонилирования гистона H3 нарушает процесс дифференцировки серотонинергических нейронов. В этой работе впервые было доказано, что серотонин может напрямую действовать на ядерные белки в клетках, регулируя экспрессию генов.
В эмбриональном развитии серотонилированные белки были обнаружены в ядрах клеток дробящихся зародышей морского ежа, моллюска и костистой рыбы. Паттерн распределения серотонилированных белков в ядрах клеток определялся уровнем серотонина в развивающихся зародышах (Ivashkin et al., 2019). Поскольку у зародышей серотонилирование ядерных белков происходит не только в специфических уже дифференцированных клетках (нейронах), но и в плюрипотентных клетках эмбриона, можно предположить, что такой механизм выступает потенциальным регулятором дифференцировки широкого спектра клеток и тканей.
ЗНАЧЕНИЕ МОНОАМИН-ОПОСРЕДОВАННЫХ РЕГУЛЯЦИЙ В РАЗВИТИИ МЛЕКОПИТАЮЩИХ
Проблема влияния моноаминов на процесс развития сохраняет свою актуальность также и для млекопитающих и человека. Моноаминергическая система является высоко консервативной, что дает возможность не только соотносить данные, полученные на далеко отстоящих в филогенетическом отношении организмах, но и проводить прямые параллели между экспериментами на модельных организмах и отклонениями в экспрессии компонентов моноаминергической системы у человека. Значение активности моноаминергической системы во время раннего развития изучено менее всего и часто не учитывается в разработке подходов к терапии взрослого организма.
Как уже упоминалось выше, на самых ранних этапах онтогенеза, подобно беспозвоночным животным с анцестральным типом развития, созревающий ооцит, зигота и ранний зародыш млекопитающих находятся под непосредственным влиянием моноаминов материнского организма. Серотонин в физиологических концентрациях определяется в гонадах и половых путях млекопитающих (Amireault, Dubé, 2005b; Dubé, Amireault, 2007). Созревающий ооцит и ранний зародыш способны захватывать серотонин из окружающей среды (Никишин и др., 2018a).
В дальнейшем (на постимплантационных стадиях развития) ведущим источником серотонина для плода становится плацента. Экспрессия обоих ферментов синтеза серотонина (триптофангидроксилазы 1 и декарбоксилазы ароматических аминокислот), а также синтез серотонина были подтверждены в плаценте с 10-го дня эмбрионального развития у мышей (Bonnin et al., 2011) и около 11-й гестационной недели у человека (Bonnin et al., 2011). В этот период материнский триптофан (и, возможно, 5-НТР, непосредственный биохимический предшественник синтеза серотонина) может служить источником серотонина у плода. Плацента непроницаема для серотонина матери, что было подтверждено экспериментами на животных с нокаутами SERT и прямой перфузией плаценты ex vivo (Bonnin, Levitt, 2011; St-Pierre et al., 2016), но сама способна синтезировать серотонин из поступающих из материнского организма биохимических предшественников. Ведущая роль плаценты в поддержании необходимого уровня серотонина у плода сохраняется у мышей в период с 10-го по 15-й день эмбрионального развития. Позднее возрастает синтез серотонина собственными тканями плода, в частности, клетками мозга и желудочно-кишечного тракта. Таким образом, в развитии млекопитающих происходит прогрессивное переключение источников серотонина с материнского на плацентарный и на эндогенные источники плода.
Существенно, что каждый из этих этапов по-разному чувствителен к внешним факторам: как сигналам, поступающим из организма матери, так и к сигналам из окружающей среды. Например, показано, что умеренный стресс или воспаление у матери приводит к повышению синтеза серотонина в плаценте и возрастанию его уровня в тканях плода (St-Pierre et al., 2016; Chen et al., 2020). При этом именно плацентарный серотонин, поступающий к формирующимся корковым структурам плода значительно раньше, чем серотонин, синтезируемый нервными клетками самого эмбриона, оказывает ключевое влияние на формирование нейрональных структур в коре (Vitalis et al., 2013). У человека описаны многочисленные мутации генов SERT и рецепторов серотонина, а также их связь с нарушениями процесса развития, приводящими к поведенческим расстройствам в постнатальном онтогенезе. Во многих исследованиях подчеркивается значение изменений уровня серотонина в организме матери, плаценте и тканях плода в критические периоды развития и связь этих изменений с возрастающими рисками таких психических заболеваний, как тревожность, депрессия, расстройства аутичного спектра. Авторы обзоров по данной тематике призывают к более глубокому исследованию всех компонентов серотонинергической системы как матери, так и плода, участвующих в таком перинатальном программировании, предлагают обращать больше внимания на модификации этой системы с целью выявления ранних биомаркеров психических расстройств (Sato, 2013; St-Pierre et al., 2016).
Предварительные результаты транскриптомного анализа, полученные в нашей лаборатории, показывают, что модуляция уровня материнского серотонина на самых ранних стадиях развития млекопитающих способна оказывать значимое влияние на уровень экспрессии большого количества генов в бластоцистах мышей, вовлеченных в процессы миграции, клеточной адгезии, ионного транспорта. Все перечисленные выше факты позволяют говорить о моноаминах и в первую очередь о серотонине как о значимых эпигенетических факторах в процессе развития. При этом конкретный эффект действия определяется стадией развития эмбриона, моментом дифференцировки определенных структур и экспрессией рецепторов и транспортеров моноаминов на специфических клетках.
Несмотря на широкий спектр описанных физиологических эффектов серотонина в развитии млекопитающих и человека, до настоящего времени нет ясности, какие именно молекулярные механизмы лежат в основе обнаруженных эпигенетических эффектов. Долговременные трансглутаминаз-опосредованные модификации белков представляются нам одними из вероятных кандидатов на такую роль. Как видно из приведенной выше литературы, локальный уровень моноаминов и, в частности, серотонина как субстрата для трансглутминаз является значимым модулятором формирования различных функциональных систем. Опять же подчеркнем, что на разных стадиях эмбрионального развития затрагиваются компоненты разных функциональных систем, не ограниченных только нервной тканью. Кроме эффектов на собственно серотонинергические нейроны (Farrelly et аl., 2019), показано, что серотонилирование задействовано в формировании иммунной системы (Ahern, 2011), развитии иммунного ответа (Abdala-Valencia et al., 2012), осуществлении связи нервной и иммунной систем (Baganz, Blakely, 2012), вовлечено в регуляцию привлечения остеокластов при росте и развитии костной ткани (Chabbi-Achengli et al., 2012). Эти данные позволяют говорить о целой аутокринно-паракринной сети на основе локальных серотонинергических систем, тесно скоординированных с нервной системой (Amireault et al., 2013).
ВЫВОДЫ
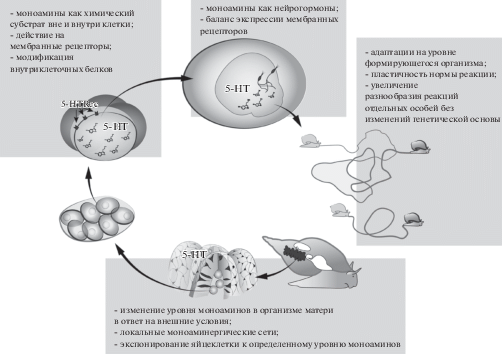
Таким образом, активация разных мембранных рецепторов, повышение внутриклеточного уровня серотонина (моноаминов) за счет работы транспортеров, моноаминилирование структурных и регуляторных белков, зависимость уровня моноаминов матери и плода от внешних факторов – совокупность и взаимосвязь этих механизмов создают молекулярно-биохимическую основу для формирования адаптивной пластичности в процессе развития (рис. 1). Можно считать, что в настоящее время открывается новая страница в исследовании эффектов моноаминов. Становится понятным, в каком месте искать механизмы, лежащие в основе их отставленных долговременных эффектов, которые невозможно было объяснить, основываясь только на их классическом рецепторном действии.
Рис. 1.
Представление о регуляторной роли моноаминов и механизмах их действия на разных стадиях развития на примере жизненного цикла моллюска.
Fig. 1. Schematic representation of monoamines regulatory role and suggested mechanisms in the course of development (on the example of the molluskan life cycle).
Список литературы
Бузников Г.А., Манухин Б.Н. Серотонин-подобные вещества в эмбриогенезе некоторых брюхоногих моллюсков. Журн. общ биол. 1961. 22: 223–229.
Бузников Г.А. Донервные трансмиттеры как регуляторы эмбриогенеза. Современное состояние проблемы. Онтогенез. 2007. 38 (4): 262–270.
Воронежская Е.Е., Хабарова М.Ю. Функция апикального органа в развитии беспозвоночных. ДАН. 2003. 390 (2): 272–275.
Воронежская Е.Е., Хабарова М.Ю., Чабан А.К., Незлин Л.П. Влияние химической сигнализации на реализацию моторных программ в эмбриогенезе легочных моллюсков Lymnaea stagnalis и Helisoma trivolvis. Онтогенез. 2007. 38 (2): 94–104.
Дьяконова В.Е. Нейротрансмиттерные механизмы контекст-зависимого поведения. Журнал высшей нервной деятельности им. И.П. Павлова. 2012. 62 (6): 1–17.
Мещеряков В.Н. Прудовик Lymnaea stagnalis L. Объекты биологии развития: справ.-метод. пособие. М. Наука. 1975. 53–94.
Никишин Д.А., Алешина Н.М., Шмуклер Ю.Б. Синтез и мембранный транспорт серотонина в развивающемся овариальном фолликуле мыши. ДАН. 2018а. 478 (1): 103–106.
Никишин Д.А., Храмова Ю.В., Багаева Т.С., Семенова М.Л., Шмуклер Ю.Б. Экспрессия компонентов серотонинергической системы в фолликулогенезе и доимплантационном развитии мыши. Онтогенез. 2018b. 49 (3): 208–216.
Сахаров Д.А. Множественность нейротрансмиттеров: функциональное значение Журн. эвол. биохим. физиол. 1990. 26: 733–740.
Сахаров Д.А. Биологический субстрат генерации поведенческих актов. Ж. общ. биол. 2012. 73 (5) 334–348.
Шмуклер Ю.Б., Никишин Д.А. О внутриклеточной рецепции медиаторов. Нейрохимия. 2018. 35 (4): 289–293.
Abdala-Valencia H., Berdnikovs S., McCary C.A., Urick D., Mahadevia R., Marchese M.E., Swartz K., Wright L., Mutlu G.M., Cook-Mills J.M. Inhibition of allergic inflammation by supplementation with 5-hydroxytryptophan.Am. J. Physiol. Lung Cell. Mol. Physiol. 2012. 303 (8): L642–60.
Ahern G.P. 5-HT and the immune system. Curr Opin Pharmacol. 2011 11 (1): 29–33.
Amireault P., Dubé F. Intracellular cAMP and Calcium Signaling by Serotonin in Mouse Cumulus-Oocyte Complexes. Mol. Pharmacol. 2005a. 68 (6): 1678–1687.
Amireault P., Dubé F. Serotonin and its Antidepressant-Sensitive Transport in Mouse Cumulus-Oocyte Complexes and Early Embryos. Biol. Reprod. 2005b. 73: 358–365.
Amireault P., Sibon D., Côté F. Life without Peripheral Serotonin: Insights from Tryptophan Hydroxylase 1 Knockout Mice Reveal the Existence of Paracrine/Autocrine Serotonergic Networks. ACS Chem. Neurosci. 2013. 4 (1): 64–71.
Aonuma H., Mezheritskiy M., Boldyshev B., Totani Y., Vorontsov D., Zakharov I., Ito E., Dyakonova V. The role of serotonin in the influence of intense locomotion on the behavior under uncertainty in the mollusk Lymnaea stagnalis. Frontiers in Physiology. 2020. 11. Art. 221. https://doi.org/10.3389/fphys.2020.00221
Bader M. Serotonylation: Serotonin Signaling and Epigenetics. Front. Mol. Neurosci. 2020. 12: 288.
Baganz N.L., Blakely R.D. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci. 2013. 4 (1): 48–63.
Basu B., Desai R., Balaji J., Chaerkady R., Sriram V., Maiti S., Panicker M. M. Serotonin in pre-implantation mouse embryos is localized to the mitochondria and can modulate mitochondrial potential. Reproduction. 2008. 135: 657–669.
Beyer T., Danilchik M., Thumberger T., Vick P., Tisler M., Schneider I., Bogusch S., Andre P., Ulmer B., Walentek. P, Niesler B., Blum M., Schweickert A. Serotonin signaling is required for Wnt-dependent GRP specification and leftward flow in Xenopus. Curr. Biol. 2012. 22 (1): 33–39.
Bonnin A., Goeden N., Chen K., Wilson M.L., King J., Shih J.C., Blakely R.D., Deneris E.S., Levitt P. A transient placental source of serotonin for the fetal forebrain. Nature. 2011. 472 (7343): 347–350.
Bonnin A., Levitt P. Fetal, maternal and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 2011. 197: 1–7.
Burden H.W., Lawrence I.E. Jr. Presence of biogenic amines in early rat development. Am. J. Anat. 1973. 136 (2): 251–257.
Buznikov G.A., Chudakova I.V., Zvezdina N.D. The role of neurohumours in early embryogenesis. i. serotonin content of developing embryos of sea urchin and loach. J. Embryol. Exp. Morphol. 1964. 12: 563–573.
Buznikov G.A., Lambert H.W., Lauder J.M. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell and Tissue Res. 2001.305 (2): 177–186.
Buznikov G.A., Nikitina L.A., Voronezhskaya E.E., Bezuglov V.V., Willows A.D., Nezlin L.P. Localization of serotonin and its possible role in early embryos of Tritonia diomedea (Mollusca: Nudibranchia). Cell and Tissue Research. 2003. 311: 259–266.
Candiani S., Augello A., Oliveri D., Passalacqua M., Pennati R., De Bernardi F., Pestarino M. Immunocytochemical localization of serotonin in embryos, larvae and adults of the lancelet. Branchiostoma floridae. Histochem. J. 2001. 33: 413–420.
Chabbi-Achengli Y., Coudert A.E., Callebert J., Geoffroy V., Côté F., Collet C., de Vernejoul M.C. Decreased osteoclastogenesis in serotonin-deficient mice. Proc. Natl. Acad. Sci. U S A. 2012. 109 (7): 2567–2572.
Chen H.J., Antonson A.M., Rajasekera T.A., Patterson J.M., Bailey M.T., Gur T.L. Prenatal stress causes intrauterine inflammation and serotonergic dysfunction, and long-term behavioral deficits through microbe- and CCL2-dependent mechanisms. Transl. Psychiatry. 2020. 10 (1): 191.
Cooney C.A., Dave A.A., Wolff G.L. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J. Nutr. 2002. 132: 2393S–2400S.
Dias B.G., Ressler K.J. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 2014. 17 (1): 89–96.
Dubé F., Amireault P. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life Sci. 2007. 81 (25–26): 1627–1637.
Duerschmied D., Bode C. The role of serotonin in haemostasis. Hamostaseologie. 2009. 29 (4): 356–359.
Emanuelsson H. Localization of serotonin in cleavage embryos of Ophryotrocha labronica La Greca and Bacci. 1974. Dev. Genes Evol. 175: 253–271.
Emanuelsson H., Carlberg M., Lowkvist B. Presence of serotonin in early chick embryos. Cell. Differ. 1988. 24: 191–199.
Farrelly L.A., Thompson R.E., Zhao S., Lepack A.E., Lyu Y., Bhanu N.V., Zhang B., Loh Y.-H.E., Ramakrishnan A., Vadodaria K.C., Heard K.J., Erikson G., Nakadai T., Bastle R.M., Lukasak B.J., Zebroski H., Alenina N., Bader M., Berton O., Roeder R.G., Molina H., Gage F.H., Shen L., Garcia B.A., Li H., Muir T.W., Maze I. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature. 2019. 567: 535–539.
Fossat P., Bacqué-Cazenave J., De Deurwaerdère P., Delbecque J.P., Cattaert D. Comparative behavior. Anxiety-like behavior in crayfish is controlled by serotonin. Science. 2014. 344 (6189): 1293–1297.
Fukumoto T., Kema I.P., Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr. Biol. 2005. 15: 794–803.
Glebov K., Voronezhskaya E.E., Khabarova M.Y., Ivashkin E., Nezlin L.P., Ponimaskin E.G. Mechanisms underlying dual effects of serotonin during development of Helisoma trivolvis (Mollusca). 2014. BMC Dev. Biol. 14: 14.
Guilluy C., Rolli-Derkinderen M., Tharaux P.L., Melino G., Pacaud P., Loirand G. Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J. Biol. Chem. 2007. 282 (5): 2918–2928.
Hummerich R., Schloss P. Serotonin-more than a neurotransmitter: transglutaminase-mediated serotonylation of C6 glioma cells and fibronectin. Neurochem. Int. 2010. 57 (1): 67–75.
Hummerich R., Thumfart J.O., Findeisen P., Bartsch D., Schloss P. Transglutaminase-mediated transamidation of serotonin, dopamine and noradrenaline to fibronectin: evidence for a general mechanism of monoaminylation. FEBS Lett. 2012. 586 (19): 3421–3428.
Hummerich R., Costina V., Findeisen P., Schloss P. Monoaminylation of Fibrinogen and Glia-Derived Proteins: Indication for Similar Mechanisms in Posttranslational Protein Modification in Blood and Brain. ACS Chem. Neurosci. 2015. 6 (7): 1130–1136.
Hinckley M., Vaccari S., Horner K., Chen R., Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev. Biol. 2005. 287 (2): 249–261.
Il’kova G., Rehak P., Vesela J., Cikos S., Fabian D., Czikkova S., Koppel J. Serotonin localization and its functional significance during mouse preimplantation embryo development. Zygote. 2004. 12 (3): 205–213.
Issa T.B., Sagaama A., Issaoui N. Computational study of 3-thiophene acetic acid: Molecular docking, electronic and intermolecular interactions investigations. Comput. Biol. Chem. 2020. 86: 107268.
Ivashkin E., Khabarova M., Voronezhskaya E. Serotonin transport and synthesis systems during early development of invertebrates: Functional analysis on a bivalve model. Acta Biol. Hung. 2012. 63: 217–220.
Ivashkin E., Khabarova M., Melnikova V., Nezlin L., Kharchenko O., Voronezhskaya, E., Adameyko I. Serotonin mediates maternal effects and directs developmental and behavioral changes in the progeny of snails. Cell Rep. 2015. 12: 1144–1158.
Ivashkin E., Melnikova V., Kurtova A., Brun N.R., Obukhova A., Khabarova M.Y., Voronezhskaya E.E. Transglutaminase activity determines nuclear localization of serotonin immunoreactivity in the early embryos of invertebrates and vertebrates. ACS Chem. Neurosci. 2019. 10: 3888–3899.
Jong Y.I., Harmon S.K., O’Malley K.L. GPCR signalling from within the cell. Br. J. Pharmacol. 2018. 175 (21): 4026–4035.
Khabarova M.Y., Voronezhskaya E.E. Pharmacological analysis of locomotion and heart contraction during the development of Helisoma (Mollusca: Gastropoda). Acta Biol. Hung. 2012. 63 (2): 206–209.
Koene J.M. Neuro-endocrine control of reproduction in hermaphroditic freshwater snails: mechanisms and evolution. Frontiers in behavioral neuroscience 2010. 4: 167.
Mercado C.P., Ziu E., Kilic F. Communication between 5-HT and small GTPases. Curr Opin Pharmacol. 2011. 11 (1): 23–28.
Mousseau T.A., Fox C.W. The adaptive significance of maternal effects. Trends Ecol Evol. 1998. 13 (10): 403–407.
Muma N.A., Mi Z. Serotonylation and transamidation of other monoamines. ACS Chem. Neurosci. 2015. 6: 961–969.
Neilson L., Andalibi A., Kang D., Coutifaris C., Strauss J.F., Stanton J.A., Green D.P. Molecular phenotype of the human oocyte by PCR-SAGE. Genomics. 2000. 63 (1): 13–24.
Nikishin D.A., Kremnyov S.V., Konduktorova V.V., Shmukler Y.B. Expression of serotonergic system components during early Xenopus embryogenesis. Int. J. Dev. Biol. 2012. 56 (5): 385–391.
Nikishin D.A., Milošević I., Gojković M., Rakić L., Bezuglov V.V., Shmukler Y.B. Expression and functional activity of neurotransmitter system components in sea urchins' early development. Zygote. 2016. 24 (2): 206–218.
Nikishin D.A., Alyoshina N.M., Semenova M.L., Shmukler Y.B. Analysis of Expression and Functional Activity of Aromatic L-Amino Acid Decarboxylase (DDC) and Serotonin Transporter (SERT) as Potential Sources of Serotonin in Mouse Ovary. Int. J. Mol. Sci. 2019. 20 (12): 3070.
Numanoi N. Studies on the fertilization substance. V. Distribution of acetycholine esterase in egg particles of the sea urchin, Hemicentrotus pulcherrhimus. 1955. Scient. Papers Coll. Gen. Educ. Univ. Tokyo. 5: 37–41.
Pavone L.M., Norris R.A. Distinct signaling pathways activated by “extracellular” and “intracellular” serotonin in heart valve development and disease. Cell. Biochem. Biophys. 2013. 67 (3): 819–828.
Paulmann N., Grohmann M., Voigt J.P., Bert B., Vowinckel J., Bader M., Skelin M., Jevsek M., Fink H., Rupnik M., Walther D.J. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009. 7 (10): e1000229.
Räsänen K., Kruuk L.E.B. Maternal effects and evolution at ecological time-scales. Functional Ecology. 2007. 21 (3): 408–421.
Sakharov D.A. Integrative function of serotonin common to distantly related invertebrate animals. Early Brain. Ed. Gustafsson M., Reuter M. Abo: Abo Akademi Press. 1990. 73–88 pp.
Sato K. Placenta-derived hypo-serotonin situations in the developing forebrain cause autism. Med. Hypotheses. 2013. 80 (4): 368–372.
Schmidt-Rhaesa A., Harzsch S., Purschke G. Structure and Evolution of Invertebrate Nervous Systems. Oxford: Oxford University Press. 2016.
St-Pierre J., Laurent L., King S., Vaillancourt C. Effects of prenatal maternal stress on serotonin and fetal development. Placenta. 2016. 48(1): S66-S71.
Sze J.Y., Victor M., Loer C., Shi Y., Ruvkun G. Food and metabolic signaling defects in a serotonin-synthesis mutant. Nature. 2000. 403: 560–564.
Tanaka T., Doe J.M., Horstmann S.A., Ahmad S., Ahmad A., Min S.J., Reynolds P.R., Suram S., Gaydos J., Burnham E.L., Vandivier R.W. Neuroendocrine signaling via the serotonin transporter regulates clearance of apoptotic cells. J. Biol. Chem. 2014.289 (15): 10466–10475. Caenorhabditis elegans
Ugrumov M.V. Hypothalamic monoaminergic systems in ontogenesis: development and functional significance. Int. J. Dev. Biol. 1997. 41 (6): 809–816.
Vitalis T., Ansorge M.S., Dayer A.G. Serotonin homeostasis and serotonin receptors as actors of cortical construction: special attention to the 5-HT3A and 5-HT6 receptor subtypes. Front. Cell. Neurosci. 2013. 7: 93.
Voronezhskaya E.E., Nezlin L.P., Khabarova M.Yu. Apical sensory neurons mediate developmental retardation induced by conspecific environmental stimuli in freshwater pulmonate snails. Development. 2004. 131: 3671–3680.
Voronezhskaya E.E., Khabarova M.Y., Nezlin L.P., Ivashkin E.G. Delayed action of serotonin in molluscan development. Acta Biol. Hung. 2012. 63 (2): 210–216.
Walther D.J., Peter J.U., Winter S., Höltje M., Paulmann N., Grohmann M., Vowinckel J., Alamo-Bethencourt V., Wilhelm C.S., Ahnert-Hilger G., Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet a-granule release. Cell. 2003. 115: 851–862.
Walther D.J., Stahlberg S., Vowinckel J. Novel roles for biogenic monoamines: from monoamines in transglutaminase-mediated posttranslational protein modification to monoaminylation deregulation diseases. FEBS J. 2011. 278: 4740–4755.
Waterland R.A., Jirtle R.L. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 2003. 23 (15): 5293–5300.
Watts S.W., Priestley J.R., Thompson J.M. Serotonylation of vascular proteins important to contraction. PLoS One. 2009. 4: e5682.
Weaver I.C., Cervoni N., Champagne F.A., D’Alessio A.C., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004. 7 (8): 847–854.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


