Журнал высшей нервной деятельности им. И.П. Павлова, 2021, T. 71, № 2, стр. 184-201
Память: аксиоматика и факты
Ю. И. Аршавский *
Калифорнийский Университет
г. Сан Диего, США
* E-mail: yarshavs@ucsd.edu
Поступила в редакцию 28.09.2020
После доработки 22.12.2020
Принята к публикации 22.12.2020
Аннотация
Принято считать, что нейрофизиология является в основном экспериментальной наукой, знания которой базируются на достоверных фактах. Однако, если мы обратимся к нейрофизиологии сложных и до сих пор малопонятных механизмов высших (когнитивных) функций головного мозга животных и особенно человека (таких как память, генерация и восприятие речи, формирование абстрактных понятий и т.п.), то увидим, что парадоксальным образом знания в этой области в значительной степени базируются на априорной аксиоматике. Общепризнанные аксиомы во многом определяют направление экспериментальных исследований и интерпретацию полученных результатов. Больше того, исследователи зачастую игнорируют или “забывают” полученные ими факты, если они противоречат общепризнанной аксиоматике. В настоящей статье я проиллюстрирую сказанное на примере изучения механизмов формирования и хранения памяти. Эта функция мозга исследовалась особенно интенсивно, поскольку в отличие от многих других когнитивных функций память существует как у человека, так и у животных. Хотя память исследовалась на животных, принадлежащих к разным биологическим типам, я буду обсуждать результаты, полученные при изучении декларативной (explicit) памяти у человека и млекопитающих животных.
ОСНОВНАЯ АКСИОМАТИКА
В 1949 г. D. Hebb предложил гипотезу, согласно которой память формируется в виде изменений силы синаптических связей между одновременно возбуждающимися нейронами (Hebb, 1949). Позднее его гипотеза была кратко сформулирована следующим образом: “Fire together, wire together”. Эта гипотеза синаптической пластичности (ГСП) была с энтузиазмом воспринята научной общественностью, участвующей в изучении нейронных механизмов памяти (Скребицкий, Чепкова, 1999; Asok et al., 2019; Chen, Tonegawa, 1997; Kandel et al., 2014; Martin et al., 2000; Mayford et al., 2012; Poo et al., 2016; Silva, 2003). Всеобщее признание ГСП превратили ее из гипотезы в безоговорочную аксиому. Как сказано в одном из обзоров: “It has seemed almost axiomatic that learning and memory must be expressed as a change in synaptic function and form” (Bailey, 1999). И еще одна цитата, дополняющая сказанное: “Activity-dependent synaptic plasticity is induced at appropriate synapses during memory formation, and is both necessary and sufficient for the information storage underlying the type of memory mediated by the brain area in which that plasticity is observed” (Martin et al., 2000).
ГСП не единственная аксиома, принятая исследователями, изучающими механизм памяти. T.V. Bliss и T. Lømo (1973) в опытах на наркотизированных кроликах показали, что кратковременное высокочастотное электрическое раздражение перфорантного пути, идущего к зубчатой извилине гиппокампа, вызывает продолжительную (от 30 мин до 10 ч) потенциацию постсинаптического ответа. Авторы предположили, что этот искусственный феномен, получивший название “долговременная потенциация”, ДВП (long-term potentiation, LTP), может иметь отношение к механизму формирования и хранения памяти. Это предположение было еще менее очевидным, чем предыдущее. Продолжительность ДВП измеряется часами. Между тем декларативная память о фактах и событиях, как все знают из своего повседневного опыта, может храниться годами, а иногда всю жизнь. У животных поведенческие навыки, приобретенные в раннем возрасте, также, по-видимому, сохраняются на протяжении всей жизни, поскольку от этого зависит их выживание. Так ли естественно предполагать, что длящаяся часами ДВП имеет отношение к такой перманентной памяти? Тем не менее идея, что ДВП (а также присоединившаяся к ней позднее долговременная депрессия (Collingridge et al., 2010)) является экспериментальной моделью памяти, была воспринята научной общественностью с таким же энтузиазмом, как ГСП, и также возведена в ранг непререкаемой аксиомы (Kandel et al., 2014; Mayford et al., 2012; Lynch, 2004; Neves et al., 2008; Nicoll, 2017). Для иллюстрации я приведу две цитаты, отражающие общепринятую точку зрения:
“LTP provides an important key to understanding the cellular and molecular mechanisms by which memories are formed and stored.” (Malenka, Nicoll, 1999) и
“Long-term potentiation (LTP) has been the gold standard synaptic model for mammalian memory mechanisms.” (Abraham, Williams, 2003).
В результате изучение нейронных механизмов реальной памяти было в большой степени подменено изучением механизма происхождения ДВП.
Аксиоматика, принятая при изучении механизмов памяти, наглядно показана на рис. 1. В данной статье я постараюсь показать несостоятельность этой аксиоматики. В действительности вся совокупность экспериментальных и клинических данных показывает, что память формируется и хранится не как изменение силы синаптических связей между нейронами, а на внутриклеточном уровне, и надежно защищена от текущей синаптической активности. Эту идею я уже высказывал в своей предыдущей статье, опубликованной в “Журнале высшей нервной деятельности” (Аршавский, 2011) (см. также (Peña de Ortiz, Arshavsky, 2001; Arshavsky, 2006, 2017)). Здесь я приведу дополнительные факты, в большой степени появившиеся в последнее десятилетие, которые показывают неправомочность общепринятой аксиоматики, а также проанализирую причины ее незыблемости.
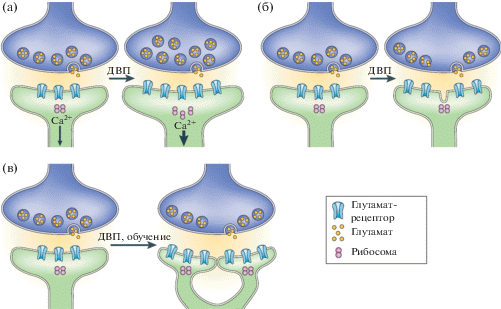
Рис. 1.
Примеры структурных изменений синапсов, образованных глутаматэргическими волокнами на грибовидных шипиках дендритов пирамидных клеток гиппокампа, при долговременной потенциации или формировании памяти. (а) – увеличение головки шипика (Van Harreveld, Fifkova, 1975; Fifkova, Anderson, 1981; Fifkova, Van Harreveld, 1977). (б) – частичное расщепление головки шипика (Toni et al., 1999). (в) – увеличение числа шипиков (Engert, Bonhoeffer, 1999; Leuner et al., 2003). Изменения морфологии шипиков могли сопровождаться увеличением количества глутаматных рецепторов и числа рибосом в шипиках. Модифицировано из (Lamprecht, LeDoux, 2004).
Fig. 1. Examples of structural changes in synapses formed by glutamatergic fibers on the mushroom-like spines of the pyramidal cell dendrites of the hippocampus during long-term potentiation or learning. (а) – increase in spine head (Van Harreveld, Fifkova, 1975; Fifkova, Anderson, 1981; Fifkova, Van Harreveld, 1977). (б) – spine perforation (Toni et al., 1999). (в) – increase in the number of spine (Engert, Bonhoeffer, 1999; Leuner et al., 2003). These changes in spine morphology are accompanied by alterations in the number of glutamate receptors and increases in ribosomes within spines. Modified from (Lamprecht, LeDoux, 2004).
ПАМЯТЬ И ГИПОТЕЗА СИНАПТИЧЕСКОЙ ПЛАСТИЧНОСТИ
В истории изучения памяти было предпринято немало попыток экспериментально подтвердить гипотезу, что в ее основе лежит ДВП. Поскольку молекулярные механизмы ДВП изучены достаточно детально, были разработаны методы фармакологического и генетического подавления или, что реже, усиления ДВП. Затем эти методы были тестированы в поведенческих опытах, чтобы оценить их влияние на память животного. Одна из первых работ такого рода была выполнена R. Morris и др. (1986). Они исследовали действие хронического внутрижелудочкового введения AP5 (amino-5-phosphonovaleric acid) – ингибитора глутаматэргических NMDA-рецепторов, активация которых необходима для возникновения ДВП в гиппокампе, – на способность крыс к обучению пространственной ориентации в водном лабиринте. Было обнаружено, что AP5 полностью блокирует способность животных к обучению этой гиппокамп-зависимой задаче. Поскольку эта работа неоднократно подвергалась критике (см. (Bannerman et al., 2014)), лаборатория, возглавляемая R. Morris, представила дополнительные доказательства, что действие AP5 на пространственную память обусловлено именно подавлением ДВП в гиппокампе, а не побочными эффектами типа нарушения моторных или сенсорных функций (Morris et al., 2013). Однако вся аргументация этих авторов базируется на аксиоме, что утрата пространственной памяти, вызванная блокадой NMDA-рецепторов, зависит исключительно от подавления ДВП. Между тем естественно предположить, что блокада этого важного класса рецепторов может вести не только к подавлению ДВП в гиппокампе, но и к серьезным нарушениям его функционирования. Это предположение означает, что эффект блокады NMDA-рецепторов может быть эквивалентен эффекту по крайней мере частичной резекции гиппокампа. В этом случае неудивительно, что хроническое действие AP5 вело к потере некоторых видов гиппокамп-зависимой памяти.
Генерация ДВП связана с активацией NMDA-рецепторов в постсинаптической мембране и вхождением в нейроны ионов Ca2+ (рис. 1 (а)), которые в свою очередь вызывают каскад реакций, ведущих к активации ряда протеинкиназ (calcium–calmodulin kinase II, CaMKII; protein kinase A, PKA; mitogen-activated protein kinase, MAPK; protein kinase C, PKC и др.), фосфатаз и транскрипционных факторов (Chen, Tonegawa, 1997; Kandel et al., 2014; Mayford et al., 2012; Poo et al., 2016; Silva, 2003). С разработкой методики получения генетически модифицированных животных (обычно мышей) появилось большое количество работ, в которых избирательно выключали (gene knockout) или увеличивали активность генов, экспрессирующих протеины, прямо или косвенно вовлеченных в генерацию ДВП. Результаты этих работ оказались неоднозначными. Одни исследователи в опытах на трансгенных животных показали, что, в соответствии с общепризнанной аксиоматикой, блокада активности NMDA-рецепторов, CaMKII, PKA и других факторов, вовлеченных в генерацию ДВП, подавляла по крайней мере некоторые виды памяти (Abel et al., 1997; Giese et al., 1998; Tsien et al., 1996)11, тогда как повышение экспрессии NMDA-рецепторов увеличивало ДВП и способность к обучению (Tang et al., 1999) (см. обзоры литературы (Kandel et al., 2014; Lynch, 2004; Mayford et al., 2012; Tsien, 2000)). Однако другие авторы не обнаружили корреляции между ДВП и памятью. Так, было показано, что нокаут NMDA-рецепторов вызывал утрату гиппокамп-зависимой памяти у мышей, содержащихся в стандартных условиях, но оказывался неэффективным у мышей, которых ежедневно помещали в условия так называемой обогащенной среды (Rampon et al., 2000). Этот факт аналогичен другому результату, показавшему, что внутрижелудочковое введение AP5 не влияет на пространственное обучение животных в лабиринте, если они предварительно обучались в другом лабиринте (Bannerman et al., 1995). Оба эти результата плохо согласуются с представлением о взаимосвязи между ДВП и памятью. Больше того, были описаны факты, прямо противоречащие этому представлению. Например, у трансгенных мышей с нокаутом гена, экспрессирующего нейротрофический фактор мозга (Montkowski, Holsboer, 1997), или с повышенной экспрессией одной из двух субъединиц (NR2D), формирующих NMDA-рецептор (Okabe et al., 1998), наблюдалось подавление ДВП, не сопровождавшееся нарушениями пространственной памяти. В других работах у мышей вызывали нокаут гена, экспрессирующего белок PSD-95, который в постсинаптической мембране связан с NMDA-рецепторами. Хотя у этих мутантных животных имело место увеличение ДВП в гиппокампе, у них отсутствовала способность к обучению пространственной ориентации в водном лабиринте (Migaud et al., 1998; Nithianantharajah et al., 2013) (другие примеры, демонстрирующие отсутствие корреляции между ДВП в гиппокампе и способностью к обучению гиппокамп-зависимым задачам, описаны в обзорах (Bannerman et al., 2014; Brakebusch et al., 2002; Gallistel, Matzel, 2013; Grant, 2018)).
Экспериментальные результаты, противоречащие идее об обязательной корреляции между ДВП и памятью, никоим образом не поколебали существующую аксиоматику. Эти результаты либо пытались объяснить, хотя и не очень вразумительно, в рамках принятых представлений, либо их просто игнорировали.
ПАМЯТЬ И ФЕНОМЕН РЕКОНСОЛИДАЦИИ
Я уже писал о феномене реконсолидации в предыдущей публикации (Аршавский, 2011) и хочу вернуться к нему здесь, поскольку обсуждение механизма реконсолидации памяти как нельзя лучше иллюстрирует ситуацию, сложившуюся в этой области. Как известно, консолидация долговременной памяти – это сложный биохимический процесс, включающий дополнительный синтез белка. Ингибиторы синтеза белка или мРНК, введенные животному непосредственно после его обучения, например, выработки условного рефлекса, блокируют консолидацию долговременной памяти, но не влияют на кратковременную память (Анохин, 1997; Davis, Squire, 1984; Hernandez, Abel, 2008; McGaugh, 2000). Этот результат обычно рассматривается как важный аргумент в пользу ГСП.
В опытах на мышах и цыплятах было показано, что ингибиторы синтеза белка не только препятствуют консолидации памяти, но также ведут к подавлению консолидированной памяти, если действовуют после ее воспроизведения (Литвин, Анохин, 1999; Муравьева, Анохин, 2006; Judge, Quartermain, 1982). Эти и другие результаты привели к заключению, что энграммы памяти дестабилизируются после их воспроизведения, и требуется дополнительный синтез белка для их реконсолидации (см. обзоры (Anokhin, 2002; Sara, 2000)). Интерес к явлению реконсолидации памяти увеличился в связи с публикацией работы K. Nader и др. (2000). В опытах на крысах они изучали эффект инъекции ингибитора белкового синтеза (анизомицина) в область миндалины после воспроизведения ранее выработанной условной оборонительной реакции на звук (“Pavlovian fear conditioning”). Если анизомицин инъецировали сразу после воспроизведения оборонительной реакции, эффект последующих условных сигналов был подавлен. Между тем аналогичная инъекция анизомицина через 6 ч после воспроизведения условной реакции не влияла на память животных. Заключая абстракт своей статьи, авторы написали: “These findings are not predicted by traditional theories of memory consolidation”. Не совсем понятно, почему слово “теория” было написано во множественном числе. Как в настоящее время, так и во время публикации статьи существовала только одна теория, точнее гипотеза, памяти – ГСП, согласно которой долговременная память консолидируется в виде структурных изменений межнейронных синаптических связей (рис. 1). Надо признать, что феномен реконсолидации действительно никак не согласуется с ГСП. Трудно представить, что структурные изменения синаптических связей, сформировавшиеся в процессе консолидации памяти, нарушаются после ее воспроизведения, а затем самопроизвольно восстанавливаются за счет дополнительного белкового синтеза.
Хотя K. Nader и соавт. признали, что полученные ими результаты не предсказываются традиционными теориями консолидации памяти, уже в своей следующей статье они утверждали, что в основе формирования условной оборонительной реакции лежат синаптическая пластичность и ДВП:
“Progress in elucidating the neural system underlying fear conditioning has in recent years been paralleled by great strides in our understanding of the cellular and molecular basis of synaptic plasticity, including LTP” (Schafe et al., 2001).
При этом авторы отмечают, что существуют трудности в интерпретации феномена реконсолидации, но считают, что они должны быть преодолены исключительно в рамках ГСП. Приверженность общепризнанной аксиоматике оказалась сильнее экспериментальных фактов.
ПАМЯТЬ И СТАБИЛЬНОСТЬ СИНАПТИЧЕСКИХ СВЯЗЕЙ
Как упоминалось выше, память отличается высокой стабильностью. Она может храниться на протяжении многих лет, а зачастую и всю жизнь. Подобно тому как люди различаются по своим музыкальным, математическим и другим способностям, они различаются по своей памяти. Встречаются не только люди с абсолютным музыкальным слухом, но и с абсолютной памятью. Они помнят наизусть прочитанные книги или услышанные тексты, могут в деталях описать каждый день своей жизни и т.п. (Лурия, 1968; Parker et al., 2006; Treffert, Christensen, 2005). Например, человек по имени Kim Peek помнил наизусть все (~9000) прочитанные им книги, начиная с книг, которые были прочитаны ему матерью в возрасте 1.5 лет (Treffert, Christensen, 2005). Это не единственный случай такой феноменальной памяти. Люди моего поколения хорошо знакомы с рассказами Ираклия Андрониковa. Один из них посвящен известному музыковеду И.И. Соллертинскому. По свидетельству Андроникова, Соллертинский мог воспроизвести наизусть наугад названную страницу из произведения русской классики (к этому можно добавить, что он знал, т.е. хранил в своей памяти, двадцать шесть языков и сто диалектов). Совершенно очевидно, что в основе такой стабильной памяти должны лежать столь же стабильные механизмы. В настоящем разделе я рассмотрю вопрос о стабильности предполагаемых структурных изменений синаптических связей, которые, согласно ГСП, являются носителями (энграммами) памяти.
Долговечность памяти всегда представляла проблему для ГСП, и исследователи, работающие в этой области, были вынуждены создавать дополнительные гипотезы для ее объяснения. Я приведу несколько примеров таких гипотез. Анализируя механизм консолидации долговременной памяти, H. Wang и др. (2006) пришли к заключению, что однократного каскада реакций, инициируемых активацией NMDA-рецепторов, ведущего к дополнительному синтезу соответствующих белков, недостаточно для устойчивого изменения синаптических связей. Для объяснения долговременных изменений структуры синапсов они предложили гипотезу повторного синаптического подкрепления (synaptic reentry reinforcement hypothesis). По мнению авторов, такие повторные подкрепления могут иметь место при спонтанных воспроизведениях памяти или во время фазы быстрого сна, когда происходит синхронное возбуждение больших массивов нейронов.
Другие исследователи предполагают, что долговременные структурные изменения синаптических связей контролируются строго локализованным дендритным синтезом соответствующих белковых молекул (Asok et al., 2019; Bramham, 2008; Rayman, Kandel, 2017; Si, Kandel, 2016; Steward, Schuman, 2001; см. рис. 1). Согласно E.R. Kandel и соавт., эта устойчивая, локальная трансляция мРНК поддерживается цитоплазматическими полиаденил элемент-связывающими протеинами (cytoplasmic polyadenylation element-binding proteins, CPEBs), обладающими прион-подобными свойствами (Asok et al., 2019; Rayman, Kandel, 2017; Si, Kandel, 2016).
Наиболее перспективная гипотеза была предложена F. Crick (1984) и H.P. Davis и L.R. Squire (1984), которые предположили, что механизм консолидации долговременной памяти базируется на модификации молекулы ДНК. Эта идея была конкретизирована R. Holliday (1999), считавшим, что консолидация памяти обусловлена метилированием ДНК. К настоящему времени гипотеза о эпигенетических механизмах памяти подтверждена экспериментально. В опытах, выполненных главным образом на грызунах, показано, что формирование памяти связано с метилированием/деметилированием ДНК и изменением (ацетилированием, фосфорилированием и метилированием) ассоциированных с ней гистонов (Бородинова, Балабан, 2020; Burns, Gräff, 2021; Campbell, Wood, 2019; Gräff, Mansuy, 2008; Levenson, Sweatt, 2005; Li et al., 2013; Miller et al., 2010; Zovkic, 2021; Zovkic et al., 2013). В соответствии с существующей аксиоматикой считается, что эти эпигенетические модификации хроматина ведут к устойчивым изменениям структуры синаптических связей.
Фактически все эти гипотезы негласно подразумевают, что у взрослых организмов, имеющих определенную память, существует два типа синапсов: обладающие высокой пластичностью нативные синапсы, сформировавшиеся в процессе развития мозга в результате реализации генетических программ, и более ригидные синапсы, сформировавшиеся в процессе обучения и консолидации памяти. К этой проблеме я вернусь ниже, а сейчас рассмотрю вопрос о том, что реально известно о структурных изменениях синапсов в процессе формирования памяти. Декларативная память формируется и хранится главным образом в медиальной височной доле, включающей гиппокамп, и в ассоциативных зонах префронтальной, височной, теменной и затылочной долей неокортекса (Eichenbaum, 2000; Frankland, Bontempi, 2005; Squire, 2009, Squire et al., 2015). Когда говорят о структурных изменениях синапсов в процессе формирования памяти, в основном имеют в виду синапсы, образованные глутаматэргическими волокнами на шипиках апикальных и базальных дендритов пирамидных клеток, локализованных в этих областях мозга (рис. 1). Дендритные шипики имеют разную форму: нитевидные шипики с небольшим утолщением на конце, короткие шипики без выраженной шейки, грибовидные шипики (рис. 1) и более сложные, разветвленные шипики (Holtmaat, Svoboda, 2009). Основные результаты о структурных изменениях синапсов получены не при изучении реальной памяти, а при изучении ДВП. В этом можно убедиться, обратившись к обзорам литературы на данную тему (Bailey et al., 2015; Lamprecht, LeDoux, 2004; Suratkal et al., 2021). Иллюстрацией сказанного является также рис. 1. Изменения структуры синапсов, показанные на этом рисунке, базируются на шести экспериментальных работах, пять из которых были посвящены изучению ДВП. При этом в качестве одного из излюбленных объектов использовали тонкие (~350 микрон толщиной) срезы гиппокампа грызунов. Только результаты одной из работ были получены при реальном обучении животных, причем в этой работе морфологию синапсов исследовали уже на следующий день после окончания обучения (Leuner et al., 2003). Таким образом, ни о какой долговременности структурных изменений синаптических связей в этих работах речи не идет.
Сравнительно немногочисленные исследования структурных изменений синапсов при формировании реальной долговременной памяти дали неоднозначные результаты. Я приведу только несколько примеров. L. Restivo и др. (2009) изучали влияние выработки условной оборонительной реакции у мышей на плотность шипиков пирамидных клеток в гиппокампе и неокортексе (передняя поясная кора). В соответствии с предсказаниями ГСП они нашли, что выработка оборонительной реакции ведет к быстрому и сравнительно непродолжительному увеличению плотности шипиков в гиппокампе и к более медленному, но устойчивому увеличению плотности шипиков в неокортексе. Однако другие авторы получили прямо противоположный результат. Так, J. Sanders и др. (2012), изучавшие условную оборонительную реакцию в тех же экспериментальных условиях, что и предыдущие авторы, обнаружили, что выработка оборонительной реакции ведет не к увеличению, а к уменьшению числа шипиков у пирамидных клеток гиппокампа. Mетодом двухфотонной лазерной сканирующей микроскопии (2PLSM) было показано, что оборонительная реакция также сопровождается устойчивым уменьшением числа шипиков в ассоциативной области фронтальной доли неокортекса (Lai et al., 2012). Наконец, авторы недавней работы вообще не обнаружили изменений плотности шипиков у пирамидных клеток гиппокампа при обучении крыс пространственной ориентации в лабиринте (Craig et al., 2020). Единственный вывод, который можно сделать из этих противоречивых фактов – в настоящее время невозможно сделать никаких определенных выводов о структурных изменениях синапсов при формировании декларативной (explicit) памяти у животных.
Между тем результаты, полученные с изобретением двухфотонной лазерной микроскопии, позволяющей в течение длительного времени (от нескольких дней до месяца) прижизненно наблюдать морфологию шипиков дендритов клеток коры головного мозга, показали, что стабильная память едва ли хранится в виде изменения силы синаптических связей. Было обнаружено, что у взрослых животных синапсы, образованные глутаматэргическими волокнами на шипиках дендритов пирамидных клеток, крайне нестабильны: одни шипики втягиваются и вместо них вырастают другие шипики, с которыми приходящие волокна формируют новые синапсы. Согласно A. Attardo и соавт. (2015), продолжительность жизни шипиков пирамидных клеток гиппокампа равна одной-двум неделям; шипики сложной формы более устойчивы, чем простые шипики. Сходные результаты, свидетельствующие о нестабильности шипиков пирамидных клеток гиппокампа в отсутствие какого-либо специального обучения, были получены другими авторами (Gu et al., 2014; Pfeiffer et al., 2018).
Более стабильными оказались синапсы пирамидных клеток в неокортексе. По данным J.T. Trachtenberg и соавт. (2002), изучавших дендриты пирамидных клеток, локализованных в 5-м слое соматосенсорной коры мышей, только ~50% шипиков сохранялись на протяжении месяца. Согласно расчетам авторов, средняя продолжительность жизни этой устойчивой фракции шипиков равна 120 дням. В этой же лаборатории было обнаружено, что в зрительной коре устойчивая фракция составляет ~73% (Holtmaat et al., 2005), тогда как, по данным J. Grutzendler и соавт. (2002), устойчивая фракция шипиков, время полужизни которых оценивается в 13 мес, равна ~96%. Наименьшая устойчивость синапсов пирамидных клеток была найдена в слуховой коре, где ~60% шипиков обновляется в течение трех недель (Loewenstein et al., 2015). Как отмечает большинство авторов, даже шипики устойчивой фракции нельзя назвать полностью стабильными, поскольку они непрерывно меняют свои размеры и форму. В свою очередь это ведет к изменению количества глутаматных рецепторов в постсинаптической мембране и, следовательно, к изменению силы синаптических связей (Noguchi et al., 2011). Описанная изменчивость шипиков, по-видимому, обусловлена собственными свойствами пирамидных клеток, поскольку она наблюдалась не только в нормальных условиях, но и при блокаде синаптической активности (Nagaoka et al., 2016).
Экспериментальные результаты, свидетельствующие о спонтанной изменчивости синаптических связей, детально описаны в обзорах (Choquet, Triller, 2013; Mongillo et al., 2017; Ziv, Brenner, 2018). Эти результаты никак не согласуются с ГСП. Память, дающая возможность хранить в мозге содержание тысяч книг, десятки различных языков, события каждого прожитого дня, едва ли базируется на такой зыбкой основе, как изменение синаптических связей. Для подтверждения этих слов я процитирую авторов одного из упомянутых обзоров:
“We reviewed some of the recent evidence supporting the synaptic trace theory of memory. This theory agrees with our everyday intuition that information is stored for long-periods of time in elements that are physically as stable as possible … . Yet, we pointed out a substantial challenge to this theory, the fact that the ‘stable’ elements of this theory – the synapses, are in fact highly volatile. … Thus, the question of how the brain maintains functional stability with volatile elements remains a fundamental puzzle in neuroscience” (Mongillo et al., 2017).
Несмотря на убедительность такого заключения, факты, описанные в данном разделе, также не оказали никакого влияния на существующую аксиоматику. Напротив, были предприняты достаточно искусственные теоретические попытки совместить факты, свидетельствующие о стабильности декларативной памяти и имманентной нестабильности синаптических связей, с ГСП (Acker et al., 2019; Fauth, van Rossum, 2019).
ПАМЯТЬ И ЭПИЛЕПСИЯ
Основным свидетельством неправомочности ГСП является устойчивость памяти к генерализованным эпилептическим припадкам, в частности, к припадкам, источник которых находится в медиальной височной доле. В основе этих припадков лежат повторяющиеся, высокочастотные, синхронные разряды нейронов (Alarcón et al., 2012; Alvarado-Rojas et al., 2013). Частота импульсов внутри нейронных разрядов сопоставима с частотой стимулов, используемой для получения ДВП. В опытах на животных было показано, что фармакологически вызванные приступы эпилептоподобной активности вызывают в гиппокампе ДВП (epileptic long-term potentiation), аналогичную той, которая имеет место при электрической стимуляции нервных волокон (Ben-Ari, Represa, 1990; Lopantsev et al., 2009). С точки зрения существующей аксиоматики это означает, что у больных эпилепсией каждый приступ должен вызывать хаотические изменения силы межнейронных синаптических связей в структурах, вовлеченных в эпилептическую активность. В свою очередь это должно вести к деструкции энграмм памяти, сформировавшихся в нейронных сетях в результате изменения силы синаптических связей после предыдущего приступа. Тем самым, согласно предсказанию общепризнанной аксиоматики, у людей, страдающих от периодически повторяющихся генерализованных припадков, берущих начало в медиальной височной доле и распространяющихся на близлежащие неокортикальные структуры, включая височную и фронтальную доли, память должна полностью или почти полностью отсутствовать. Однако это предсказание не соответствует действительности. Даже после очень сильных припадков больные ничего не помнят только о том, что происходило непосредственно перед и во время эпилептического приступа, тогда как память обо всем произошедшем в течение основного времени, прошедшего после предыдущего приступа, не страдает. Наблюдающиеся иногда после эпилептического приступа более серьезные провалы в памяти обычно носят обратимый характер. Я приведу два примера, свидетельствующих об устойчивости памяти к эпилептическим припадкам.
Первый пример – это случай пациента, известного под инициалами H.M. Важность этого случая определяется тем, что он воочию показал, что память является самостоятельной функцией мозга с собственной локализацией. По свидетельству L.R. Squire (2009), только его первое описание (Scoville, Milner, 1957) цитировалось ~2500 раз. Однако здесь я хочу обратить внимание на другой аспект этого случая. Небольшие эпилептические припадки появились у пациента H.M. в 10 лет. В 15 лет к ним добавились сильные генерализованные приступы, частота которых с возрастом увеличивалась. К 27 годам, когда ему осуществили двустороннюю резекцию медиальной височной доли, включая большую часть гиппокампа, частота сильных приступов достигла примерно одного раза в неделю. Как известно, операция привела к полной антероградной амнезии (Scoville, Milner, 1957). Послеоперационное исследование памяти H.M. показало, что операция вызвала не только антероградную, но и частичную ретроградную амнезию. Тем не менее H.M. помнил очень многие факты и события из своей предоперационной жизни, включая период, когда у него появились сильные генерализованные припадки. Это позволило заключить, что его память была устойчива к эпилепсии (Аршавский, 2011). После смерти H.M. были опубликованы дополнительные факты его биографии, подтверждающие правильность этого заключения (Dossani et al., 2015). Примерно в 15 лет он бросил школу, где его дразнили из-за его недуга. Позднее, в возрасте 17 лет, он вновь поступил в школу, которую окончил в 21 год. Таким образом, в период, когда у него уже появились довольно часто повторяющиеся сильные эпилептические припадки, H.M. мог учиться и закончить школу. Это было бы невозможно, если бы каждый последующий приступ вызывал деструкцию памяти о знаниях, приобретенных им после предыдущего приступа, что предсказывает ГСП.
Второй пример – открытие так называемых “концептуальных клеток” (“concept cells”) в медиальной височной доле эпилептических больных (Quiroga, 2012; Quiroga et al., 2009, 2013; Rey et al., 2020)). У этих больных частота припадков варьировала от нескольких раз в день до одного раза в месяц, тогда как очаг эпилептогенной активности как правило находился в медиальной височной доле (G. Kreiman, личное сообщение). Реакции одиночных нейронов чаще всего исследовали на предъявление пациентам изображений известных им людей (членов семьи, политических деятелей, актеров и т.п.). Оказалось, что в медиальной височной доле существуют нейроны, избирательно реагирующие на изображения только какого-то одного человека (рис. 2 (а)). Реакция не зависела от деталей изображения, таких как размер или поворот лица, мимики, одежды, было ли изображение цветной фотографией или карандашным наброском и т.п. Часть этих нейронов реагировала не только на изображения данного человека, но и на его имя, написанное и/или произнесенное вслух. Таким образом, можно полагать, что эти нейроны являются носителями энграмм абстрактной памяти (“концепции”) об отдельных людях (“memory engram neurons”, согласно термину, предложенному S. Tonegawa и соавт. (2015)). Были также найдены концептуальные клетки, избирательно реагирующие на изображение какого-либо животного или известного архитектурного памятника. Сходные результаты были получены другими авторами (Reber et al., 2019; Steinmetz et al., 2011; Suthana et al., 2015; рис. 2 (б)). Больше того, в одной из работ эпилептическим больным показывали не фотографии, а короткие (5–10 сек) видеоклипы (эпизоды из популярных фильмов, съемки известных людей, животных, достопримечательностей и т.п.); каждый клип показывался в случайном порядке от 5 до 10 раз (Gelbard-Sagiv et al., 2008). В медиальной височной доле были обнаружены нейроны, избирательно возбуждающиеся при демонстрации какого-то одного клипа и не реагирующие на другие клипы. Пример такого нейрона показан на рис. 3. Эта клетка возбуждалась при демонстрации эпизода из мультипликационного комического телесериала “Симпсоны”, слабо реагировала на демонстрацию эпизода из другой комедии и не реагировала на 46 других клипов. После сеанса пациента просили вспомнить просмотренные им клипы. Клетка возбуждалась, когда пациент вспоминал эпизод из комедии “Симпсоны”, но не любой другой клип.
Рис. 2.
Две концептуальные клетки, зарегистрированные в гиппокампе. (а) – нейрон избирательно реагировал на предъявление пациенту различных фотографий (стимулы 4–6) и написанное и произнесенное имя (стимулы 13 и 17) артиста Джеки Чан (Jackie Chan) и не реагировал на предъявление фотографий и имя (стимулы 1–3, 15 и 19) артиста Лучиано Кастро (Luciano Castro), а также других известных людей (Rey et al., 2020). (б) – нейрон, избирательно реагировавший на предъявление пациенту фотографий президента США Клинтона (стимул 1). Этот нейрон не реагировал на фотографию президента Буша-младшего (стимул 5), но слабо реагировал на демонстрацию фотографий трех людей, внешне похожих на Клинтона (стимулы 2–4) (Suthana et al., 2015). Под каждым стимулом показаны ответы нейронов и усредненная частота нейронной активности в Гц. Ноль на шкале времени – начало предъявления стимула.
Fig. 2. Two concept cells from the hippocampus. (а) – the neuron selectively responded to pictures (stimuli 4–6) and written and spoken name (stimuli 13 and 17) of the actor Jackie Chan and did not react to pictures and names of the actor Luciano Castro (stimuli 1–3, 15 and 19) and other celebrities (Rey et al., 2020). (б) – the neuron that selectively responded to pictures of US President Clinton (stimulus 1). This neuron did not react to a picture of President Bush Jr. (stimulus 5), but reacted although weaker to pictures of three people who looked like Clinton (stimuli 2–4) (Suthana et al., 2015). Neuron responses and the average frequency of neuronal activity are shown under each stimulus. Zero on the time scale is the beginning of stimulus presentation.
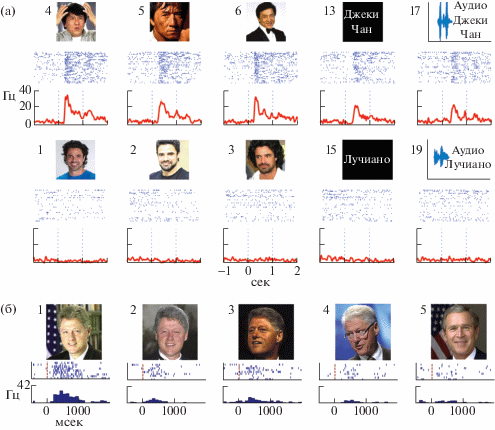
Рис. 3.
Нейрон из энторинальной коры, избирательно реагировавший на демонстрацию пациенту эпизода из мультипликационного комического телесериала “Симпсоны”. Нейрон не реагировал на демонстрацию других 46 клипов и слабо реагировал на демонстрацию эпизода из другой комедии – “Seinfeld”. Приведены 8 примеров реакций нейронов на демонстрацию разных клипов. Верхний ряд – номера клипов: 35 – Madonna; 36 – Luther King; 37 – Marilyn Monroe; 38 – Michael Jordan; 40 – Bin Laden; 41 – Pyramids; 46 – Simpsons; 48 – Wall Street. Ниже показаны частота активности нейрона и электронейрограмма (Gelbard-Sagiv et al., 2008).
Fig. 3. The neuron in the entorhinal cortex that was selectively activated during viewing of an episode from the animated comic TV series “The Simpsons”. Neuron did not react during viewing of other 46 movie clips and reacted poorly to an episode from the comedy “Seinfeld”. Eight examples are presented. The upper row – clip numbers: 35 – Madonna; 36 – Luther King; 37 – Marilyn Monroe; 38 – Michael Jordan; 40 – Bin Laden; 41 – Pyramids; 46 – Simpsons; 48 – Wall Street. Below, the frequency of neuron activity and electroneurogram are shown (Gelbard-Sagiv et al., 2008).

Ясно, что каждая концепция представлена в медиальной височной доле не одним или несколькими, а множеством нейронов. По расчетам авторов, чтобы вероятность зарегистрировать концептуальную клетку за время тестирования не была близкой к нулю, количество нейронов, представляющих каждую концепцию, должно быть не менее двадцати тысяч (Quiroga et al., 2013). Эти нейроны расположены не компактной группой, а разбросаны в медиальной височной доле, поскольку в случаях, когда одним электродом регистрировали две концептуальные клетки, они никогда не реагировали на изображение одного и того же человека, но только разных людей (например, один нейрон реагировал на фотографию матери Терезы, а другой – актрисы Хэлл Берри, или один на фотографию актера Майкла Дугласа, другой – одного из участников данного исследования).
Согласно R.Q. Quiroga (2012), все нейроны, представляющие данную концепцию, образуют единую сеть. Тем самым молчаливо предполагается, что абстрактные концепции формируются и хранятся в памяти на уровне нейронной сети и что регистрируемые концептуальные клетки отражают активность соответствующей сети. Однако идея, что нейроны, представляющие одну и ту же концепцию, преимущественно связаны друг с другом и образуют единую сеть, никак не следует из полученных результатов. Это не больше чем дань существующей аксиоматике. С таким же успехом можно предположить, что эти нейроны образуют связи не столько друг с другом, сколько с нейронами, представляющими другие концепции. В зависимости от характера воспоминаний активируются те или иные ансамбли разно-концептуальных нейронов.
Факт, что концептуальные нейроны были обнаружены у больных, страдающих от частых эпилептических припадков, которые берут начало в медиальной височной доле, прямо доказывает несостоятельность ГСП. Теоретический анализ показывает, что на уровне нейронных сетей память может храниться исключительно в виде изменения силы межнейронных связей (например, (Evans, Stringer, 2012)). Как подчеркивалось выше, в этом случае энграммы памяти не могли бы храниться в медиальной височной доле исследуемых пациентов – они должны были бы разрушаться при очередном эпилептическом приступе. Можно, конечно, предположить, что синапсы, участвующие в формировании памяти, не чувствительны к эпилептической активности, поскольку они переходят из пластичного состояния в ригидное (см. выше). Однако это предположение не выдерживает критики. В этом случае у больных, страдающих от хронической эпилепсии, все синапсы должны были бы достаточно быстро перейти в ригидное состояние, что привело бы к утрате способности формировать новые энграммы памяти. Между тем такая антероградная амнезия отсутствовала у исследуемых пациентов. Например, у одного из них был зарегистрирован концептуальный нейрон, реагировавший на изображение и имя участника данного исследования, хотя он ранее был неизвестен пациенту (Quiroga et al., 2009).
Таким образом, случай пациента H.M. и открытие концептуальных нейронов у больных, страдающих от хронической эпилепсии, с неизбежностью приводят к заключению, что энграммы памяти формируются не на уровне нейронных сетей благодаря изменению силы межнейронных синаптических связей, а на внутриклеточном уровне, где они надежно защищены от текущей синаптической активности, включая патологическую эпилептическую активность. Упомянутые выше результаты, показавшие, что формирование памяти базируется на эпигенетических модификациях молекулы ДНК, не только поддерживают это заключение, но и объясняют долговечность памяти (Бородинова, Балабан, 2020; Burns, Gräff, 2021; Campbell, Wood, 2019; Gräff, Mansuy, 2008; Levenson, Sweatt, 2005; Li et al., 2013; Miller et al., 2010; Zovkic, 2021; Zovkic et al., 2013).
В конце этого раздела хочу сделать одно замечание. Я прочел не одну сотню статей и книг о физиологии памяти. При этом я ни разу не столкнулся с обсуждением вопроса о причинах устойчивости памяти к эпилепсии. Лишь одна из статей начиналась словами:
“The human brain consists of 1011 neurons connected by 1015 synapses. This awesome network has a remarkable capacity to translate experiences into vast numbers of memories, some of which can last an entire lifetime. These long-term memories survive surgical anaesthesia and epileptic episodes …” (Chklovskii et al., 2004).
Однако в дальнейшем авторы статьи больше не возвращались к вопросу об устойчивости памяти к эпилепсии. Не должно ли такое положение вещей вызывать по крайней мере чувство недоумения?
ПРИЧИНЫ НЕЗЫБЛЕМОСТИ ОБЩЕПРИЗНАННОЙ АКСИОМАТИКИ
В данной статье я попытался привести ряд аргументов, свидетельствующих о несостоятельности ГСП. Дополнительные аргументы были приведены в предыдущих публикациях (Аршавский, 2011; Arshavsky, 2006, 2017; Peña de Ortiz, Arshavsky, 2001). Все эти аргументы базируются на общеизвестных фактах. Очевидность по крайней мере части из этих аргументов, как я думаю, должна бросаться в глаза. Тем не менее в литературе можно встретить лишь одиночные публикации, подвергающие сомнению правильность ГСП (Albo, Gräff, 2018; Gallistel, 2013, 2017; Langille, Gallistel, 2020; Trettenbrein, 2016).
Существует несколько причин незыблемости аксиоматики, принятой при изучении механизмов памяти. По-видимому, главная причина состоит в том, что эти аксиомы являются частью общей парадигмы, известной также как коннекционистская концепция, на которой в настоящее время базируется физиология высшей нервной деятельности. Согласно этой парадигме, высшие функции мозга осуществляются исключительно на уровне нейронных сетей в результате взаимодействия между нейронами. При этом сами нейроны рассматриваются как примитивные элементы, функция которых сводится к генерации электрических потенциалов и передаче сигналов к другим нервным клеткам с помощью нейротрансмиттеров. Краткая формулировка этой парадигмы дана в учебнике по нейрофизиологии, по которому учатся аспиранты во многих странах: “Mental processes are the end product of the interactions between elementary processing units” (Kandel et al., 2013). Ясно, что в рамках этой парадигмы память может формироваться и храниться только в виде изменений силы межнейронных связей.
Логическое развитие основной парадигмы физиологии высшей нервной деятельности привело к точке зрения, что мозг является вычислительной системой, состоящей из гигантского числа двоичных элементов. Эта точка зрения была четко сформулирована в недавней статье: “The distinctive function of the brain is computation” (Huys et al., 2020). Другой пример – в книге “Cerebral Cortex: Principles of Operation” E.T. Rolls (2016) пишет:
“Understanding at the molecular level is important for helping to understand how these large-scale computational processes are implemented in the brain, but will not by itself give any account of what computations are performed to implement the cognitive functions”.
Число подобных высказываний может быть многократно увеличено22. Соответственно, существует бесчисленное количество работ, авторы которых надеются понять механизм когнитивных функций с помощью компьютерных моделей (например, журнал Current Opinion in Neurobiology, т. 46, 2017 г. и т. 58, 2019 г.). Я уже писал, что одним из авторов, критически относившихся к попыткам использовать компьютерные модели для понимания когнитивных функций, был выдающийся математик И.М. Гельфанд (Аршавский, 201033). Согласно Гельфанду, сложность нейрофизиологических процессов, лежащих в основе когнитивных функций мозга, несопоставима со сложностью технических систем и для их описания требуется иной математический аппарат. Поэтому компьютерное моделирование когнитивных функций, как правило, представляет собой попытку низвести неформализуемую работу мозга до уровня сложности работы технических систем (Gelfand, 1991). Сходную идею позднее высказали D. Aur и M.S. Jog, которые писали:
“Models are able to characterize only a few properties of real physical phenomena. Many scientists believe that coding and decoding information in the brain is governed by classical physics. However, at the microscopic level many interacting particles, molecular machines, proteins, genes and specific regulatory mechanisms may actually naturally display quantum properties” (Aur, Jog, 2010).
К последнему пункту этого высказывания я вернусь позднее.
Несостоятельность основной парадигмы когнитивной нейрофизиологии и попыток представить мозг как простую вычислительную систему следует из того факта, что нейроны как “elementary processing units” являются крайне медленными элементами (Аршавский, 2018; Arshavsky, 2017). В то время как даже в самых медленных компьютерах характерные времена работы processing units измеряются в наносекундах, характерные времена работы нейронов измеряются миллисекундами. Однако, несмотря на медленную работу нейронов как электрофизиологических элементов нейронных сетей, мозг способен работать очень быстро. Для примера я обычно приводил результат двух матчей между Гарри Каспаровым и суперкомпьютером Deep Blue, которые окончились в пользу Каспарова, хотя скорость работы компьютера была такова, что он анализировал 2 × 108 позиций в секунду. Другой пример – работа синхронных переводчиков высшего класса, обслуживающих такие организации, как ООН или ЮНЕСКО. В считанные секунды они находят не только нужные слова и грамматические конструкции, но и зачастую адекватные шутки и идиоматические выражения в целевом языке. Для сравнения могу привести компьютерную программу translate.google.com, над которой работало множество специалистов. Тем не менее при переводе письменного текста с русского на английский эта программа делает элементарные грамматические ошибки (не говоря о семантических, что естественно).
Как согласовать медленную работу нейронов с быстрой работой мозга? Для ответа я обращусь к опыту, полученному при изучении физиологии моторной активности. Мои коллеги и я изучали нейронные механизмы, контролирующие “автоматизированные” ритмические движения, такие как дыхание, локомоция, жевание и т.п. В частности, обсуждался вопрос, как нейроны с их миллисекундными потенциалами действия контролируют движения, осуществляемые с периодами, измеряемыми секундами или сотнями миллисекунд. Работавшие с физиологами представители физико-математических наук пытались создать модели нейронных сетей, включающие сложное взаимодействие возбуждающих и тормозных нейронов, которые были бы способны генерировать такого рода ритмическую активность. Однако решение вопроса пришло с другой стороны. Многими исследователями на беспозвоночных и позвоночных животных было показано, что работа центральных механизмов, генерирующих основную картину моторного выхода, лежащего в основе ритмических движений (central pattern generators), базируется главным образом на эндогенной, пейсмекерной активности специфических групп “медленных” интернейронов. Будучи изолированными от всех синаптических входов, эти интернейроны генерируют продолжительные разряды импульсов или медленные потенциалы с частотой, характерной для данного движения. Таким образом, для управления медленными (по сравнению с миллисекундными потенциалами действия нейронов) движениями в процессе естественного отбора возникли медленные нейроны, работающие в тех же временных интервалах, что и контролируемые ими движения. Что касается взаимодействия между нейронами в пределах нервных центров или сетей, управляющими ритмическими движениями, его основная функция состоит не в генерации ритма, а в формировании моторного выхода. Благодаря межнейронному взаимодействию осуществляется одновременное возбуждение синергичных нейронов, реципрокное возбуждение нейронов-антагонистов, последовательное вовлечение в активность нейронов, работающих в разные фазы движения и т.п. (Аршавский и др. 2015; Arshavsky, 2003).
Не следует ли, исходя из этого опыта, предположить, что скорость когнитивных функций обусловлена тем, что они осуществляются не на уровне нейронных сетей, а в результате быстрых внутринейрональных процессов, механизм которых в настоящее время неизвестен. Что касается межнейронного взаимодействия, оно обеспечивает кооперативную работу нейронов, выполняющих элементарные, специализированные когнитивные функции. Иначе говоря, предполагается, что для осуществления быстрых функций необходимы “быстрые” нейроны и что механизм высокой скорости работы нейронов следует искать на внутриклеточном уровне, поскольку, как хорошо известно, на экстра-клеточном уровне нейроны работают медленно. В этой связи я хочу опять вернуться к описанным выше концептуальным клеткам. По моему убеждению, все значение открытия концептуальных нейронов – носителей памяти об отдельных людях – не было достаточно оценено в литературе. Как уже говорилось, поскольку эти нейроны были зарегистрированы у больных, страдающих от частых эпилептических припадков, берущих начало в медиальной височной доле, мы должны заключить, что эта память формируется и хранится не на уровне межнейронных связей, а на внутриклеточном уровне. Однако концептуальные клетки являются не просто memory engram neurons. Память клетки о данном человеке не зависит от выражения его лица, одежды и других деталей, она включает не только внешний облик данного человека, но и его имя. Следовательно, на внутриклеточном уровне осуществляется не только формирование памяти, но и такая когнитивная функция, как формирование обобщенного (абстрактного) образа данной личности.
Открытие концептуальных нейронов было одной из причин, почему математики Д.Ю. и Ю.И. Манины пришли к заключению, что мозг правильнее сравнивать не с компьютером, а с интернетом (the World Wide Web), где роль компьютеров играют специализированные нейроны или небольшие группы нейронов типа концептуальных клеток (Manin, Manin, 2017). Близкую идею, что мозг правильнее сравнивать с интернетом, высказали позднее биологи J.J. Langille и C.R. Gallistel (2020). Можно назвать ряд теоретических работ, авторы которых пытаются обосновать гипотезу, что механизм когнитивных функций, включая механизм сознания, базируется на квантовых процессах, происходящих внутри клеток мозга (Либерман и др. 1987; Bruza, Busemeyer, 2012; Gunji 2016; Hameroff et al., 2002; Hameroff, Penrose, 2014; Igamberdiev, Shklovskiy-Kordi, 2017; Korf, 2015; Liberman et al., 1989). Иными словами, эти авторы предполагают, что нейроны, вовлеченные в осуществление когнитивных функций, можно уподобить быстродействующим квантовым компьютерам.
Из всего сказанного в этом разделе следует, что понимание истинных механизмов памяти может радикально изменить всю физиологию высшей нервной деятельности. В настоящей статье я пытался показать, что дальнейший прогресс в этой области требует полного отказа от существующей априорной аксиоматики и обращения в сторону внутриклеточной физиологии памяти.
Список литературы
Анохин К.В. Молекулярные сценарии консолидации долговременной памяти. Журнал высшей нервной деятельности им. И.П. Павлова. 1997. 47: 261 –279.
Аршавский Ю.И. И.М. Гельфанд о математике и нейрофизиологии. Вестник РАН. 2010. 80 (10): 937–941.
Аршавский Ю.И. Нейронные механизмы памяти: синаптическая и геномная гипотезы. Журнал высшей нервной деятельности им. И.П. Павлова. 2011. 61 (6): 660–674.
Аршавский Ю.И. Можно ли судить о сознании животных на основании их поведения? Журнал высшей нервной деятельности им. И.П. Павлова. 2018. 68 (2): 152–162.
Аршавский Ю.И., Делягина Т.Г., Орловский Г.Н. Центральные генераторы: механизм работы и их роль в управлении автоматизированными движениями. Журнал высшей нервной деятельности им. И.П. Павлова. 2015. 65 (2): 156–187.
Бородинова А.А., Балабан П.М. Эпигенетическая регуляция как основа долговременных изменений в нервной системе: В поисках механизмов специфичности. Биохимия. 2020. 85 (9): 1139–1158.
Либерман Е.А., Минина С.В., Шкловский-Корди Н.Е. Мозг как система квантовых компьютеров и путь к объединению наук. Москва: ИППИ АН СССР, 1987.
Литвин О.О., Анохин К.В. Механизмы реорганизации памяти при извлечении приобретенного поведенческого опыта у цыплят: эффекты блокады синтеза белка в мозге. Журнал высшей нервной деятельности им. И.П. Павлова. 1999. 49 (4): 554–565.
Лурия А.Р. Маленькая книжка о большой памяти. Москва: Изд. МГУ, 1968.
Муравьева Е.В., Анохин К.В. Участие синтеза белка в реконсолидации памяти в разное время после обучения условно-рефлекторному замиранию у мышей. Журнал высшей нервной деятельности им. И.П. Павлова. 2006. 56 (2): 274–281.
Скребицкий В.Г., Чепкова А.Н. Синаптическая пластичность в аспекте обучения и памяти. Успехи физиол. наук. 1999. 30: 3–13.
Abel T., Nguyen P.V., Barad M., Deuel T.A., Kandel E.R., Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997. 88 (5): 615–626.
Abraham W.C., Williams J.M. Properties and mechanisms of LTP maintenance. Neuroscientist. 2003. 9 (6): 463–474.
Acker D., Paradis S., Miller P. Stable memory and computation in randomly rewiring neural networks. J. Neurophysiol. 2019. 122 (1): 66–80.
Alarcón G., Martinez J., Kerai S.V., Lacruz M.E., Quiroga R.Q., Selway R.P., Richardson M.P., García Seoane J.J., Valentín A. In vivo neuronal firing patterns during human epileptiform discharges replicated by electrical stimulation. Clin. Neurophysiol. 2012. 123 (9):1736–1744.
Albo Z., Gräff J. The mysteries of remote memory. Philos. Trans. R. Soc. Lond. Biol. Sci. 2018. 373 (1742): 20170029.
Alvarado-Rojas C., Lehongre K., Bagdasaryan J., Bragin A., Staba R., Engel J.Jr., Navarro V., Le Van Quyen M. Single-unit activities during epileptic discharges in the human hippocampal formation. Front. Comput. Neurosci. 2013 7: 140.
Anokhin K., Litvin O., Radyushkin K. Memory retranscription at the time of retrieval: a clue to dynamic nature of memory. In: Memory and Emotions. Ed. P. Calabrese, A. Neugebauer, World Scientific Publ, New Jersey, 2002. 45–61.
Arshavsky Y.I. Cellular and network properties in the functioning of the nervous system: from central pattern generators to cognition. Brain Res. Rev. 2003. 41 (2-3): 229–267.
Arshavsky Y.I. “The seven sins” of the Hebbian synapse: can the hypothesis of synaptic plasticity explain long-term memory consolidation? Prog. Neurobiol. 2006. 80: 99–113.
Arshavsky Y.I. Neurons versus networks: The interplay between individual neurons and neural networks in cognitive functions. Neuroscientist. 2017. 23 (4): 341–355.
Asok A., Leroy F., Rayman J.B., Kandel E.R. Molecular mechanisms of the memory trace. Trends Neurosci. 2019. 42 (1): 14–22.
Attardo A., Fitzgerald J.E., Schnitzer M.J. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature. 2015. 523: 592–596.
Aur D., Jog M.S. Neuroelectrodynamics, Understanding the Brain Language. Amsterdam: IOS Press. 2010.
Bailey C.H. Structural changes and the storage of long-term memory in Aplysia. Can. J. Physiol. Pharmacol. 1999. 77: 738–747.
Bailey C.H., Kandel E.R., Harris K.M. Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb. Perspect. Biol. 2015. 7 (7): a021758.
Bannerman D.M., Good M.A., Butcher S.P., Ramsay M., Morris R.G. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995. 378: 182–186.
Bannerman D.M., Sprengel R., Sanderson D.J., McHugh S.B., Rawlins J.N., Monyer H., Seeburg P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014. 15 (3): 181–192.
Ben-Ari Y., Represa A. Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends Neurosci. 1990. 13 (8): 312–318.
Bliss T.V., Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. (Lond.) 1973. 232: 331–356.
Brakebusch C., Seidenbecher C.I., Asztely F., Rauch U., Matthies H., Meyer H., Krug M., Böckers T.M., Zhou X., Kreutz M.R., Montag D., Gundelfinger E.D., Fässler R. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol. Cell Biol. 2002. 22 (21): 7417–7427.
Bramham C.R. Local protein synthesis, actin dynamics, and LTP consolidation. Curr. Opin. Neurobiol. 2008. 18 (5): 524–531.
Bruza P.D., Busemeyer J.R. Quantum models of cognition and decision. Cambridge, Engl.: Cambridge University Press. 2012.
Burns A.M., Gräff J. Cognitive epigenetic priming: leveraging histone acetylation for memory amelioration. Curr. Opin. Neurobiol. 2021. 67: 75–84.
Campbell R.R., Wood M.A. How the epigenome integrates information and reshapes the synapse. Nat. Rev. Neurosci. 2019. 20 (3): 133–147.
Chen C., Tonegawa S. Molecular genetic analysis of synaptic plasticity, activity-dependent neural development, learning, and memory in the mammalian brain. Annu. Rev. Neurosci. 1997. 20: 157–184.
Chklovskii D.B., Mel B.W., Svoboda K. Cortical rewiring and information storage. Nature. 2004. 431 (7010): 782–788.
Choquet D., Triller A. The dynamic synapse. Neuron. 2013. 80 (3): 691–703.
Clandinin T.R., Marder E. Editorial overview: Microcircuit evolution and computation. 2016. Curr. Opin, Neurobiol. 2016. 41: 188–190.
Collingridge G.L., Peineau S., Howland J.G., Wang Y.T. Long-term depression in the CNS. Nat. Rev. Neurosci. 2010. 11 (7): 459–473.
Craig E., Dillingham C.M., Milczarek M.M., Phillips H.M., Davies M., Perry J.C., Vann S.D. Lack of change in CA1 dendritic spine density or clustering in rats following training on a radial-arm maze task. Wellcome Open Res. 2020. 5: 68.
Crick F. Memory and molecular turnover. Nature. 1984. 312: 101.
Davis H.P., Squire L.R. Protein synthesis and memory: a review. Psychol. Bull. 1984. 96: 518–559.
Dossani R.H., Missios S., Nanda A. The legacy of Henry Molaison (1926–2008) and the impact of his bilateral mesial temporal lobe surgery on the study of human memory. World Neurosurg. 2015. 84 (4): 1127–1135.
Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000. 1 (1): 41–50.
Engert F., Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999. 399: 66–70.
Evans B.D., Stringer S.M. Transformation-invariant visual representations in self-organizing spiking neural networks. Front. Comput. Neurosci. 2012. 6: 46.
Fauth M.J., van Rossum M.C. Self-organized reactivation maintains and reinforces memories despite synaptic turnover. Elife. 2019. 8: e43717.
Fifkova E., Anderson C.L. Stimulation-induced changes in dimensions of stalks of dendritic spines in the dentate molecular layer. Exp. Neurol. 1981. 74: 621–627.
Fifkova E., Van Harreveld A. Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. J. Neurocytol. 1977. 6: 211–230.
Frankland P.W., Bontempi B. The organization of recent and remote memories. Nat. Rev. Neurosci. 2005. 6 (2): 119–130.
Gallistel C.R. The neurobiological bases for the computational theory of mind. In: On concepts, modules, and language. Eds. De Almeida R.G., Gleitman L. New York: Oxford University Press, 2017. 275–296.
Gallistel C.R., Matzel L.D. The neuroscience of learning: beyond the Hebbian synapse. Annu. Rev. Psychol. 2013. 64: 169–200.
Gelbard-Sagiv H., Mukamel R., Harel M., Malach R., Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008. 322 (5898): 96–101.
Gelfand I.M. Two archetypes in the psychology of man. Nonlinear Sci. Today. 1991. 1 (5): 11–16.
Giese K.P., Fedorov N.B., Filipkowski R.K., Silva A.J. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998. 279 (5352): 870–873.
Gräff J., Mansuy I.M. Epigenetic codes in cognition and behaviour. Behav. Brain Res. 2008. 192 (1): 70–87.
Grant S.G. Synapse molecular complexity and the plasticity behaviour problem. Brain Neurosci. Adv. 2018. 2: 1–7.
Grutzendler J., Kasthuri N., Gan W.B. Long-term dendritic spine stability in the adult cortex. Nature. 2002. 420 (6917): 812–816.
Gu L., Kleiber S., Schmid L., Nebeling F., Chamoun M., Steffen J., Wagner J., Fuhrmann M. Long-term in vivo imaging of dendritic spines in the hippocampus reveals structural plasticity. J. Neurosci. 2014. 34 (42): 13948–13953.
Gunji Y.P., Sonoda K., Basios V. Quantum cognition based on an ambiguous representation derived from a rough set approximation. Biosystems. 2016. 141: 55–66.
Hameroff S., Nip A., Porter M., Tuszynski J. Conduction pathways in microtubules, biological quantum computation, and consciousness. Biosystems. 2002. 64 (1–3): 149–168.
Hameroff S., Penrose R. Consciousness in the universe: a review of the 'Orch OR’ theory. Phys. Life Rev. 2014. 11 (1): 39–78.
Hebb D.O. The Organization of Behavior. New York: Wiley, 1949.
Hernandez P.J., Abel T. The role of protein synthesis in memory consolidation: progress amid decades of debate. Neurobiol. Learn. Mem. 2008. 89 (3): 293–311.
Holliday R. Is there an epigenetic component in long-term memory? J. Theor. Biol. 1999. 200: 339–341.
Holtmaat A., Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009. 10 (9): 647–658.
Holtmaat A.J., Trachtenberg J.T., Wilbrecht L., Shepherd G.M., Zhang X., Knott G.W., Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005. 45 (2): 279–291.
Huys Q.J.M., Browning M., Paulus M., Frank M.J. Advances in the computational understanding of mental illness. Neuropsychopharmacology. 2020. 46: (in press).
Igamberdiev A.U., Shklovskiy-Kordi N.E. The quantum basis of spatiotemporality in perception and consciousness. Prog. Biophys. Mol. Biol. 2017. 130 (Pt A): 15–25.
Judge M.E., Quartermain D. Characteristics of retrograde amnesia following reactivation of memory in mice. Physiol. Behav. 1982. 28: 585–590.
Kandel E.R., Dudai Y., Mayford M.R. The molecular and systems biology of memory. Cell. 2014. 157 (1): 163–186.
Korf J. Quantum and multidimensional explanations in a neurobiological context of mind. Neuroscientist. 2015. 21 (4): 345–355.
Lai C.S., Franke T.F., Gan W.B. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012. 483 (7387): 87–91.
Lamprecht R., LeDoux J. Structural plasticity and memory. Nat. Rev. Neurosci. 2004. 5 (1): 45–54.
Langille J.J., Gallistel C.R. Locating the engram: Should we look for plastic synapses or information-storing molecules? Neurobiol. Learn. Mem. 2020. 169: 107164.
Leuner B., Falduto J., Shors T.J. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 2003. 23: 659–665.
Levenson J.M., Sweatt J.D. Epigenetic mechanisms in memory formation. Nat. Rev. Neurosci. 2005. 6: 108–118.
Li X., Wei W., Ratnu V.S., Bredy T.W. On the potential role of active DNA demethylation in establishing epigenetic states associated with neural plasticity and memory. Neurobiol. Learn. Mem. 2013. 105:125–132.
Liberman E.A., Minina S.V., Shklovsky-Kordi N.E. Quantum molecular computer model of the neuron and a pathway to the union of the sciences. Biosystems. 1989. 22 (2): 135–154.
Loewenstein Y., Yanover U., Rumpel S. Predicting the dynamics of network connectivity in the neocortex. J. Neurosci. 2015. 35 (36): 12535–12544.
Lopantsev V., Both M., Draguhn A. Rapid plasticity at inhibitory and excitatory synapses in the hippocampus induced by ictal epileptiform discharges. Eur. J. Neurosci. 2009. 29 (6): 1153–1164.
Lynch M.A. Long-term potentiation and memory. Physiol. Rev. 2004. 84 (1): 87–136.
Malenka R.C., Nicoll R.A. Long-term potentiation–a decade of progress? Science. 1999 285 (5435): 1870–1874.
Manin D.Yu., Manin Yu.I. Cognitive networks: brains, internet, and civilizations. In: Humanizing Mathematics and its Philosophy. Ed. Sriraman B. Berlin: Springer, 2017. 85–96.
Martin S.J., Grimwood P.D., Morris R.G. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 2000. 23: 649–711.
Mayford M., Siegelbaum S.A., Kandel E.R. Synapses and memory storage. Cold Spring Harb. Perspect. Biol. 2012. 4 (6): a005751.
McGaugh J.L. Memory – a century of consolidation. Science 2000. 287: 248–251.
Migaud M., Charlesworth P., Dempster M., Webster L.C., Watabe A.M., Makhinson M., He Y., Ramsay M.F., Morris R.G., Morrison J.H., O’Dell T.J., Grant S.G. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998. 396 (6710): 433–439.
Miller C.A., Gavin C.F., White J.A. Cortical DNA methylation maintains remote memory. Nat. Neurosci. 2010. 13: 664–666.
Mongillo G., Rumpel S., Loewenstein Y. Intrinsic volatility of synaptic connections – a challenge to the synaptic trace theory of memory. Curr. Opin. Neurobiol. 2017. 46: 7–13.
Montkowski A., Holsboer F. Intact spatial learning and memory in transgenic mice with reduced BDNF. Neuroreport. 1997. 8 (3): 779–782.
Morris R.G., Anderson E., Lynch G.S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986. 319 (6056): 774–776.
Morris R.G., Steele R.J., Bell J.E., Martin S.J. N-methyl-d-aspartate receptors, learning and memory: chronic intraventricular infusion of the NMDA receptor antagonist d-AP5 interacts directly with the neural mechanisms of spatial learning. Eur. J. Neurosci. 2013. 37 (5): 700–717.
Nader K., Schafe G.E., Le Doux J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000. 406: 722–726.
Nagaoka A., Takehara H., Hayashi-Takagi A., Noguchi J., Ishii K., Shirai F., Yagishita S., Akagi T., Ichiki T., Kasai H. Abnormal intrinsic dynamics of dendritic spines in a fragile X syndrome mouse model in vivo. Sci. Rep. 2016. 6: 26651.
Neves G., Cooke S.F., Bliss T.V. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat. Rev. Neurosci. 2008. 9 (1): 65–75.
Nicoll R.A. A brief history of long-term potentiation. Neuron. 2017. 93 (2): 281–290.
Nithianantharajah J., Komiyama N.H., McKechanie A., Johnstone M., Blackwood D.H., St Clair D., Emes R.D., van de Lagemaat L.N., Saksida L.M., Bussey T.J., Grant S.G. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat. Neurosci. 2013. 16 (1): 16–24.
Noguchi J., Nagaoka A., Watanabe S., Ellis-Davies G.C., Kitamura K., Kano M., Matsuzaki M., Kasai H. In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J. Physiol. 2011. 589 (10): 2447–2457.
Okabe S., Collin C., Auerbach J.M., Meiri N., Bengzon J., Kennedy M.B., Segal M., McKay R.D. Hippocampal synaptic plasticity in mice overexpressing an embryonic subunit of the NMDA receptor. J. Neurosci. 1998. 18 (11): 4177–4188.
Parker E.S., Cahill L., McGaugh J.L. A case of unusual autobiographical remembering. Neurocase. 2006. 12: 35–49.
Peña de Ortiz S., Arshavsky Y.I. DNA recombination as a possible mechanism in declarative memory: a hypothesis. J. Neurosci. Res. 2001. 63: 72–81.
Pfeiffer T., Poll S., Bancelin S., Angibaud J., Inavalli V.K., Keppler K., Mittag M., Fuhrmann M., Nägerl U.V. Chronic 2P-STED imaging reveals high turnover of dendritic spines in the hippocampus in vivo. Elife. 2018. 7: e34700.
Poo M.M., Pignatelli M., Ryan T.J., Tonegawa S., Bonhoeffer T., Martin K.C., Rudenko A., Tsai L.H., Tsien R.W., Fishell G., Mullins C., Gonçalves J.T., Shtrahman M., Johnston S.T., Gage F.H., Dan Y., Long J., Buzsáki G., Stevens C. What is memory? The present state of the engram. BMC Biol. 2016. 14: 40.
Queenan B.N., Ryan T.J., Gazzaniga M.S., Gallistel C.R. On the research of time past: the hunt for the substrate of memory. Ann. N. Y. Acad. Sci. 2017. 396: 108–125.
Quiroga R.Q. Concept cells: the building blocks of declarative memory functions. Nat. Rev. Neurosci. 2012. 13 (8): 587–597.
Quiroga R.Q., Fried I., Koch C. Brain cells for grandmother. 2013. Sci. Am. 308 (2): 30–35.
Quiroga R.Q., Kraskov A., Koch C., Fried I. Explicit encoding of multimodal percepts by single neurons in the human brain. Curr. Biol. 2009. 19 (15): 1308–1313.
Rampon C., Tang Y.P., Goodhouse J., Shimizu E., Kyin M., Tsien J.Z. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat. Neurosci. 2000. 3 (3): 238–244.
Rayman J.B., Kandel E.R. Functional prions in the brain. Cold Spring Harb. Perspect. Biol. 2017. 9 (1): a023671.
Reber T.P., Bausch M., Mackay S., Boström J., Elger C.E., Mormann F. Representation of abstract semantic knowledge in populations of human single neurons in the medial temporal lobe. PLoS Biol. 2019. 17 (6): e3000290.
Restivo L., Vetere G., Bontempi B., Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J. Neurosci. 2009. 29 (25): 8206–8214.
Rey H.G., Gori B., Chaure F.J., Collavini S., Blenkmann A.O., Seoane P., Seoane E., Kochen S., Quian Quiroga R. Single neuron coding of identity in the human hippocampal formation. Curr. Biol. 2020. 30 (6): 1152–1159.
Rolls E.T. Cerebral Cortex: Principles of Operation. Oxford: Oxford University Press, 2016.
Sanders J., Cowansage K., Baumgärtel K., Mayford M. Elimination of dendritic spines with long-term memory is specific to active circuits. J. Neurosci. 2012. 32 (36): 12570–12578.
Sara S.J. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn. Mem. 2000. 7: 73–84.
Schafe G.E., Nader K., Blair H.T., LeDoux J.E. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001. 24: 540–546.
Scoville W.B., Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiat. 1957. 20: 11–21.
Si K., Kandel E.R. The role of functional prion-like proteins in the persistence of memory. Cold Spring Harb Perspect Biol. 2016. 8 (4): a021774.
Silva A.J. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J. Neurobiol. 2003. 54 (1): 224–237.
Squire L.R. The legacy of patient H.M. for neuroscience. Neuron. 2009. 61 (1): 6–9.
Squire L.R., Genzel L., Wixted J.T., Morris R.G. Memory consolidation. Cold Spring Harb Perspect Biol. 2015. 7 (8): a021766.
Steinmetz P.N., Cabrales E., Wilson M.S., Baker C.P., Thorp C.K., Smith K.A., Treiman D.M. Neurons in the human hippocampus and amygdala respond to both low- and high-level image properties. J. Neurophysiol. 2011. 105 (6): 2874–2884.
Steward O., Schuman E.M. Protein synthesis at synaptic sites on dendrites. Annu. Rev. Neurosci. 2001. 24: 299–325.
Suratkal S.S., Yen Y.H., Nishiyama J. Imaging dendritic spines: molecular organization and signaling for plasticity. Curr Opin Neurobiol. 2021. 67: 66–74.
Suthana N.A., Parikshak N.N., Ekstrom A.D., Ison M.J., Knowlton B.J., Bookheimer S.Y., Fried I. Specific responses of human hippocampal neurons are associated with better memory. Proc. Natl. Acad. Sci. U. S. A. 2015. 112 (33): 10503–10508.
Tang Y.P., Shimizu E., Dube G.R., Rampon C., Kerchner G.A., Zhuo M., Liu G., Tsien J.Z. Genetic enhancement of learning and memory in mice. Nature. 1999. 401 (6748): 63–69.
Tonegawa S., Liu X., Ramirez S., Redondo R. Memory engram cells have come of age. Neuron. 2015. 87 (5): 918–931.
Toni N., Buchs P.A., Nikonenko I., Bron C.R., Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999. 402: 421–425.
Trachtenberg J.T., Chen B.E., Knott G.W., Feng G., Sanes J.R., Welker E., Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002. 420 (6917): 788–794.
Treffert D.A., Christensen D.D. Inside the mind of a savant. Sci. Am. 2005. 293: 108–113.
Trettenbrein P.C. The demise of the synapse as the locus of memory: A looming paradigm shift. Front. Syst. Neurosci. 2016. 10: 88.
Tsien J.Z. Linking Hebb’s coincidence-detection to memory formation. Curr, Opin. Neurobiol. 2000. 10 (2): 266–273.
Tsien J.Z., Huerta P.T., Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996. 87 (7): 1327–1338.
Van Harreveld A., Fifkova E. Swelling of dendritic spines in the fascia dentata after stimulation of the perforant fibers as a mechanism of post-tetanic potentiation. Exp. Neurol. 1975. 49: 736–749.
Wang H., Hu Y., Tsien J.Z. Molecular and systems mechanisms of memory consolidation and storage. Prog. Neurobiol. 2006. 79 (3): 123–135.
Ziv N.E., Brenner N. Synaptic tenacity or lack thereof: Spontaneous remodeling of synapses. Trends Neurosci. 2018. 41 (2): 89–99.
Zovkic I.B. Epigenetics and memory: an expanded role for chromatin dynamics. Curr Opin Neurobiol. 2021. 67: 58–65.
Zovkic I.B., Guzman-Karlsson M.C., Sweatt J.D. Epigenetic regulation of memory formation and maintenance. Learn. Mem. 2013. 20 (2): 61–74.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


