Журнал высшей нервной деятельности им. И.П. Павлова, 2021, T. 71, № 2, стр. 164-183
Роль стриатума в организации произвольного движения
Н. Ю. Ивлиева *
Федеральное государственное бюджетное учреждение науки Институт высшей нервной деятельности
и нейрофизиологии РАН
Москва, Россия
* E-mail: nivlieva@mail.ru
Поступила в редакцию 21.07.2020
После доработки 16.10.2020
Принята к публикации 22.12.2020
Аннотация
Стриопаллидарная система является ключевой структурой в регуляции моторного поведения (Базян и др., 2011), однако механизмы и специфика ее участия в организации движения не прояснены даже в общих чертах. За последние годы были разработаны многочисленные молекулярно-генетические подходы к исследованию функций стриатума; результаты этих исследований пролили свет на организацию релевантных связей и на функционирование отдельных его элементов и этим еще больше заострили основные противоречия во взглядах на роль стриопаллидарной системы в двигательном поведении; в первую очередь это касается функций нейронов, дающих начало прямому и непрямому пути стриатума, а также участия дофаминергической системы в организации движения. В этой работе приведен краткий обзор новых данных о связях стриатума и рассмотрены последние исследования, в которых, во-первых, в центре внимания находилась двигательная функция и, во-вторых, в экспериментальной парадигме в явном виде не присутствовало научение.
ОБЩИЕ СВЕДЕНИЯ О СТРУКТУРЕ И СВЯЗЯХ СТРИОПАЛЛИДАРНОЙ СИСТЕМЫ
Базальные ганглии (БГ) – эволюционно довольно консервативный структурный модуль, сохраняющий общие черты организации от ланцетника до человека (Grillner, Robertson, 2016). Ключевым образованием базальных ганглиев является полосатое тело (стриатум (corpus striatum)), часто подразделяемое на дорзальный и вентральный стриатум (последний также называют прилежащим ядром (nucleus accumbens)). Также базальные ганглии включают бледный шар (globus pallidus) и вентральный паллидум, ретикулярную часть черного вещества (substantia nigra pars reticulata (SNr)). В состав стриопаллидарной системы также входит субталамическое ядро (nucleus subthalamicus (STN)) (рис. 1). В зависимости от вида животного состав и названия структур, образующих базальные ганглии, могут варьироваться; иногда к БГ относят среднемозговой локомоторный регион. Мы ради простоты не станем включать эти вариации в общую схему, а уточнения будем вводить в тексте по мере необходимости. Сокращенные названия структур в русскоязычной литературе довольно разнообразны и являются плохо узнаваемыми на письме, поэтому далее мы будем использовать аббревиатуры, основанные на латинских названиях.
Рис. 1.
Схема основных связей базальных ганглиев. Кружками обозначены стриато-нигральные (нейроны прямого пути, экспрессирующие D1-рецепторы к дофамину) и стриато-паллидарные нейроны (нейроны непрямого пути, экпрессирующие D2-рецепторы). ILTh – интраламинарные ядра таламуса (являются источниками основных таламических проекций в стриатум), MTh – преимущественно моторные ядра таламуса. STN – субталамическое ядро, SNr – ретикулярная часть черного вещества среднего мозга, SNc – компактная (дофаминергическая) часть черного вещества, VTA – вентральная область покрышки среднего мозга.
Fig. 1. Diagram of the main connections of the basal ganglia. The circles indicate striato-nigral (direct pathway neurons expressing D1 receptors) and striato-pallidal neurons (indirect pathway neurons expressing D2 receptors). ILTh – intralaminar nuclei of the thalamus (the sources of the main thalamic projections into the striatum), MTh – mainly the motor nuclei of the thalamus. STN – the subthalamic nucleus, SNr – substantia nigra pars reticulata, SNc – substantia nigra pars compacta (dopaminergic), VTA – ventral tegmental area.
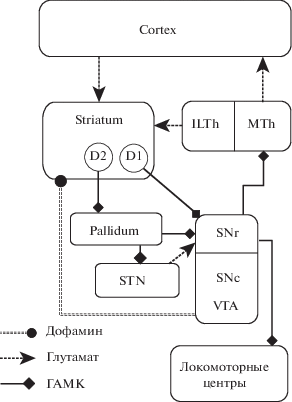
Основные возбуждающие входы в стриатум образованы глутаматергическими нейронами коры больших полушарий, в прилежащее ядро приходят проекции главным образом из гиппокампа, префронтальной коры и миндалевидного тела. Помимо корковых входов, в дорзальную и вентральные части стриатума проецируются нейроны таламических ядер (Haber, 2016), наиболее представленными из которых являются входы от интраламинарных ядер (прежде всего, из парафасцикулярного ядра (Wall et al., 2013; Mandelbaum et al., 2019)), ядер средней линии (Fujiyama et al., 2019) и от медиодорзального ядра таламуса (Wall et al., 2013; Hunnicutt et al., 2016).
Также стриатум в целом является главной мишенью для дофаминергических нейронов среднего мозга, где VTA (ventral tegmental area) отправляет проекции главным образом в вентральный, а SNc (substantia nigra pars compacta) – в дорзальный стриатум (рис. 1). Дофаминовая модуляция является ключевой в работе БГ и опосредуется главным образом D1- и D2-рецепторами, или, точнее, соответствующими семействами рецепторов, связанных с G-белком, у первого из которых сродство к дофамину существенно ниже (Neve, Neve, 1997; Richfield et al., 1989; но см. также обсуждение у Yapo et al., 2017).
Большая часть дорзального стриатума имеет неоднородную структуру и представлена матриксом и стриосомами, выделяемыми на основе нейрохимических особенностей (Brimblecombe, Cragg, 2017). Исключение составляют дорзолатеральные области, в которых стриосомы отсутствуют (Miyamoto et al., 2018). Прилежащее ядро подразделяется на собственно “ядро” (core) и “оболочку” (shell).
Подавляющее большинство нейронов стриатума (около 90–95%, соотношение несколько варьирует у представителей разных видов) представлено проекционными ГАМК-ергическими клетками, так называемыми средними шипиковыми нейронами (medium spiny neurons, MSN), многочисленные коллатерали которых заканчиваются и внутри стриатума. Среди интернейронов стриатума в большей степени исследованы, во-первых, холинергические клетки, их с высокой степенью достоверности соотносят с тонически активными нейронами, во-вторых, ГАМК-ергические парвальбуминовые клетки, соотносимые с быстро разряжающимися интернейронами.
Аксоны проекционных нейронов стриатума довольно четко подразделяются на две группы: те, что направляются сразу в ретикулярную часть черного вещества, являющуюся главным выходным звеном базальных ганглиев, и этим формируют так называемый прямой путь, и те, которые идут в бледный шар, и таким образом дают начало непрямому пути. После переключения в бледном шаре проекции направляются либо напрямую в ретикулярную часть черного вещества, либо через переключение в субталамическом ядре (Haber, 2016). Важной особенностью этих путей является то, что для них (в большей степени в дорзальном стриатуме) установлена существенная сегрегация D1- и D2-рецепторов, экспрессирующихся на клетках, соответственно, прямого и непрямого пути (Gerfen, Surmeier, 2011; Gong et al., 2003; но см. также Cazorla et al., 2014). Проекционные нейроны бледного шара и ретикулярной части черного вещества являются автономными пейсмейкерами. Разделяемая большинством исследователей точка зрения состоит в том, что в покое локомоторные центры среднего и промежуточного мозга (двигательные таламические ядра) находятся под влиянием тонического торможения со стороны базальных ганглиев. Также многие авторы сходятся на том, что ключевая роль базальных ганглиев состоит в организации действия, и посредством прямого пути движение облегчается, а непрямой путь движение затрудняет, а дофамин в свою очередь способствует проведению по прямому пути, повышая возбудимость экспрессирующих D1-рецепторы MSNs, и уменьшает активность непрямого пути, снижая возбудимость D2-экспрессирующих нейронов (Bariselli et al., 2018; Gerfen, Surmeier, 2011; Kreitzer, 2009).
На основе этих хрестоматийных сведений о структуре стриопаллидарной системы было предложено немало моделей, объясняющих работу стриатума в норме и при паркинсонизме (Albin et al., 1989), хорее Хантингтона (Plotkin, Goldberg, 2019), шизофрении (Maia, Frank, 2017), зависимости (Nestler, Lüscher, 2019), заикании (Civier et al., 2013) и т.д. Однако накопившиеся к настоящему моменту данные, уточняющие свойства ключевых элементов базальных ганглиев и особенности их связей с выше- и нижележащими структурами, предполагают гораздо более сложную картину. Ниже мы кратко остановимся на этих данных и рассмотрим исследования, в которых, во-первых, в центре внимания находилась двигательная функция и, во-вторых, в экспериментальной парадигме в явном виде не присутствовало научение. Цель статьи – обратить внимание на парадокс одновременной активации нейронов, дающих начало прямому и непрямому пути стриатума при движении.
ВХОДЫ В СТРИАТУМ ИЗ КОРЫ БОЛЬШИХ ПОЛУШАРИЙ И ТАЛАМУСА
Стриатум представляет собой массивное и, как уже отмечалось, неоднородное образование, получающее множество корковых входов. При этом в силу сложности организации он не может быть четко подразделен на подструктуры, такие как ядра, и даже на более или менее определенные области, получающие, например, преимущественные входы из сенсорной, моторной, ассоциативной или лимбической коры (Miyamoto et al., 2018).
Подробнейший анализ картины кортико-стриатных проекций мозга мыши был проведен Хинтирян и соавт. (Hintiryan et al., 2016). В дополнение к корковым входам Ханникатт с сотрудниками проанализировали также распределение таламических входов (Hunnicutt et al., 2016). Было показано, что все области коры посылают проекции в стриатум. Обе группы исследователей полагают возможным условное деление стриатума на сенсомоторный, ассоциативный и лимбический, c определенными оговорками, но обращают особое внимание на каудальную область стриатума, уникальную сочетанием выраженных лимбических входов и входов из зрительной и слуховой коры. Приблизительно эту же область выделяют Миямото и соавт. (Miyamoto et al., 2018) как зону, богатую энкефалином. Интересно, что в нее проецируется подкласс дофаминовых нейронов, обладающих связями, существенно отличными от связей большинства дофаминовых нейронов (Menegas et al., 2015). И более того, самая каудальная часть этой области, получающая входы от первичной слуховой коры, по данным Гангароссы и соавт., в отличие от всего остального стриатума, совсем не содержит D2-экспрессирующих нейронов (Gangarossa et al., 2013).
Сенсомоторные области довольно компактно представлены в промежуточном дорзальном стриатуме, однако они также имеют представительства в передних и задних его областях, из которых последние характеризуются высочайшей степенью взаимодействия различных входов. Входы из отделов коры, которые могут быть вовлечены в обеспечение взаимодействия между различными корковыми сетями, проецируются в центральные, очень ограниченные регионы дорзального стриатума – по контрасту со входами из отделов коры, более тесно связанных с сенсорными проекциями, широко распространяющимися по периферии стриатума (Hintiryan et al., 2016). Авторы предполагают, что такая организация в общих чертах сходна со структурой связей в прилежащем ядре. Интересная особенность исследования Хинтирян с соавторами состоит в том, что они попытались соотнести организацию входов из коры в стриатум со структурной организацией собственно корковых сетей, исследованной ими ранее (Zingg et al., 2014). Также авторами продемонстрирована соматотопия для входов из моторной и соматосенсорной коры для мозга мыши и показана высокая степень взаимосвязанности всех соответствующих определенным частям тела областей (Hintiryan et al., 2016). Ранее соматотопическая организация базальных ганглиев была подробно разобрана на приматах (Nambu, 2011). Детальный анализ входов в стриатум из парафасцикулярного ядра таламуса мыши также подтвердил его деление на лимбические, ассоциативные и соматосенсорные области (Mandelbaum et al., 2019); авторы исследования показали, что в эти области стриатума проецируются существенно различающиеся по электрофизиологическим свойствам, а также по паттерну транскрипции нейроны парафасцикулярного ядра, также они показали, что эти нейроны синаптически не связаны между собой внутри таламуса, но реципрокно связаны с корковыми областями, проецирующимися в соответствующие области стриатума.
Помимо возбудительных корковых входов в стриатум описаны также прямые тормозные входы из слуховой и моторной коры к проекционным нейронам (Melzer et al., 2017; Rock et al., 2016), и, в частности, показано, что парвальбуминовые и соматостатиновые ГАМК-ергические нейроны моторной коры дают проекции в дорзальный стриатум и устанавливают там связи как с проекционными стриато-нигральными и стриато-паллидарными нейронами, так и с холинергическими интернейронами (Melzer et al., 2017).
ОСОБЕННОСТИ ВХОДОВ К РАЗНЫМ ПОДРАЗДЕЛЕНИЯМ И ЭЛЕМЕНТАМ СТРИАТУМА
Как уже упоминалось, на основании нейрохимических маркеров стриатум может быть поделен на матрикс и стриосомы. Гистохимически стриосомы определяются по высокому содержанию μ-опиоидных рецепторов, вещества P, дофаминовых D1-рецепторов, мет-энкефалина и других веществ. Матрикс содержит существенно больше калбиндина (calbindin), соматостатина, энкефалина, D2-рецепторов дофамина и выделяется, в первую очередь, на основании высокого содержания холинергических маркеров: холинацетилтрансферазы и холинестеразы, но такое подразделение на два компартмента не является безусловным, поэтому иногда выделяют дополнительные области или промежуточные зоны, не вписывающиеся однозначно в имеющиеся для двух описанных компартментов характеристики (Brimlecombe, Cragg, 2016). Миямото и соавт. (Miyamoto et al., 2018) не рассматривают и собственно стриосомы как однородные образования, но подразделяют их на пять типов, также на основе гистохимических критериев. Пропорции D1-экспрессирующих нейронов в стриосомах, содержащих вещество P, в областях, свободных от стриосом, и в матриксе составили, по оценке авторов исследования, приблизительно 70, 30 и 50% соответственно, а пропорции D2-экспрессирующих нейронов были им комплементарны. Помимо этого авторы отдельно выделяют дорзолатеральную зону, свободную от стриосом, которая получает входы преимущественно от первичных соматосенсорной и моторной областей коры, и указывают на то, что регионы с большим числом стриосом получают в основном афференты от ассоциативных и лимбических областей коры. Сходные с этими выводы делают и Смит и соавт. (Smith et al., 2016), а также они обращают внимание на существенное преобладание входов из подкорковых лимбических структур (в том числе и таламических) в стриосомы по сравнению с матриксом. С помощью электронного микроскопа показано, что в матриксе таламические входы заканчиваются в 70% случаев на стволах дендритов проекционных нейронов, а в стриосомах 84% входов из таламуса заканчиваются на дендритных шипиках (Fujiyama et al., 2019). Также здесь отметим, что основным таламическим источником окончаний, формирующих синапсы на стволе дендрита, является парафасцикулярное ядро таламуса (Smith et al., 2009). Важной особенностью стриосом является то, что именно в них берут начало прямые проекции из стриатума к дофаминергическим нейронам среднего мозга (Smith et al., 2016; Watabe-Uchida et al., 2012).
Проекционные нейроны, дающие начало прямому и непрямому пути, во многом разделяют общие входы из коры, однако существуют и определенные особенности: входы из первичной моторной коры существенно преобладают у нейронов непрямого пути, они опосредованы коллатералями пирамидного тракта и интерпретируются как передающие сигнал об эфферентной копии запускаемого через моторную кору движения (Reiner et al., 2010; Wall et al., 2013). Нейроны же прямого пути получают заметно больше проекций из миндалевидного тела и лимбических областей коры (Wall et al., 2013).
Взаимные связи стриатума и дофаминергических проекций организованы так, что вентральный стриатум может влиять на дорзальный. Проекции из оболочки прилежащего ядра в средний мозг направляются как в VTA, так и в медиальную часть SNc, а проекции из VTA направляются обратно в оболочку, формируя замкнутую петлю, но также они проецируются более латерально к клеткам стриатума, которые в свою очередь проецируются к более латерально расположенным дофаминовым клеткам; и такая спиральная организация присуща всем взаимным связям стриатума и дофаминергического среднего мозга (Haber et al., 2000).
ИНТЕРНЕЙРОНЫ СТРИАТУМА
Как уже говорилось, интернейроны составляют не более 5% от клеточного состава стриатума у грызунов и, по разным оценкам, около 20% у приматов. Они подразделяются на крупные холинергические нейроны и гораздо более мелкие ГАМК-ергические клетки. Холинергические клетки обладают радиально разветвленными дендритами без шипиков. Посредством длинных ветвящихся аксонов эта небольшая популяция интернейронов выделяет ацетилхолин во всем стриатуме (Abudukeyoumu et al., 2019). Благодаря этим нейронам стриатум является одной из богатейших ацетилхолином структур мозга. Большинство синаптических контактов на них – от тормозных нейронов. Помимо внутренних нейронов стриатума тормозные входы к ним представлены аксонами клеток наружного сегмента бледного шара (Mallet et al., 2012; Klug et al., 2018), а также из моторной коры (Melzer et al., 2017). Из возбудительных входов доминируют связи с таламическими нейронами (Abudukeyoumu et al., 2019; Assous, Tepper, 2019), хотя в недавнем исследовании показано общее превалирование входов из ассоциативных областей коры (Klug et al., 2018). Холинергические нейроны не формируют многочисленных структурно определяемых синаптических контактов, что позволяет говорить скорее о медленной “объемной трансмиссии” (volume transmission) ацетилхолина в стриатуме. Многократно показано, что эти нейроны могут также выделять и глутамат (Abudukeyoumu et al., 2019). В электрофизиологических исследованиях холинергические нейроны стриатума чаще всего упоминаются как тонически активные нейроны (однако это не единственный тип тонически активных нейронов в стриатуме).
Классификация тормозных интернейронов является чрезвычайно сложной задачей, так как эти клетки существенно различаются по своему химическому составу, размеру, морфологии, связям, электрофизиологическим свойствам, паттерну транскрипции и т.д., и по многим параметрам их свойства перекрываются. Новые подходы к классификации находятся в стадии становления. Число выделяемых типов варьирует в разных источниках. Исходя из принятой на сегодняшний день классификации, на основании нейрохимического состава более или менее определенно выделяют следующие типы клеток: нейроны, содержащие (1) парвальбумин, (2) соматостатин (а также синтазу оксида азота и нейропептид У), (3) калретинин и (4) тирозин-гидроксилазу; в дополнение к ним в последние годы выделяют клетки, экспрессирующие ионный серотониновый рецептор 5-HT3a, а также нейроглиаформные клетки, каждые из которых также подразделяются на подтипы; и в дополнение к этому новые подтипы выделяют среди клеток, принадлежащих к ранее выделенным типам (Tepper et al., 2018). Отдельной проблемой является соотнесение этих выделенных типов клеток с классификацией на основе физиологических критериев (Burke et al., 2017).
Большинство ГАМК-ергических интернейронов стриатума получают входы как из коры, так и из таламуса. Однако соотношение этих входов для каждого типа интернейронов существенно варьируется. Например, нейроглиаформные клетки (NGF) получают существенно больше входов из таламуса, а парвальбуминовые нейроны – из коры (преимущественно из соматосенсорной и соотносящейся с ней моторной коры (Klug et al., 2018), причем эти входы оказывают на них более сильное возбуждающее влияние, чем на соседние проекционные нейроны (Johansson, Silberberg, 2020)). Интересно, что парвальбуминовые нейроны, помимо прочего, получают тормозные входы из ретикулярного ядра таламуса (Klug et al., 2018). Для соматостатиновых нейронов у грызунов подтверждены почти исключительно корковые входы (эти клетки описаны как физиологически идентифицированные LTS-нейроны у Assous и Tepper (2019)).
Парвальбуминовые нейроны связаны между собой электрическими синапсами; эти клетки также тесно связаны с проекционными нейронами стриатума (Hjorth et al., 2009; Tepper et al., 2018), при этом – почти исключительно с проекционными нейронами, формируя сильные синапсы в проксимальной части их дендритов (Straub et al., 2016), это отличает парвальбуминовые нейроны от всех остальных интернейронов, связанных друг с другом в различных комбинациях, детально описанных Ассусом и Теппером (Assous, Tepper, 2019). Здесь добавим также, что было продемонстрировано существенное преимущество в числе входов от быстро разряжающихся тормозных интернейронов стриатума (ассоциирующихся с парвальбуминовыми нейронами) к проекционным нейронам прямого пути по сравнению с нейронами непрямого пути (Gerfen, Surmeier, 2011).
ДОФАМИНЕРГИЧЕСКИЕ ВХОДЫ В СТРИАТУМ
Как уже упоминалось, стриатум является ключевой мишенью для дофаминергических нейронов среднего мозга. VTA направляет проекции главным образом в прилежащее ядро, а SNc – в дорзальный стриатум. По уровню содержанию дофамина стриатум стоит на первом месте в мозге. Дофамин выделяется как в синаптических окончаниях, так и в местах расширения аксонов; интересно, что и через такие аксонные расширения в стриатуме осуществляется как адресная передача, так и объемная трансмиссия дофамина (Liu et al., 2018). Основной мишенью этого медиатора являются проекционные нейроны стриатума и холинергические нейроны, однако рецепторы к нему присутствуют практически на всех интернейронах, а также на пресинаптических окончаниях (Abudukeyoumu et al., 2019; Burke et al., 2017; Chuhma et al., 2018). В недавней работе было показано, что дофаминовые нейроны VTA, SNc и ретро-рубрального поля могут быть поделены на подтипы на основании молекулярных маркеров, а клетки – представители разных подтипов имеют разнонаправленные проекции и некоторые из них, в частности, могут выделять в качестве медиатора также и глутамат (Poulin et al., 2018). Эти данные дополняются описаниями разнородных дофаминергических входов в разных отделах стриатума и сложного паттерна котрансмиссии в нем (Menegas et al., 2015; Trudeau et al., 2014; Chuhma et al., 2017, 2018).
НЕКОТОРЫЕ ОСОБЕННОСТИ СВЯЗЕЙ
Также важно отметить, что бледный шар, особенно его наружный сегмент, а также вентральный паллидум не являются исключительно внутренними структурами базальных ганглиев, но, напротив, обладают широкой сетью связей с ниже- и вышележащими отделами мозга. Кроме того, внешний сегмент бледного шара проецируется назад в стриатум, где устанавливает связи как с проекционными нейронами, так и с интернейронами (Mallet et al., 2012). Продемонстрировано преимущественное число входов от быстро разряжающихся тормозных интернейронов стриатума к проекционным нейронам прямого пути, а источником важнейших проекций к этим интернейронам является бледный шар, в свою очередь контролируемый нейронами непрямого пути стриатума (Gerfen, Surmeier, 2011). Также показано, что в реципрокных тормозных отношениях между проекционными нейронами внутри стриатума D2-экспрессирующие нейроны направляют больше коллатералей к D1-экспрессирующим нейронам, чем последние к ним (Taverna et al., 2008).
РОЛЬ НЕЙРОНОВ СТРИАТУМА В ОРГАНИЗАЦИИ ДВИЖЕНИЯ
Согласно классическим представлениям, отчасти упомянутым ранее, прямой путь базальных ганглиев опосредует движение посредством растормаживания входов в кору из моторного таламуса, а непрямой путь препятствует осуществлению движения, усиливая торможение таламуса (Albin et al., 1989; Alexander, Crutcher, 1990) (рис. 1). Аналогично влияние обоих путей на стволовые локомоторные центры, чему также получено недавнее подтверждение (Roseberry et al., 2016). Эта общая модель опирается на богатый клинический и экспериментальный материал (Albin et al., 1989, DeLong, 1990; Plotkin, Goldberg, 2019). Существуют определенные вариации модели, уточняющие свойства компонентов, временные соотношения активности двух путей и функциональную приуроченность этой активности (например, Mink et al., 1996; Hikosaka et al., 2000; Silkis, 2001; Bariselli et al., 2018). Данная модель нашла надежное подтверждение и в физиологических свойствах нейронов прямого и непрямого пути стриатума, и в первую очередь в существенной сегрегации на них D1- и D2-рецепторов (Gong et al., 2003) (cегрегация не является полной, в частности, около 2% проекционных нейронов дорзального стриатума и до 15% нейронов оболочки прилежащего ядра экспрессируют оба типа рецепторов (Gagnon et al., 2017), эти нейроны отличаются морфологически, эффектами дофамина на их активность (Gerfen, Surmeier, 2011) и некоторыми другими свойствами, и, таким образом, могут быть выделены в отдельный подкласс проекционных нейронов (см. также Cazorla et al., 2014)).
В последние годы с использованием молекулярно-генетических подходов к исследованиям функций стриатума были получены новые доводы в пользу таких воззрений (Alcacer et al., 2017; Bateup et al., 2010; Durieux et al., 2012; Bay Konig et al., 2019; Kravitz et al., 2010; Lemos et al., 2016; Roseberry et al., 2016). Однако существенная доля недавних исследований вынуждает также поставить под сомнение классическую точку зрения (Barbera et al., 2016; Cui et al., 2013, Fobbs et al., 2020; Isomura et al., 2013; Klaus et al., 2017; Meng et al., 2018; Parker et al., 2018; Tecuapetla et al., 2016). Вероятно, речь в данном случае не должна идти о том, какая чаша весов перевесит; и представляется важным проанализировать детали проведенных экспериментов. Подавляющее большинство исследований двигательных функций стриатума проводится с использованием поведенческих методик, эксплицитно включающих процедуру двигательного обучения. Из-за существенного многообразия таких методик, варьирования их параметров от исследования к исследованию возникает значительное число дополнительных переменных, делающих анализ чрезвычайно громоздким, в связи с чем этим данным будет посвящено отдельное исследование. В этой же статье мы предполагаем в первую очередь остановиться на работах, в которых экспериментальных животных целенаправленно не подвергали процедуре научения.
Одной из ключевых работ с применением современных методов, подтверждающих классические представления, стало исследование Кравиц и соавт., показавших, что билатеральная оптогенетическая стимуляция нейронов прямого пути стриатума способствует усилению локомоции в открытом поле, а непрямого пути – уменьшению двигательной активности (Kravitz et al., 2010). Существенный перевес в обоих случаях во многом определялся почти полным исчезновением доли мелких движений (fine movements), не связанных со значительным перемещением, характер таких движений не анализировался. В согласии с этими данными исследователи также показали, что при унилатеральной стимуляции прямого или непрямого пути у животных наблюдается соответствующее классической модели поведение вращения в ту или иную сторону.
Одной из проблем интерпретации результатов этого исследования является то, что авторы использовали достаточно продолжительную стимуляцию постоянно действующим светом: позже было убедительно продемонстрировано (группой исследователей, в которую отчасти вошли те же авторы), что в условиях такой стимуляции в стриатуме активность проекционных нейронов существенно меняется не только в результате запланированных в эксперименте эффектов действия канального родопсина, но и в результате нагревания ткани за счет действия света (Owen et al., 2019). Основным механизмом обусловленной нагреванием динамки является изменение работы калиевых выпрямляющих каналов (Kir), по структуре близких к терморецепторам; особенно важно то, что именно этими каналами определяются некоторые ключевые электрофизиологические свойства проекционных нейронов (Nisenbaum, Wilson, 1995). В более поздних исследованиях для возбуждения нейронов стали применять периодическую стимуляцию короткими световыми импульсами, однако для торможения нейронов по-прежнему часто используют стимуляцию постоянно действующим светом (Owen et al., 2019).
Другой проблемой, связанной с интерпретацией такого рода исследований, является то, что одновременной неизбирательной стимуляции подвергаются большие близко расположенные пулы клеток, что с небольшой вероятностью может соответствовать физиологически значимым пространственным и временным паттернам активации нейронов стриатума, тем более что в некоторых исследованиях отмечается, что фундаментальной чертой проекционных нейронов стриатума является очень избирательная и разреженная активация в связи с различными контролируемыми в эксперименте событиями (Yamin et al., 2013).
Непосредственное влияние уровня активности прямого и непрямого пути на локомоцию также апробировали Алкацер и соавт. (Alcacer et al., 2017), они показали, что хемогенетическая активация прямого пути стимулирует локомоцию, а непрямого – тормозит. При односторонних воздействиях поведение вращения мышей также соответствовало классической модели. А в исследовании Бай Кениг и соавт. унилатеральное торможение приблизительно 20% нейронов прямого пути посредством DREADD наряду с нормальной в остальном локомоцией приводило к непродолжительным периодам ипсилатеральных вращений, при этом, по наблюдениям авторов, использование передней конечности животным при опоре на стенку не изменялось (Bay Konig et al., 2019).
Также помимо прямых воздействий на активность проекционных нейронов были испытаны другие экспериментальные воздействия на клетки стриатума. Так, Лемос и соавт. показали, что генетически обусловленное устранение D2-рецепторов на нейронах непрямого пути приводит к паркинсоноподобным нарушениям локомоции, а последующая имитация сигнала, опосредуемого D2-рецепторами, с помощью DREADD в дорзальном стриатуме, частично улучшает показатели локомоторной активности (Lemos et al., 2016). Генетическое устранение ключевого внутриклеточного посредника DARPP-32 в нейронах прямого и непрямого пути также приводило к последствиям, полностью согласующимся, по мнению авторов, с классической моделью (Bateup et al., 2010). Противоположные эффекты на локомоторное поведение оказывало и повреждение ERK/MAPK-внутриклеточного каскада в нейронах прямого и непрямого пути (Hutton et al., 2017).
Все упомянутые выше в этом разделе исследования с учетом методических ограничений и нежелательных эффектов так или иначе подтверждают классические представления о роли прямого и непрямого пути в организации движения. Интересно отметить, что в каждом из этих исследований в качестве основных подходов применены те или иные виды непосредственных экспериментальных воздействий на объект исследования, но ситуация с подтверждением классической модели оказывается практически противоположной при рассмотрении работ, основным методом в которых было изучение корреляционных взаимоотношений между поведением и активностью нейронов прямого и непрямого пути.
Основным мотивом такого рода исследований явилось намерение проследить, каким образом нейроны прямого и непрямого пути стриатума участвуют в организации движения, в каких из аспектов движения роль стриатума является критичной и как это участие проявляется на выходе из базальных ганглиев. Главным результатом исследования Фризе и соавт. (Freeze et al., 2013), совместившими стимуляцию и регистрацию нейронной активности у мышей в свободном поведении, явилось то, что световая стимуляция и прямого, и непрямого путей стриатума приводит к очень похожей картине популяционной реакции нейронов ретикулярной части черного вещества: в ответ на стимуляцию обоих путей нейроны SNpr активируются и тормозятся в приблизительно равных соотношениях. Примирить этот факт с классической моделью авторам помогает установление значимых избирательных корреляционных взаимоотношений между поведением животных и активностью именно тех нейронов, которые разряжаются в соответствии с моделью, и отсутствие значимой корреляции поведения и активности нейронов, меняющих частоту разряда противоположным предсказаниям модели образом. Отметим, что в этом исследовании также применялась стимуляция постоянным светом (Freeze et al., 2013).
Одним из первых исследований, проведенных с применением генетических методов и поставивших под сомнение основной постулат классических представлений о роли прямого и непрямого пути, была работа Куи и соавт. по регистрации нейронной активности стриатума при выполнении животным двигательной задачи, предполагающей самопроизвольное начало движения животным. Они обнаружили, что нейроны обоих путей активируются примерно в одно и то же время в период инициации движения (Cui et al., 2013), также они продемонстрировали существенную активацию нейронов обоих путей непосредственно перед и во время поворота животного в сторону, противоположную области регистрации. Ввиду высокой степени модуляции активности нейронов стриатума, сопровождающей движение, со стороны процессов вознаграждения (Hikosaka et al., 2000; Isomura et al., 2013), важно установить, не является ли такая синхронная активность в первую очередь проявлением процессов ожидания вознаграждения. Поэтому необходимо в первую очередь рассмотреть активность проекционных нейронов стриатума при спонтанных движениях в экспериментальных ситуациях, эксплицитно не включающих вознаграждение. Однако важно при этом понимать, что разделить на нормальном бодрствующем животном движение и вознаграждение – непростая задача даже в такого рода ситуациях. Связь активности нейронов прямого и непрямого пути со спонтанной локомоцией была исследована, в частности, в открытом поле и у животных с закрепленной головой на подвижном шаре. И нам на настоящий момент не известно ни одного исследования, в котором бы наблюдение Куи и соавт. (Cui et al., 2013) об одновременной активации нейронов обоих путей в связи с движением было бы опровергнуто. И, таким образом, данные с регистрацией клеточной активности стриатума в целом не подтверждают устоявшейся модели функционирования базальных ганглиев.
Интересную закономерность описывают Менг и соавт. (Meng et al., 2018): по их наблюдению, превалирование активности нейронов прямого пути приводит к повороту и продолжению движения, а непрямого – к повороту с остановкой.
В большинстве из только что упомянутых исследований также ставился вопрос о преимущественной приуроченности активности стриатных нейронов к кинематическим характеристикам движения. Ответ о наличии такой связи скорее положительный, но исследователи существенно расходятся в деталях. Например, в исследовании Марковица и соавт. (Markowitz et al., 2018) говорится об обнаруженной ими корреляции скорости и активности нейронов обоих путей на секундной временной шкале при достаточно грубой оценке двигательной активности (главным образом, по треку в открытом поле). Когда же они охарактеризовали движение более детально (с помощью специально разработанной программы анализа поведения по трехмерной видеозаписи) и попытались разбить его на унитарные поведенческие акты (на поведенческие слоги, по терминологии авторов) на шкале из долей секунды, им удалось выявить более тонкие закономерности: в частности, они показали, что на протяжении одного поведенческого акта нейроны обоих путей демонстрируют сходную динамику активации, однако в ней устойчиво выделяется комплементарная (некоррелированная) активность, учет которой позволяет значительно точнее предсказать поведение; при смене поведения активность нейронов обоих путей изменяется, интересно, что высоковероятные смены поведенческих актов сопровождаются заметно меньшими изменениями в активности нейронов, по сравнению с низковероятными переходами от одного поведения к другому. Последнее упомянутое наблюдение заслуживает отдельного внимания, так как оно стало результатом предпринятого авторами анализа последовательностей естественного поведения; развитие систем такого анализа и соотнесение его с записями нейрофизиологической активности обещает в перспективе существенное углубление понимания связи поведения с активностью нейронов. В работе Марковица и соавт. такой анализ подтверждает точку зрения, согласно которой одной из важнейших функций дорзального стриатума является участие в формировании двигательных последовательностей целостного поведенческого акта (Graybiel, 1998); с этим согласуется и наблюдаемое авторами повышение поведенческой энтропии в результате частичного разрушения дорзолатерального стриатума.
На вопрос о том, в какой степени активность нейронов связана с кинематическими характеристиками движений или с последовательностями движений, пытаются ответить и Фоббс и соавт. (Fobbs et al., 2020). По их данным, активность обоих путей тесно связана с началом и завершением поведенческих последствий, но также они сообщают и об определенной, как правило, нелинейной связи активности проекционных нейронов стриатума со скоростью перемещения животного. Хочется особо отметить одно наблюдение этих исследователей: они регистрировали активность стриатных нейронов у животных в открытом поле, а также на подвижном в трех измерениях шаре, где мыши находятся с фиксированной головой, и показали, что частота разряда подавляющего большинства нейронов в открытом поле значительно выше частоты разряда при нахождении животного на шаре. Это в очередной раз ставит вопрос о принципиальной возможности изучения механизмов движения/локомоции в чистом виде. В исследовании Клауса и соавт. (Klaus et al., 2017) определенные кинематические характеристики движения были интегрированы авторами в числовые последовательности из 300-миллисекундных бинов, описывающие общую картину поведения; авторам удалось показать, что активность проекционных нейронов, возбуждающихся во время определенного поведения, в большей степени коррелирует между собой и такие клеточные ансамбли кодируют конкретное движение вне зависимости от его скорости. Эти ансамбли состоят как из нейронов, расположенных поблизости друг от друга, так и из довольно удаленных клеток. Это последнее наблюдение существенно расходится с тем, что было описано Барбера и соавт. (Barbera et al., 2016), показавшими присутствие довольно компактных кластеров нейронов в дорзальном стриатуме, активность которых коррелирует со скоростью локомоции животных. Интересно, что обе группы исследователей использовали метод оценки активности нейронов по кальциевому сигналу и, более того, применяли сходные способы обработки исходных данных; и, по мнению Клауса и соавт., такое расхождение возможно из-за различий в способе выявления динамики внутриклеточной концентрации кальция на основе сырых данных микроскопии.
Существенный интерес представляет исследование, в котором избирательное повреждение нейронов прямого и непрямого пути дорзального стриатума производили у взрослых животных посредством локального введения дифтерийного токсина, при этом была также предпринята попытка исследовать эффекты избирательного разрушения нейронов дорзомедиального и дорзолатерального стриатума (Durieux et al., 2012). Авторами было обнаружено, что основным источником влияния на общий уровень спонтанной локомоции в открытом поле является дорзомедиальный стриатум, при этом, в полном соответствии с классической моделью, поражение нейронов прямого пути ведет к снижению уровня локомоторной активности, а нейронов непрямого пути – к резкому повышению. Также в этом исследовании наблюдали поведение животных в ответ на установку в центре открытого поля нового объекта. Оказалось, что поражение проекционных нейронов именно дорзомедиального стриатума также оказывает сильное влияние на это поведение: было обнаружено, что при разрушении нейронов прямого пути животное значимо меньше времени проводит, обследуя новый объект, а животные с разрушением нейронов непрямого пути находятся в центральной зоне гораздо дольше контрольных животных, когда там присутствует новый объект. Интересно, что, по их данным, эти животные значительно меньше времени, по сравнению с контрольными, проводят в центре открытого поля до установки там нового объекта, а также то, что при установке этого объекта повторно мыши приближаются к нему еще чаще, чем в первый раз. Авторы предполагают, что такое поведение, по крайней мере отчасти, определяется изначально высокой аверсией в отношении новизны у этих животных. Надо отметить, что некоторые побочные наблюдения в процитированных ранее работах также указывают на определенные изменения исследовательского поведения при направленных воздействиях на проекционные нейроны стриатума (Lemos et al., 2016; Bay Konig et al., 2019; Alcacer et al., 2017). Здесь также стоит отметить, что в исследовании Ямин и соавт. (Yamin et al., 2013), регистрировавших активность нейронов стриатума у животных в свободном поведении при смене обстановки, была выделена отдельная группа нейронов, активирующихся почти исключительно при такой смене.
На определенные аспекты поведения, которые могут повлечь за собой изменения спонтанной локомоции в отсутствие специфичных для локомоции изменений как таковых, указывают также ЛеБланк и соавт. (LeBlanc et al., 2020). Основным объектом их исследования являются нокаутные мыши без D2-рецепторов к дофамину на нейронах непрямого пути в стриатуме; авторы утверждают, что у этих животных изменений собственно локомоции не наблюдается. В ранее упомянутой нами работе, имеющей общих авторов с обсуждаемой здесь (Lemos et al., 2016), было, однако, показано, что у этих животных наблюдаются нарушения локомоции, подобные описанным при паркинсонизме. В исследовании ЛеБланк и соавт. (LeBlanc et al., 2019) апробируется идея о том, что существенную роль здесь может играть состояние повышенной тревоги, присущей нокаутным животным. При том, что основания, которые они приводят в этой работе, далеко не всегда выглядят убедительно (в единственном числе предоставлены показатели локомоции там, где поведение может быть оценено только по совокупности показателей, не представлены возможные объяснения противоречивых результатов воздействий), и сами авторы не обсуждают различия в выводах в этих двух исследованиях, эту идею нельзя оставлять без внимания.
В самых общих чертах укладывается в представления классической модели и влияние дофамина на активность нейронов обоих путей стриатума (Shen et al., 2008; Gerfen, Surmeier, 2011; Parker et al., 2018). В данном контексте отдельно необходимо упомянуть исследование Паркера и соавт. (Parker et al., 2018), которые, с одной стороны, продемонстрировали практически идентичную картину активности обоих путей в связи с движением при свободном поведении животного и с фиксированной головой на подвижном колесе (практически в полном соответствии с другими исследованиями по регистрации клеточной активности стриатума), а с другой – после воздействия 6-OHDA, уже в соответствии с классической моделью, показали снижение активности нейронов прямого пути и повышение – непрямого. Помимо этого они отмечают, что после поражения дофаминовой системы активность нейронов непрямого пути, приуроченная к движению, полностью нивелировалась; интересно, что подобную закономерность они подмечают и в активности нейронов прямого пути в условиях дискинезии, вызванной воздействием высокой дозы L-DOPA. Также хочется отметить, что в связи с проявлениями исследовательской активности животных (при стойках на задних лапах) упомянутые исследователи зафиксировали активацию только в нейронах непрямого пути; значимых изменений в нейронах прямого пути они не обнаружили. На этом примере в очередной раз обнаруживаются существенная противоречивость данных и потребность в более глубоком понимании роли стриатума в организации движения.
Еще более противоречивыми оказались данные недавних исследований роли дофаминового сигнала в стриатуме при спонтанной локомоции животных в очень сходных условиях – у мышей с закрепленной головой на подвижном шаре. Так, Хоу и Домбек (Howe, Dombek, 2016), регистрируя кальциевую активность аксонов дофаминовых нейронов в дорзальном стриатуме, показали, что активация дофаминергических проекций в стриатум предшествует инициации спонтанного движения и сопровождает движение. А Додсон и соавт., используя ту же поведенческую модель, но регистрируя электрическую активность дофаминовых нейронов в VTA и SNpc, выявили фазное снижение активности большинства исследованных нейронов черного вещества непосредственно перед движением (Dodson et al., 2016). Обсуждая эти расхождения, среди методических различий авторы указывают на полное отсутствие в экспериментальной обстановке у Додсона и соавт. стимулов, способных вызвать реакцию приближения. Однако нельзя исключить, что, находясь на шаре с фиксированной головой, животное может начинать двигаться для устранения неудобства позы или для избегания самой по себе неприятной ситуации. Рассуждая о возможной функциональной роли дофаминовой паузы перед движением, Додсон и соавт. высказывают предположение о ее значении в обеспечении точности движения с участием нейронов непрямого пути. Именно на активацию дофаминовой системы перед спонтанной инициацией движения в открытом поле указывают также да Сильва и соавт. (da Silva et al., 2018, они регистрировали экстраклеточную активность нейронов, идентифицируя их медиаторную природу с помощью оптогенетики). Здесь важно отметить, что, говоря о спонтанности близких по моторике действий, нельзя не учитывать критического различия ситуаций, когда животное свободно перемещается в обстановке и когда оно пребывает на месте с фиксированной головой, тем более что основной целью локомоции является перемещение в пространстве.
Мы уже упоминали, что активность нейронов стриатума существенно регулируется процессами вознаграждения. Это же утверждение даже в большей степени справедливо в отношении дофаминового сигнала. А так как роль среднемозгового дофамина в организации действия многие авторы ставят на первое место, здесь мы кратко остановимся на сути ключевых разногласий по этой проблеме.
Основные точки зрения на функции дофамина в стриатуме таковы: дофамин опосредует удовольствие (Wise, 1985); дофамин служит универсальным обучающим сигналом, критичным для научения (Schultz, 2013, Langdon et al., 2018); дофамин опосредует мотивацию (Berridge, 2007); дофамин обеспечивает усилие для выполнения движения (Salamone, 2007; Pasquereau, Turner, 2014; Varazzani et al., 2015; Ивлиева, 2010; Майоров, 2018). Каждая из точек зрения опирается на обширный экспериментальный материал (Ivlieva, Ivliev, 2014), и существует мнение, что они не являются взаимоисключающими (Beeler, 2012), однако концептуальная противоположность таких функций как, например, мотивация и подкрепление, очевидна. Более того, тот факт, что активация одного и того же мозгового субстрата является и подкрепляющей, и вызывающей драйв, представляется парадоксальным (Wise, 2013).
Не менее противоречивой является и наиболее распространенная среди исследователей научения концепция о том, что дофаминовый сигнал кодирует ошибку предсказания вознаграждения (Schultz, 2013). С одной стороны, теория находит все новые подтверждения (Watabe-Uchida et al., 2017), в том числе – и в исследованиях с применением новых генетических методов (Steinberg et al., 2013; Chang et al., 2016). С другой стороны, быстро накапливаются данные, противоречащие этой теории, или, как минимум, требующие ее уточнения (Howe et al., 2013). Так, например, показано, что идентифицированные дофаминергические нейроны могут активироваться в ответ на вредоносные стимулы (Moriya et al., 2018; Tsai et al., 2009), в то время как в теории об ошибке предсказания вознаграждения положительный или отрицательный знаки реакции нейронов являются принципиальными. И в контексте нашей работы особенно важно отметить, что активация нейронов дофаминергических областей в связи с движением представляет наиболее серьезную проблему для теории об ошибке предсказания вознаграждения, ведь такого рода активность предшествует во времени ожидаемому сигналу об ошибке, а не следует за ним, а если предположить, что проекционные структуры способны эти два сигнала различать, то эти сигналы должны быть четко разграничены. Существенно то, что данные в пользу участия дофамина в инициации движения получены, как правило, на свободноподвижных животных, вовлеченных в близкие к естественным формы поведения, такие как движения, направленные на получение вознаграждения или избегание вреда; и в них систематически демонстрируется, что выброс дофамина/активация дофаминергической системы предшествует/сопутствует движению (Roitman et al., 2004; Puryear et al., 2010; Cacciapaglia et al., 2011; Flagel et al., 2011; Ivlieva et al., 2014; Oleson et al., 2012; Pasquereau, Turner, 2014; Schultz et al., 1983; Майоров, Серков, 2016; Howe, Dombeck, 2016; da Silva et al., 2018; Tye et al., 2013). В то время как активность, ассоциирующаяся с ошибкой предсказания вознаграждения, была выявлена и активно исследована, главным образом, в условиях классического условного рефлекса, где движение не значимо. Насколько определенно можно судить о системе, появившейся сотни миллионов лет назад и контролирующей подвижность у всех многоклеточных животных (Caveney et al., 2006), по исследованиям на поведенческих моделях, в которых движение не критично или вообще отсутствует?
Действие аденозина на внутриклеточные каскады реакций нейронов прямого и непрямого пути стриатума характеризуется эффектами, противоположными дофаминовым: стриато-нигральные клетки экспрессируют D1- и А1-рецепторы, а стриато-паллидарные – D2- и А2А-рецепторы, они взаимодействуют в составе образуемых ими гетеромеров, а также на уровне вторичных посредников, в частности, оказывая разнонаправленное действие на синтез цАМФ (Fuxe et al., 2007). Можно сказать, что особенности организации аденозиновой сигнализации в стриатуме в общих чертах подтверждают классическую схему двух путей. С этим согласуется и обобщение, сделанное Данвидди и Масино, о том, что значительно увеличивают выброс аденозина те манипуляции, которые приводят к превышению потребности мозга в энергии над его способностями синтезировать АТФ (Dunwiddie, Masino, 2001).
Таким образом, классическая модель с участием двух ключевых популяций проекционных нейронов стриатума в организации движения в самом общем виде сохраняет свою актуальность, однако она требует существенного уточнения.
И в завершение мы кратко остановимся на исследованиях интернейронов стриатума в связи с локомоцией. В большей степени с движением соотносится активность быстро разряжающихся нейронов (Marche, Apicella, 2017; Assous, Tepper, 2019), что предопределено характером их связей (Assous, Tepper, 2019; Klug et al., 2018; Johansson, Silberberg, 2020), однако с движением, безусловно, связана и активность холинергических интернейронов, так как самые воспроизводимые эффекты воздействий на ацетилхолиновую передачу в стриатуме – это двигательные эффекты (Abudukeynoumu et al., 2019). Серьезного внимания в этом отношении заслуживает исследование Гриттона и соавт. (Gritton et al., 2019): они регистрировали кальциевую динамику в идентифицированных проекционных нейронах, в парвальбуминовых нейронах и в холинергических нейронах дорзального стриатума у мыши с закрепленной головой на подвижном шаре при спонтанной локомоции животного и также перемежали регистрацию с оптогенетической стимуляцией интернейронов стриатума. Ими было показано, что каждая изученная популяция нейронов демонстрирует повышение кальциевой активности в связи с движением; максимальные изменения, начинающиеся существенно раньше инициации движения, проявили парвальбуминовые нейроны, сохраняющие высокие показатели активности на протяжении всего движения; пик активности холинергических нейронов в большей степени приурочен к завершению движения. При стимуляции парвальбуминовых нейронов наибольшие изменения в сторону усиления локомоции проявлялись на фоне состояния неподвижности животного, напротив, эффект от стимуляции холинергических нейронов наблюдался на фоне активной фазы и состоял в снижении скорости движения. Интересно, что стимуляция парвальбуминовых нейронов закономерно могла бы приводить к снижению активности проекционных нейронов, однако вместо этого наблюдался гетерогенный ответ, включающий также повышение уровня активности части проекционных нейронов, и авторы исследования делают вывод о том, что активность этих клеток важна для подавления фонового шума и выделения сигнала, значимого для инициируемого движения. Важной является выявленная исследователями связь между активностью обоих типов интернейронов и последующей популяционной активностью проекционных нейронов: было показано, что активность парвальбуминовых нейронов является предиктором снижения синхронности кальциевых событий проекционных клеток, и наоборот, активация холинергических интернейронов предшествует повышению их уровня коактивации. На основании этих данных авторы исследования делают вывод о том, что у обоих популяций интернейронов есть своя уникальная роль в процессах организации движения: парвальбуминовые нейроны способствуют инициации и поддержанию движения, а холинергические нейроны обеспечивают завершение движения, синхронизируя проекционные нейроны. В этой работе не проводилось различения между D1- и D2-экспрессирующими нейронами, и, возможно, в последующем такое уточнение прояснит механизм участия интернейронов стриатума в движении.
ЗАКЛЮЧЕНИЕ
Таким образом, с одной стороны, результаты различных воздействий в целом скорее свидетельствуют в пользу классических представлений о функциях прямого и непрямого пути в базальных ганглиях, с другой стороны, регистрация клеточной активности или ее коррелятов почти без исключений демонстрирует очень сходные паттерны активации двух путей в связи с движением. Последнее, в принципе, также может быть увязано с существующими взглядами на функции прямого и непрямого путей, но гипотезы такого рода предполагают исключительную степень избирательности в активности клеток стриатума (например, James et al., 2018); но тогда приходится признать названные первыми эффекты многочисленных экспериментальных манипуляций совершенно не физиологическими и, следовательно, мало что объясняющими.
На основании рассмотренных работ можно допустить, что, возможно, имеет смысл отказаться от дихотомии: способствует/препятствует выполнению движения/движений (в том числе и конкурентных), в пользу участия прямого пути в запуске спроектированного в определенном контексте действия при ключевом участии дофаминового сигнала, а для непрямого пути – в пользу его роли в обеспечении дополнительных петель обратной связи при выполнении движений, в контроле над сенсорной информацией, непосредственно связанной с выполняемым действием (что ассоциируется с функцией внимания), в координации действия и, возможно, в итоге в выработке высокоспециализированного навыка. Так, нейроны непрямого пути преимущественно получают информацию об эфферентной копии двигательной команды (Reiner et al., 2010; Wall et al., 2013). Из-за более высокого сродства D2-рецепторов к дофамину (Richfield et al., 1989, но см. также обсуждение Yapo et al., 2017) они модулируются как в результате повышения, так и посредством снижения концентрации медиатора (Yapo et al., 2017). В приведенных в обзоре позднейших исследованиях именно манипуляции с нейронами непрямого пути часто приводили к изменениям исследовательского поведения (Alcacer et al., 2017; Durieux et al., 2012, LeBlanc et al., 2020; Lemos et al., 2016), хотя вектор этих изменений и был мало предсказуем. Именно нейроны непрямого пути проявляли изменение активности в связи со стойками в исследовании Паркера и соавт. (Parker et al., 2018). На связь с точностью действия указывают Додсон и соавт. (Dodson et al., 2016), когда обсуждают паузу в активности дофаминовых нейронов перед движением, а именно нейроны непрямого пути в большей степени чувствительны к снижению концентрации дофамина (Yapo et al., 2017). Также и мы при исследовании требующего ловкости пищедобывательного навыка у крысы выявили значимое снижение активности нейронов VTA в ключевой фазе движения животного (Ивлиева, Ивлиев, 2018).
В пользу участия непрямого пути в контроле такого рода говорят установленные недавно прямые проекции из наружного сегмента бледного шара во фронтальную кору (Saunders et al., 2015), а также тесная анатомическая (Ingham et al., 1985) и функциональная связь бледного шара с базальным передним мозгом, часто рассматриваемым как область, критичную для процессов внимания (Ingham et al., 1985; Lin et al., 2015; Ивлиев, Ивлиева, 2019; Gielow, Zaborszky, 2017). Показано, что активация нейронов бледного шара вызывает снижение импульсной активности нейронов ретикулярного ядра таламуса, также вовлеченного в функции внимания (Villalobos et al., 2016), и то, что бледный шар является главным источником когерентной активности в БГ в период глобальной мозговой активации (Sharott et al., 2005). Также было показано, что бледный шар участвует в фильтрации нерелевантной сенсорной информации (Nakajima et al., 2019) и таким образом контролирует доступ к рабочей памяти (Mc Nab, Klingberg, 2008), активность же нейронов бледного шара в определенной ситуации может предсказывать последующий шаг при выполнении животным поведенческой задачи с выбором, и, кроме того, наибольшая модуляция их активности связана с моментами, требующими максимального внимания (Schechtman et al., 2016).
Движение – ключевое звено во взаимодействии организма с окружающим миром. Это взаимодействие требует большой точности, а движение – тончайшей настройки. Из клинических исследований известно огромное множество примеров нарушений настройки такого рода, часто приводящих к катастрофическим последствиям. Возможно, одновременная активация при движении двух противоположно направленных механизмов является гарантией управляемости этого движения, по аналогии с функционированием двухпартийных систем в странах с развитой демократией.
Список литературы
Базян А.С., Григорьян Г.А., Иоффе М.Е. Регуляция моторного поведения. Усп. физиол. наук. 2011. 41 (1): 3–25.
Ивлиев Д.А., Ивлиева Н.Ю. Плавное снижение активности нейронов вентральной области покрышки среднего мозга в процессе выполнения пищедобывательного движения. Журнал высшей нервной деятельности им. И.П. Павлова. 2018. 68 (3): 265–272. https://doi.org/10.7868/S0044467718030012
Ивлиев Д.А., Ивлиева Н.Ю. Активность нейронов базального крупноклеточного ядра переднего мозга крысы предсказывает результат пищедобывательного движения. Журнал высшей нервной деятельности им. И.П. Павлова. 2019. 69(4): 479–492. https://doi.org/10.1134/S0044467719040051
Ивлиева Н.Ю. Участие мезокортико-лимбической дофаминергической системы в адаптивном поведении. Журнал высшей нервной деятельности им. И.П. Павлова. 2010. 60 (3): 259–278.
Майоров В.И. Функции дофамина в инструментальном условном рефлексе. Журнал высшей нервной деятельности им. И.П. Павлова. 2018. 68 (4): 404–414.
Майоров В.И., Серков А.Н. Активность нейронов вентральной тегментальной области среднего мозга при первом выполнении условного рефлекса активного избегания. Журнал высшей нервной деятельности им. И.П. Павлова. 2016. 66 (6): 725–729.
Albin R.L., Young A.B., Penney J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989. 12: 366–375.
Alcacer C., Andreoli L., Sebastianutto I., Jakobsson J., Fieblinger T., Cenci M.A. Chemogenetic stimulation of striatal projection neurons modulates responses to Parkinson’s disease therapy. J Clin Invest. 2017. 127 (2): 720–734. https://doi.org/10.1172/JCI90132
Alexander G.E., Crutcher M.D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990. 13 (7): 266–271.
Abudukeyoumu N., Hernandez-Flores T., Garcia-Munoz M., Arbuthnott G.W. Cholinergic modulation of striatal microcircuits. Eur J Neurosci. 2019. 49 (5): 604–622. https://doi.org/10.1111/ejn.13949
Assous M., Tepper J.M. Excitatory extrinsic afferents to striatal interneurons and interactions with striatal microcircuitry. Eur J Neurosci. 2019. 49 (5): 593–603. https://doi.org/10.1111/ejn.13881
Barbera G., Liang B., Zhang L., Gerfen C.R., Culurciello E., Chen R., Li Y., Lin D.T. Spatially Compact Neural Clusters in the Dorsal Striatum Encode Locomotion Relevant Information. Neuron. 2016. 92 (1): 202–213. https://doi.org/10.1016/j.neuron.2016.08.037
Bariselli S., Fobbs W.C., Creed M.C., Kravitz A.V. A competitive model for striatal action selection. Brain Res. 2019. 1713: 70–79. https://doi.org/10.1016/j.brainres.2018.10.009
Bateup H.S., Santini E., Shen W., Birnbaum S., Valjent E., Surmeier D.J., Fisone G., Nestler E.J., Greengard P. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci U S A. 2010. 107 (33): 14845–14850. https://doi.org/10.1073/pnas.1009874107
Bay Kønig A., Ciriachi C., Gether U., Rickhag M. Chemogenetic Targeting of Dorsomedial Direct-pathway Striatal Projection Neurons Selectively Elicits Rotational Behavior in Mice. Neuroscience. 2019. 401: 106–116. https://doi.org/10.1016/j.neuroscience.2019.01.013
Beeler J.A. Thorndike’s law 2.0: Dopamine and the regulation of thrift // Frontiers in neuroscience. 2012. 6: 116. https://doi.org/10.3389/fnins.2012.00116
Berridge K. C. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007. 191 (3): 391–431.
Brimblecombe K.R., Cragg S.J. The Striosome and Matrix Compartments of the Striatum: A Path through the Labyrinth from Neurochemistry toward Function. ACS Chem Neurosci. 2017. 8 (2): 235–242. https://doi.org/10.1021/acschemneuro.6b00333
Burke D.A., Rotstein H.G., Alvarez V.A. Striatal Local Circuitry: A New Framework for Lateral Inhibition. Neuron. 2017. 96 (2): 267–284. https://doi.org/10.1016/j.neuron.2017.09.019
Cacciapaglia F., Wightman R.M., Carelli R.M. Rapid dopamine signaling differentially modulates distinct microcircuits within the nucleus accumbens during sucrose-directed behavior. Journal of Neuroscience. 2011. 31 (39): 13860–13869.
Caveney S., Cladman W., Verellen L., Donly C. Ancestry of neuronal monoamine transporters in the Metazoa. Journal of Experimental Biology. 2006. 209: 4858–4868. https://doi.org/10.1242/jeb.02607
Cazorla M., de Carvalho F.D., Chohan M.O., Shegda M., Chuhma N., Rayport S., Ahmari S.E., Moore H., Kellendonk C. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014. 81 (1): 153–164. https://doi.org/10.1016/j.neuron.2013.10.041
Chang C.Y., Esber G.R., Marrero-Garcia Y., Yau H.J., Bonci A., Schoenbaum G. Brief optogenetic inhibition of dopamine neurons mimics endogenous negative reward prediction errors. Nature neuroscience. 2016. 19 (1): 111–116. https://doi.org/10.1038/nn.4191
Chuhma N., Mingote S., Kalmbach A., Yetnikoff L., Rayport S. Heterogeneity in Dopamine Neuron Synaptic Actions Across the Striatum and Its Relevance for Schizophrenia. Biol Psychiatry. 2017. 81 (1): 43–51. https://doi.org/10.1016/j.biopsych.2016.07.002
Chuhma N., Mingote S., Yetnikoff L., Kalmbach A., Ma T., Ztaou S., Sienna A.C., Tepler S., Poulin J.F., Ansorge M., Awatramani R., Kang U.J., Rayport S. Dopamine neuron glutamate cotransmission evokes a delayed excitation in lateral dorsal striatal cholinergic interneurons. Elife. 2018. 7. pii: e39786. https://doi.org/10.7554/eLife.39786
Civier O., Bullock D., Max L., Guenther F.H. Computational modeling of stuttering caused by impairments in a basal ganglia thalamo-cortical circuit involved in syllable selection and initiation. Brain Lang. 2013. 126 (3): 263–278. https://doi.org/10.1016/j.bandl.2013.05.016
Cui G., Jun S.B., Jin X., Pham M.D., Vogel S.S., Lovinger D.M., Costa R.M. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013. 494 (7436): 238–242. https://doi.org/10.1038/nature11846
DeLong M.R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990. 13 (7): 281–285.
Dodson P.D., Dreyer J.K., Jennings K.A., Syed E.C., Wade-Martins R., Cragg S.J., Bolam J.P., Magill P.J. Representation of spontaneous movement by dopaminergic neurons is cell-type selective and disrupted in parkinsonism. Proceedings of the National Academy of Sciences. 2016. 113 (15): E2180–E2188. https://doi.org/10.1073/pnas.1515941113
Durieux P.F., Schiffmann S.N., de Kerchove d’Exaerde A. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J. 2012. 31 (3): 640–653. https://doi.org/10.1038/emboj.2011.400
Dunwiddie T.V., Masino S.A. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001. 24: 31–55. https://doi.org/10.1146/annurev.neuro.24.1.31
Flagel S.B., Clark J.J., Robinson T.E., Mayo L., Czuj A., Willuhn I., Akers C.A., Clinton S.M., Phillips P.E., Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011. 469 (7328): 53–57. https://doi.org/10.1038/nature09588
Fobbs W.C., Bariselli S., Licholai J.A., Miyazaki N.L., Matikainen-Ankney B.A., Creed M.C., Kravitz A.V. Continuous Representations of Speed by Striatal Medium Spiny Neurons. J Neurosci. 2020. 40 (8): 1679–1688. https://doi.org/10.1523/JNEUROSCI.1407-19.2020
Freeze B.S., Kravitz A.V., Hammack N., Berke J.D., Kreitzer A.C. Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013. 33. (47): 18531–18539. https://doi.org/10.1523/JNEUROSCI.1278-13.2013
Fujiyama F., Unzai T., Karube F. Thalamostriatal projections and striosome-matrix compartments. Neurochem Int. 2019. 125: 67–73. https://doi.org/10.1016/j.neuint.2019.01.024
Fuxe K., Ferré S., Genedani S., Franco R., Agnati L.F. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007. 92 (1–2): 210–217. https://doi.org/10.1016/j.physbeh.2007.05.034
Gagnon D., Petryszyn S., Sanchez M.G., Bories C., Beaulieu J.M., De Koninck Y., Parent A., Parent M. Striatal Neurons Expressing D(1) and D(2) Receptors are Morphologically Distinct and Differently Affected by Dopamine Denervation in Mice. Sci Rep. 2017. 7 (41432). https://doi.org/10.1038/srep41432
Gangarossa G., Espallergues J., Mailly P., De Bundel D., de Kerchove d’Exaerde A., Hervé D., Girault J.-A., Valjent E., Krieger P. Spatial distribution of D1R- and D2R-expressing medium-sized spiny neurons differs along the rostro-caudal axis of the mouse dorsal striatum. Front. Neural Circuits. 2013. 7: 124. https://doi.org/10.3389/fncir.2013.00124
Gerfen C.R., Surmeier D.J. Modulation of striatal projection systems by dopamine. Annual review of neuroscience. 2011. 34: 441–466. https://doi.org/10.1146/annurev-neuro-061010-113641
Gielow M.R., Zaborszky L. The input-output relationship of the cholinergic basal forebrain. Cell reports. 2017. 18 (7): 1817–1830. https://doi.org/10.1016/j.celrep.2017.01.060
Gong S., Zheng C., Doughty M.L., Losos K., Didkovsky N., Schambra U.B., Nowak N.J., Joyner A., Leblanc G., Hatten M.E., Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003. 425 (6961): 917–925. https://doi.org/10.1038/nature02033
Graybiel A.M. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998. 70 (1–2): 119–136.
Grillner S., Robertson B. The Basal Ganglia Over 500 Million Years. Curr Biol. 2016. 26 (20): R1088–R1100. https://doi.org/10.1016/j.cub.2016.06.041
Gritton H.J., Howe W.M., Romano M.F., DiFeliceantonio A.G., Kramer M.A., Saligrama V., Bucklin M.E., Zemel D., Han X. Unique contributions of parvalbumin and cholinergic interneurons in organizing striatal networks during movement. Nat Neurosci. 2019. 22 (4): 586–597. https://doi.org/10.1038/s41593-019-0341-3
Haber S.N., Fudge J.L., McFarland N.R. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000. 20 (6): 2369–2382. https://doi.org/10.1523/JNEUROSCI.20-06-02369.2000
Haber S.N. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016. 18 (1): 7–21.
Hikosaka O., Takikawa Y., Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000. 80: 953–978.
Hintiryan H., Foster N.N., Bowman I., Bay M., Song M.Y., Gou L., Yamashita S., Bienkowski M.S., Zingg B., Zhu M., Yang X.W., Shih J.C., Toga A.W., Dong H.W. The mouse cortico-striatal projectome. Nat Neurosci. 2016. 19 (8): 1100–1114. https://doi.org/10.1038/nn.4332
Hjorth J., K.T., Kotaleski J.H. Gap junctions between striatal fast-spiking interneurons regulate spiking activity and synchronization as a function of cortical activity. J Neurosci. 2009. 29 (16): 5276–5286. https://doi.org/10.1523/JNEUROSCI.6031-08.2009
Howe M.W., Tierney P.L., Sandberg S.G., Phillips P.E., Graybiel A.M. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 2013. 500 (7464): 575–579. https://doi.org/10.1038/nature12475
Howe M.W., Dombeck D.A. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature. 2016. 535 (7613): 505–510. https://doi.org/10.1038/nature18942
Hunnicutt B.J., Jongbloets B.C., Birdsong W.T., Gertz K.J., Zhong H., Mao T. A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife. 2016. 5. pii: e19103. https://doi.org/10.7554/eLife.19103
Hutton S.R., Otis J.M., Kim E.M., Lamsal Y., Stuber G.D., Snider W.D. ERK/MAPK Signaling Is Required for Pathway-Specific Striatal Motor Functions. J Neurosci. 2017. 37 (34): 8102–8115. https://doi.org/10.1523/JNEUROSCI.0473-17.2017
Ingham C.A., Bolam J.P., Wainer B.H., Smith A.D. A correlated light and electron microscopic study of identified cholinergic basal forebrain neurons that project to the cortex in the rat. J Comp Neurol. 1985. 239 (2): 176–192.
Isomura Y., Takekawa T., Harukuni R., Handa T., Aizawa H., Takada M., Fukai T. Reward-modulated motor information in identified striatum neurons. J Neurosci. 2013. 33 (25): 10209–10220. https://doi.org/10.1523/JNEUROSCI.0381-13.2013
Ivlieva N.Yu., Ivliev D.A. Specific role of dopamine in striatum during instrumental learning. Zh. Vyssh. Nerv. Deiat. Im. I.P. Pavlova. 2014. 64 (3): 251–254.
Ivlieva N.Y., Timofeeva N.O., Ivliev D.A. The Dopamine Impels Us to Action as Suggested by the Neuronal Activity in the Ventral Tegmental Area during Avoidance Conditioning. The Russian Journal of Cognitive Science. 2014. 1 (1-2): 54–64.
James S.S., Papapavlou C., Blenkinsop A., Cope A.J., Anderson S.R., Moustakas K., Gurney K.N. Integrating Brain and Biomechanical Models-A New Paradigm for Understanding Neuro-muscular Control. Front Neurosci. 2018. 12: 39. https://doi.org/10.3389/fnins.2018.00039
Johansson Y., Silberberg G. The Functional Organization of Cortical and Thalamic Inputs onto Five Types of Striatal Neurons Is Determined by Source and Target Cell Identities. Cell Rep. 2020. 30 (4): 1178–1194.e3. https://doi.org/10.1016/j.celrep.2019.12.095
Klaus A., Martins G.J., Paixao V.B., Zhou P., Paninski L., Costa R.M. The Spatiotemporal Organization of the Striatum Encodes Action Space. Neuron. 2017. 95 (5): 1171–1180.e7. https://doi.org/10.1016/j.neuron.2017.08.015
Klug J.R., Engelhardt M.D., Cadman C.N., Li H., Smith J.B., Ayala S., Williams E.W., Hoffman H., Jin X. Differential inputs to striatal cholinergic and parvalbumin interneurons imply functional distinctions. Elife. 2018. 7. pii: e35657. https://doi.org/10.7554/eLife.35657
Kravitz A.V., Freeze B.S., Parker P.R., Kay K., Thwin M.T., Deisseroth K., Kreitzer A.C. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010. 466 (7306): 622–626. https://doi.org/10.1038/nature09159
Kreitzer A.C. Physiology and pharmacology of striatal neurons. Annual review of neuroscience. 2009. 32: 127–147.
Lemos J.C., Friend D.M., Kaplan A.R., Shin J.H., Rubinstein M., Kravitz A.V., Alvarez V.A. Enhanced GABA Transmission Drives Bradykinesia Following Loss of Dopamine D2 Receptor Signaling. Neuron. 2016. 90 (4): 824–838. https://doi.org/10.1016/j.neuron.2016.04.040
Langdon A.J., Sharpe M.J., Schoenbaum G., Niv Y. Model-based predictions for dopamine. Current opinion in neurobiology. 2018. 49: 1–7. https://doi.org/10.1016/j.conb.2017.10.006
LeBlanc K.H., London T.D., Szczot I., Bocarsly M.E., Friend D.M., Nguyen K.P., Mengesha M.M., Rubinstein M., Alvarez V.A., Kravitz A.V. Striatopallidal neurons control avoidance behavior in exploratory tasks. Mol Psychiatry. 2020. 25 (2): 491–505. https://doi.org/10.1038/s41380-018-0051-3
Lin S.C., Brown R.E., Hussain Shuler M.G., Petersen C.C., Kepecs A. Optogenetic Dissection of the Basal Forebrain Neuromodulatory Control of Cortical Activation, Plasticity, and Cognition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015. 35 (41): 13896–13903. https://doi.org/10.1523/JNEUROSCI.2590-15.2015
Liu C., Kershberg L., Wang J., Schneeberger S., Kaeser P.S. Dopamine Secretion Is Mediated by Sparse Active Zone-like Release Sites. Cell. 2018. 172 (4): 706–718.e15. https://doi.org/10.1016/j.cell.2018.01.008
Maia T.V., Frank M.J. An Integrative Perspective on the Role of Dopamine in Schizophrenia. Biol. Psychiatry. 2017. 81 (1): 52–66. https://doi.org/10.1016/j.biopsych.2016.05.021
Mc Nab F., Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008. 11 (1): 103–107.
Mallet N., Micklem B.R., Henny P., Brown M.T., Williams C., Bolam J.P., Nakamura K.C., Magill P.J. Dichotomous organization of the external globus pallidus. Neuron. 2012. 74 (6): 1075–1086. https://doi.org/10.1016/j.neuron.2012.04.027
Mandelbaum G., Taranda J., Haynes T.M., Hochbaum D.R., Huang K.W., Hyun M., Umadevi Venkataraju K., Straub C., Wang W., Robertson K., Osten P., Sabatini B.L. Distinct Cortical-Thalamic-Striatal Circuits through the Parafascicular Nucleus. Neuron. 2019. 102 (3): 636–652.e7. https://doi.org/10.1016/j.neuron.2019.02.035
Marche K., Apicella P. Changes in activity of fast-spiking interneurons of the monkey striatum during reaching at a visual target. J Neurophysiol. 2017. 117 (1): 65–78. https://doi.org/10.1152/jn.00566.2016
Markowitz J.E., Gillis W.F., Beron C.C., Neufeld S.Q., Robertson K., Bhagat N.D., Peterson R.E., Peterson E., Hyun M., Linderman S.W., Sabatini B.L., Datta S.R. The Striatum Organizes 3D Behavior via Moment-to-Moment Action Selection. Cell. 2018. 174 (1): 44–58.e17. https://doi.org/10.1016/j.cell.2018.04.019
Melzer S., Gil M., Koser D.E., Michael M., Huang K.W., Monyer H. Distinct Corticostriatal GABAergic Neurons Modulate Striatal Output Neurons and Motor Activity. Cell Rep. 2017. 19 (5): 1045–1055. https://doi.org/10.1016/j.celrep.2017.04.02428467898
Menegas W., Bergan J.F., Ogawa S.K. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. Elife. 2015. V. 4. P. e10032. https://doi.org/10.7554/eLife.10032
Meng C., Zhou J., Papaneri A., Peddada T., Xu K., Cui G. Spectrally Resolved Fiber Photometry for Multi-component Analysis of Brain Circuits. Neuron. 2018. 98 (4): 707–717.e4. https://doi.org/10.1016/j.neuron.2018.04.012
Mink J.W. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996. 50 (4): 381–425. https://doi.org/10.1016/s0301-0082(96)00042-1
Miyamoto Y., Katayama S., Shigematsu N., Nishi A., Fukuda T. Striosome-based map of the mouse striatum that is conformable to both cortical afferent topography and uneven distributions of dopamine D1 and D2 receptor-expressing cells. Brain Struct Funct. 2018. 223 (9): 4275–4291. https://doi.org/10.1007/s00429-018-1749-3
Moriya S., Yamashita A., Kawashima S., Nishi R., Yamanaka A., Kuwaki T. Acute Aversive Stimuli Rapidly Increase the Activity of Ventral Tegmental Area Dopamine Neurons in Awake Mice. Neuroscience. 2018. 386: 16–23. https://doi.org/10.1016/j.neuroscience.2018.06.027
Nakajima M., Schmitt L.I., Halassa M.M. Prefrontal Cortex Regulates Sensory Filtering through a Basal Ganglia-to-Thalamus Pathway. Neuron. 2019. 103 (3): 445–458.e10. https://doi.org/10.1016/j.neuron.2019.05.026
Nambu A. Somatotopic organization of the primate Basal Ganglia. Front Neuroanat. 2011. 5: 26. https://doi.org/10.3389/fnana.2011.00026
Nestler E.J., Lüscher C. The Molecular Basis of Drug Addiction: Linking Epigenetic to Synaptic and Circuit Mechanisms. Neuron. 2019. 102 (1): 48–59. https://doi.org/10.1016/j.neuron.2019.01.016
Neve K.A., Neve R.L. Molecular biology of dopamine receptors. The dopamine receptors. Humana Press, Totowa, NJ, 1997. P. 27–76.
Nisenbaum E.S., Wilson C.J. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci. 1995. 15 (6): 4449–4463.
Oleson E.B., Gentry R.N., Chioma V.C., Cheer J.F. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. The Journal of neuroscience. 2012. 32 (42): 14804–14808. https://doi.org/10.1523/JNEUROSCI.3087-12.2012
Owen S.F., Liu M.H., Kreitzer A.C. Thermal constraints on in vivo optogenetic manipulations. Nat Neurosci. 2019. 22 (7): 1061–1065. https://doi.org/10.1038/s41593-019-0422-3
Parker J.G., Marshall J.D., Ahanonu B., Wu Y.W., Kim T.H., Grewe B.F., Zhang Y., Li J.Z., Ding J.B., Ehlers M.D., Schnitzer M.J. Diametric neural ensemble dynamics in parkinsonian and dyskinetic states. Nature. 2018. 557 (7704): 177–182. https://doi.org/10.1038/s41586-018-0090-6
Pasquereau B., Turner R.S. Dopamine neurons encode errors in predicting movement trigger occurrence. Journal of neurophysiology. 2014. 113(4): 1110–1123.
Plotkin J.L., Goldberg J.A. Thinking Outside the Box (and Arrow): Current Themes in Striatal Dysfunction in Movement Disorders. Neuroscientist. 2019. 25 (4): 359–379. https://doi.org/0.1177/1073858418807887
Poulin J.F., Caronia G., Hofer C., Cui Q., Helm B., Ramakrishnan C., Chan C.S., Dombeck D.A., Deisseroth K., Awatramani R. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci. 2018. 21 (9): 1260–1271. https://doi.org/10.1038/s41593-018-0203-4
Puryear C.B., Kim M.J., Mizumori S.J.Y. Conjunctive encoding of movement and reward by ventral tegmental area neurons in the freely navigating rodent. Behavioral neuroscience. 2010. 124 (2): 234–247. https://doi.org/10.1037/a0018865
Reiner A., Hart N.M., Lei W., Deng Y. Corticostriatal projection neurons–dichotomous types and dichotomous functions. Frontiers in neuroanatomy. 2010. 4: 142. https://doi.org/10.3389/fnana.2010.00142
Richfield E.K., Penney J.B., Young A.B. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989. 30 (3): 767–777.
Rock C., Zurita H., Wilson C., Apicella A.J. An inhibitory corticostriatal pathway. Elife. 2016. 5: pii: e15890. https://doi.org/10.7554/eLife.15890
Roitman M.F., Stuber G.D., Phillips P.E., Wightman R.M., Carelli. Dopamine operates as a subsecond modulator of food seeking. Journal of Neuroscience. 2004. 24 (6): 1265–1271.
Roseberry T.K., Lee A.M., Lalive A.L., Wilbrecht L., Bonci A., Kreitzer A.C. Cell-Type-Specific Control of Brainstem Locomotor Circuits by Basal Ganglia. Cell. 2016. 164 (3): 526–537. https://doi.org/10.1016/j.cell.2015.12.037
Salamone J.D., Correa M., Farrar A., Mingote S.M. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007. 191 (3): 461–482.
Saunders A., Oldenburg I.A., Berezovskii V.K., Johnson C.A., Kingery N.D., Elliott H.L., Xie T., Gerfen C.R., Sabatini B.L. A direct GABAergic output from the basal ganglia to frontal cortex. Nature. 2015. 521 (7550): 85–89. https://doi.org/10.1038/nature14179
Schechtman E., Noblejas M.I., Mizrahi A.D., Dauber O., Bergman H. Pallidal spiking activity reflects learning dynamics and predicts performance. Proc Natl Acad Sci U S A. 2016. 113 (41): E6281–E6289.
Schultz W., Ruffieux A., Aebischer P. The activity of pars compacta neurons of the monkey substantia nigra in relation to motor activation. Exp Brain Res. 1983. 51: 377–387.
Schultz W. Updating dopamine reward signals. Current opinion in neurobiology. 2013. 23 (2): 229–238.
Sharott A., Magill P.J., Bolam J.P., Brown P. Directional analysis of coherent oscillatory field potentials in the cerebral cortex and basal ganglia of the rat. J Physiol. 2005. 562 (Pt 3): 951–63. https://doi.org/10.1113/jphysiol.2004.073189
Shen W., Flajolet M., Greengard P., Surmeier D.J. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008. 321 (5890): 848–851.
Silkis I. The cortico-basal ganglia-thalamocortical circuit with synaptic plasticity. II. Mechanism of synergistic modulation of thalamic activity via the direct and indirect pathways through the basal ganglia. Biosystems. 2001. 59 (1): 7–14. https://doi.org/10.1016/s0303-2647(00)00135-0
da Silva J.A., Tecuapetla F., Paixão V., Costa R.M. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature. 2018. 554 (7691): 244–248. https://doi.org/10.1038/nature25457
Smith Y., Raju D., Nanda B., Pare J.F., Galvan A., Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009. 78 (2–3): 60–68. https://doi.org/10.1016/j.brainresbull.2008.08.015
Smith J.B., Klug J.R., Ross D.L., Howard C.D., Hollon N.G., Ko V.I., Hoffman H., Callaway E.M., Gerfen C.R., Jin X. Genetic-Based Dissection Unveils the Inputs and Outputs of Striatal Patch and Matrix Compartments. Neuron. 2016. 91 (5): 1069–1084. https://doi.org/10.1016/j.neuron.2016.07.046
Steinberg E.E., Keiflin R., Boivin J.R., Witten I.B., Deisseroth K., Janak P.H. A causal link between prediction errors, dopamine neurons and learning. Nature neuroscience. 2013. 16 (7): 966–973. https://doi.org/10.1038/nn.3413
Straub C., Saulnier J.L., Bègue A., Feng D.D., Huang K.W., Sabatini B.L. Principles of Synaptic Organization of GABAergic Interneurons in the Striatum. Neuron. 2016. 92 (1): 84–92. https://doi.org/10.1016/j.neuron.2016.09.007
Taverna S., Ilijic E., Surmeier D.J. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson’s disease. Journal of Neuroscience. 2008. 28 (21): 5504–5512.
Tecuapetla F., Jin X., Lima S.Q., Costa R.M. Complementary Contributions of Striatal Projection Pathways to Action Initiation and Execution. Cell. 2016. 166 (3): 703–715. https://doi.org/10.1016/j.cell.2016.06.032
Tepper J.M., Koós T., Ibanez-Sandoval O., Tecuapetla F., Faust T.W., Assous M. Heterogeneity and Diversity of Striatal GABAergic Interneurons: Update 2018. Front Neuroanat. 2018. 12 (91): https://doi.org/10.3389/fnana.2018.00091
Trudeau L.E., Hnasko T.S., Wallén-Mackenzie A., Morales M., Rayport S., Sulzer D. The multilingual nature of dopamine neurons. Prog Brain Res. 2014. 211: 141–164. https://doi.org/10.1016/B978-0-444-63425-2.00006-4
Tsai H.C., Zhang F., Adamantidis A., Stuber G.D., Bonci A., de Lecea L., Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009. 324 (5930): 1080–1084. https://doi.org/10.1126/science.1168878
Tye K.M., Mirzabekov J.J., Warden M.R., Ferenczi E.A., Tsai H.C., Finkelstein J., Kim S.Y., Adhikari A., Thompson K.R., Andalman A.S., Gunaydin L.A., Witten I.B., Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behavior. Nature. 2013. 493 (7433): 537–541. https://doi.org/10.1038/nature11740
Varazzani C., San-Galli A., Gilardeau S., Bouret S. Noradrenaline and dopamine neurons in the reward/effort trade-off: a direct electrophysiological comparison in behaving monkeys. Journal of Neuroscience. 2015. 35 (20): 7866–7877. https://doi.org/10.1523/JNEUROSCI.0454-15.2015
Villalobos N., Oviedo-Chávez A., Alatorre A., Ríos A., Barrientos R., Delgado A., Querejeta E. Striatum and globus pallidus control the electrical activity of reticular thalamic nuclei. Brain Res. 2016. 1644: 258–266. https://doi.org/10.1016/j.brainres.2016.05.032
Wall N.R., De La Parra M., Callaway E.M., Kreitzer A.C. Differential innervation of direct-and indirect-pathway striatal projection neurons. Neuron. 2013. 79 (2): 347–360. https://doi.org/10.1016/j.neuron.2013.05.014
Watabe-Uchida M., Zhu L., Ogawa S.K., Vamanrao A., Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012. 74 (5): 858–873. https://doi.org/10.1016/j.neuron.2012.03.017
Watabe-Uchida M., Eshel N., Uchida N. Neural Circuitry of Reward Prediction Error. Annu Rev Neurosci. 2017. 40: 373–394.
Wise R.A. Dual roles of dopamine in food and drug seeking: the drive-reward paradox. Biological psychiatry. 2013. 73 (9): 819–826. https://doi.org/10.1016/j.biopsych.2012.09.001
Wise R.A., Bozarth M.A. Brain mechanisms of drug reward and euphoria. Psychiatric medicine. 1985. 3 (4): 445–460.
Yamin H.G., Stern E.A., Cohen D. Parallel processing of environmental recognition and locomotion in the mouse striatum. J Neurosci. 2013. 33 (2): 473–484. https://doi.org/10.1523/JNEUROSCI.4474-12.2013
Yapo C., Nair A.G., Clement L., Castro L.R., Hellgren Kotaleski J., Vincent P. Detection of phasic dopamine by D1 and D2 striatal medium spiny neurons. J. Physiol. 2017 595 (24): 7451–7475. https://doi.org/10.1113/JP274475
Zingg B., Hintiryan H., Gou L., Song M.Y., Bay M., Bienkowski M.S., Foster N.N., Yamashita S., Bowman I., Toga A.W., Dong H.W. Neural networks of the mouse neocortex. Cell. 2014. 156 (5): 1096–1111. https://doi.org/10.1016/j.cell.2014.02.023
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


