Журнал высшей нервной деятельности им. И.П. Павлова, 2020, T. 70, № 4, стр. 473-487
THE EFFECT OF CHRONIC ADMINISTRATION OF BUSPIRONE, 8-OHDPAT AND DIFFERENT DOSES OF FLUOXETINE ON HALOPERIDOL INDUCED EXTRAPYRAMIDAL DISORDERS AND GENERAL LOCOMOTOR ACTIVITY IN MALE RATS
Mohammadmahdi Sabahi 1, Sara Ami Ahmadi 1, Rasool Haddadi 2, *, **
1 Neurosurgery Research Group (NRG), Student Research Committee, Hamadan University of Medical Sciences
Hamadan, Iran
2 Department of Pharmacology and Toxicology, Medicinal plant and natural products Research Center, School of Pharmacy, Hamadan University of Medical Sciences
Hamadan, Iran
* E-mail: haddadi.rasool@gmail.com
** E-mail: haddadi.r@umsha.ac.ir
Поступила в редакцию 28.03.2019
После доработки 1.12.2019
Принята к публикации 16.12.2019
Аннотация
Buspirone is a partial agonist of 5HT1A receptors, 8-OHDPAT (8-Hydroxy-2-(dipropylamino) tetralin) is an agonist of 5HT1A receptors, and fluoxetine is a serotonin uptake inhibitor. Among 5-HT receptor subtypes, 5-HT1A receptor plays a crucial role in modulating extrapyramidal motor disorders, including antipsychotic-induced extrapyramidal symptoms. Aim. This study aimed to investigate the effect of chronic administration of buspirone, 8-OHDPAT and different doses of fluoxetine on haloperidol-induced extrapyramidal disorders and general locomotor activity in male rats. Methods. Extrapyramidal disorders were induced by haloperidol injection 1mg/kg intraperitoneally (i.p.). To investigate the effect of serotonergic drugs on haloperidol-induced extrapyramidal disorders, we used 8-OHDPAT (1 mg/Kg), buspirone (10 mg/Kg), and fluoxetine (0.5 mg/Kg and 1 mg/Kg) as a chronic injection. Following this, the different groups of rats were placed in a rotarod, bar test and open field test apparatus, where their behavior and locomotor activity during these tasks were recorded respectively. Findings. Data analysis showed that i.p. injection of fluoxetine (0.5 mg/Kg and 1 mg/Kg), buspirone (10 mg/kg) and 8-OHDPAT (1 mg/Kg) decreased catalepsy compared with the control group (p < 0.001). The attenuation of haloperidol-induced motor imbalance was observed with fluoxetine (0.5 mg/Kg) just in 15th day of treatment (p < 0.001), fluoxetine (1 mg/Kg) in all days (p < 0.001), 8-OHDPAT (1mg/Kg) in all days (p < 0.05) and buspirone (10 mg/kg) just in 10th and 15th day of treatment (p < 0.001). Conclusion. It may be concluded that fluoxetine, buspirone, and 8-OHDPAT improve extrapyramidal disorders and locomotor impairment in haloperidol-induced Parkinsonism model through activation of nigral 5HT1A receptors.
INTRODUCTION
Psychosis disorders are situations where the mind loses its relevance to reality and experiences in appropriate perceptions of its surroundings. The most common symptoms in these patients are hallucinations and delusions [van Os et al., 2001; Perälä et al., 2007]. Neuroleptic drugs are used to treat people with psychotic disorders, and one of the most commonly used neuroleptic drugs is haloperidol [Wade et al., 2017]. Continuous and long-term consumption of neuroleptic drugs is essential for controlling symptoms and preventing recurrence of the disease. However, use of these drugs has several side effects, including extrapyramidal disorders [Association, 2006; ÜÇOk, Gaebel, 2008]. All of the neuroleptic drugs inhibit the normal signaling of dopamine D2 receptors in the brain and thus exert their effects by blocking dopaminergic receptors on the Nigro striatal pathway, these drugs can lead to extrapyramidal motor disorders [Farde et al., 1992; Shireen, 2016; Moore, Furberg, 2017].
Central serotonergic and dopaminergic systems play a critical role in the regulation of normal and abnormal behaviors. Moreover, recent evidence suggests that the dysfunction of dopamine (DA) and serotonin (5-hydroxytriptamine, 5-HT) neurotransmission might underlie the pathophysiology of neuropsychiatric disorders, including depression, schizophrenia, drug abuse and Parkinson’s disease. 5-HT pathways from the raphe nuclei have been shown to make contact with dopaminergic neurons in the nigrostriatal system and modulate, in a complex manner, dopaminergic activity. Numerous studies have suggested that the functions of 5-HT and dopamine are closely related and that they may act in a cooperative manner on striatal functions [Sandyk, Fisher, 1988; Esposito et al., 2008].
Dorsal raphe nucleus (DRN) is known as a serotonin-secreting source to the basal ganglia, which is the action site of the neuroleptic drugs [Pires et al., 1990]. Serotonin has a role of homeostatic control in the regulation of neurological complications caused by neuroleptic drugs in the cerebrospinal axis (neuroaxis) and it can regulate motor activity by affecting the globus pallidus neurons [Rao et al., 1989]. Bilateral serotonin injection effectively weakens the haloperidol-induced catalepsy [Wei, Chen, 2009]. Among the various types of serotonergic receptors, 5HT1 receptors play a vital role in modulating the effects of extrapyramidal symptoms, especially the side effects of neuroleptic drugs [Meltzer et al., 2003; Ohno et al., 2011; Shimizu et al., 2013]. On the other hand, 5HT1A receptors are widely distributed throughout the basal ganglia, which results in their inhibitory effect on releasing of dopamine and play an important role in the neuroleptic drugs induced catalepsy [Hicks, 1990; Zubkov et al., 2009]. The agonists of these receptors reduce serotonergic activity, which can inhibit the dopaminergic pathway of nigro striatal neurons, which results in an increase in the amount of dopamine in the dorsal striatal region, which can be incompletely overcome blocking of D2 receptors [Bantick et al., 2005].
It should be noted that dorsal striatal is considered as the most important brain structure responsible for extrapyramidal disorders caused by neuroleptic drugs [Dunstan et al., 1981; Ossowska et al., 1990].
In this study, we sought to find a way to reduce extrapyramidal disorders in male Wistar rats caused by long-term use of neuroleptic drugs and for this purpose, we employed serotonergic drugs such as 8-OHDPAT and Buspirone as an agonist and partial agonist of 5HT1A receptors respectively, and fluoxetine as a serotonin reuptake inhibitor.
METHODS
2.1. Animals
In this investigation, we used naïve experimental male Wistar rats were obtained from Hamadan University of medical sciences institutional animal facilities. Each group consisted of 6 rats, all in the weight range of 200–230 grams. To maintain each group, a standard plastic container was used. All rats had enough access to water and food, and 12 h: 12 h light–dark cycle was observed. Animals were kept in a quiet ambiance with proper ventilation and temperature range of 22 ± 2°C. Compliance with laboratory conditions was made one hour before each test [Haddadi et al., 2018]. Extensive efforts were made to minimize animal harassment and to select at least enough rats to conduct the test. All procedures used in the present study were done in accordance with the National Institutes of Health ethical guidelines for the Care and Use of Laboratory Animals and approved by the University of Medical Sciences of Hamadan (UMSHA) Ethical Commttee, Hamadan, Iran (ID: IR.UMSHA.REC. 1395.417).
2.2. Chemicals
Haloperidol (4-[4-(4-chlorophenyl)-4-hydroxypiperidinol]-4-fluorobutyrophenone), 8-OHDPAT (8-Hydroxy-2-(dipropylamino)tetralin), buspirone and fluoxetine were obtained from Sigma–Aldrich (St. Louis, MO, USA).
Haloperidol, 8-OHDPAT, and fluoxetine dissolved in normal saline 0.9% and buspirone were solved in polyethylene glycol.
All drugs solutions were prepared freshly on the days of experimentation and were injected intra peritoneally (i.p.).
2.3. Experimental design
The rats were randomly divided into 7 groups (n = 6) (See Fig. 1. for more details):
Fig. 1.
Schematic representation of the experimental procedure.
Рис. 1. Схематическое изображение экспериментальной процедуры.
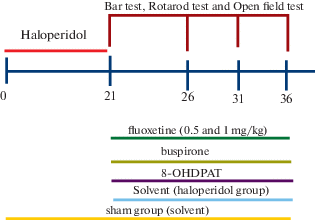
1. Normal group (healthy intact animals).
2. Sham group (receiving a solvent for 21 days).
3. Haloperidol receiving group (receiving haloperidol intraperitoneally at a dose of 1 mg/kg [Ahmadi et al., 2018] for 21 days).
4. Fluoxetine receiving group (receiving fluoxetine intraperitoneally at a dose of 1 mg/kg in healthy animals for 15 days after 21 days injection of haloperidol).
5. Fluoxetine receiving group (receiving fluoxetine intraperitoneally at a dose of 0.5 mg/kg in healthy animals for 15 days after 21 days injection of haloperidol).
6. Buspirone receiving group (receiving buspirone intraperitoneally at a dose of 1 mg/kg in healthy animals for 15 days after 21 days injection of haloperidol).
7. 8-OHDPAT receiving group (receiving 8-OHDPAT intraperitoneally at a dose of 1 mg/kg in healthy animals for 15 days after 21 days injection of haloperidol).
Schematic representation of the experimental procedure shown in Fig. 1.
2.4. Induction of Extrapyramidal Disorders
Extrapyramidal disorders were induced by administering the neuroleptic haloperidol drug (1 mg/kg intraperitoneally) [Sabahi et al., 2018] (Fig. 1).
2.5. Behavioral study
2.5.1. Assessment of catalepsy. Catalepsy was measured by bar test. Bar test is a device made up of a wooden rod 9 cm in diameter, located 9 cm above the ground. In this test, both forelimbs are placed on the rod so that the foot of the animal is completely on the ground and the animal is in a state of rest. The time that the animal stays in stagnation is calculated as the catalepsy time. The end of the test is when the animal pulls one or both of its forelimbs off the rod or shakes its head in search mode. The cut-off time was considered 720 seconds [Haddadi et al., 2018]. Before the experiment, the animals were trained to be placed in the bar test. Catalepsy was evaluated in animals after 21 days of administration of haloperidol and on days 5, 10 and 15 after starting treatment (Fig. 1).
2.5.2. Assessment of motor balance. Standard rotarod test was conducted to measure motor coordination and motor imbalance. The apparatus has a horizontal metal rod (6 cm in diameter) attached to a modifiable speed motor. In this study, the device was set to speed of 18 rpm. The rod is divided into 4 equal parts by separating plates of 10.5 cm in diameter. To avoid jumping animals, the rod is located at the height of 50 cm from the ground, which is low enough not to injure the animal. Before the initiation of the test, the animals were trained to be placed on the rotarod. At the end of the training, they could maintain their balance at constant speed of 18 rpm for 300 seconds without falling off the device [Haddadi et al., 2015]. Training procedure was done 24 h before the experiments. Motor coordination and motor imbalance of animals was evaluated similarly to catalepsy after 21 days of administration of haloperidol and also on days 5, 10 and 15 after initiation of treatment (Fig. 1).
2.5.3. Assessment of anxiety and general locomotor activity levels. The open-field test was used to assay the general locomotor activity levels. Open-Field is a square-shaped area of 70 cm × 70 cm. To prevent animal escape, this space is surrounded by walls at the height of 35 cm. The area inside this square is divided into 16 sub squares. Different colors have been used to differentiate the central square from others. The test is started by placing the rat in the central square and the animal’s behavior was recorded for 5 minutes. After 5 minutes, remove the rat from the area and carefully clean the machine to test the next rat. In reviewing the animal’s behavior, which is made by a blind observer to the potential effects of injections, crossing and rearing were recorded. This test was performed after 21 days of administration of haloperidol and also on the 15th day of treatment (Fig. 1).
The entire process was accomplished between 9:00 AM and 3:00 PM in a silent circumstance by an observer who was blind to treatments process.
2.5.4. Statistical analysis. Descriptive analysis and comparison of differences between each data set were calculated by use of SPSS-18 software. The data were expressed as mean ± SEM and were analyzed by one-way repeated measures ANOVA in each experiment. In the case of significant variation (p < 0.05), the values were compared by post-hoc Tukey test. Statistical significance was accepted at the level of p < 0.05.
RESULTS
3.1. The Effect of Haloperidol on catalepsy
The results indicated that haloperidol (1mg/kg, i.p.) as a neuroleptic drug was able to induce catalepsy significantly (p ≤ 0.001, df: 11.71) in comparison with sham and normal groups. So that, the duration of standing on the bar was increased in a significant manner (Fig. 2 (a)). Details of the data are listed in Table 1.
Fig. 2.
(a) Effect of haloperidol administration (1mg/kg) on catalepsy. Each bar represents the Mean ± SEM of elapsed time (s) in bar test. Tests have been repeated 4 times with 1 hour interval for approving of repeatability of each behavior. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following one-way ANOVA with repeated measure. †: p < 0.001. (b) Effect of chronic fluoxetine administration (0.5 mg/kg) on catalepsy, which was treated by 5, 10, 15 days injection of fluoxetine i.p. (0.5 mg/kg) after 21 days injection of haloperidol. Each bar represents the mean ± SEM of elapsed time (s) in bar test. Tests have been repeated 3 times with 1 hour interval for approving of repeatability of each behavior. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following one-way ANOVA with repeated measure. ***: p < 0.001 vs Haloperidol group. (c) Effect of chronic fluoxetine administration (1 mg/kg) on catalepsy, which was treated by 5, 10, 15 days injection of fluoxetine i.p. (1 mg/kg) after 21 days injection of haloperidol. Each bar represents the mean ± SEM of elapsed time (s) in rotarod. Tests have been repeated 3 times with 1 hour interval for approving of repeatability of each behavior. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following one-way ANOVA with repeated measure. ***: p < 0.001 vs Haloperidol group. (d) Effect of chronic buspirone administration (1 mg/kg) on catalepsy, which was treated by 5, 10, 15 days injection of buspirone i.p. (1 mg/kg) after 21 days injection of haloperidol. Each bar represents the mean ± SEM of elapsed time (s) in bar test. Tests have been repeated 3 times with 1 hour interval for approving of repeatability of each behavior. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following one-way ANOVA with repeated measure. ***: p < 0.001 vs Haloperidol group. (e) Effect of chronic 8-OHDPAT administration (1 mg/kg) on catalepsy, which was treated by 5, 10, 15 days injection of 8-OHDPAT i.p. (1 mg/kg) after 21 days injection of haloperidol. Each bar represents the mean ± SEM of elapsed time (s) in bar test. Tests have been repeated 3 times with 1 hour interval for approving of repeatability of each behavior. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following one-way ANOVA with repeated measure. ***: p < 0.001 vs Haloperidol group.
Рис. 2. (а) Влияние введения галоперидола (1 мг/кг) на каталепсию. Каждый столбец представляет среднее значение ± SEM времени. Тесты были повторены 4 раза с интервалом в 1 ч. n = 6 крыс в каждой группе. Значимость различий оценивали тестом Tukey. †: p <0.001. (b) Влияние хронического введения флуоксетина в течение 5, 10, 15 дней (0.5 мг / кг) на каталепсию, вызванную введением галоперидола в течение 21 дня. Каждый столбец представляет среднее значение ± SEM времени в тесте с перекладиной. Тесты были повторены 3 раза с 1-часовым интервалом. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. ***: р < 0.001 в сравнении с группой галоперидол. (c) Влияние хронического введения флуоксетина на каталепсию, вызванную введением галоперидола. Каждый столбец представляет среднее значение ± SEM времени (с) в ротароде. Тесты были повторены 3 раза с интервалом в 1 ч. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. ***: р < 0.001 против группы галоперидола. (d) Влияние хронического введения буспирона (1 мг/кг) на каталепсию, вызванную введением галоперидола. Каждый столбец представляет среднее значение ± SEM времени в тесте с перекладиной. Тесты были повторены 3 раза с интервалом в 1 ч. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. ***: р < 0.001 в сравнении с группой галоперидол. (e) Влияние хронического введения 8-OHDPAT (1 мг/кг) на каталепсию, вызванную введением галоперидола. Каждый столбец представляет среднее значение ± SEM времени в тесте с перекладиной. Тесты были повторены 3 раза с 1-часовым интервалом. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. ***: р < 0.001 против группы галоперидола.
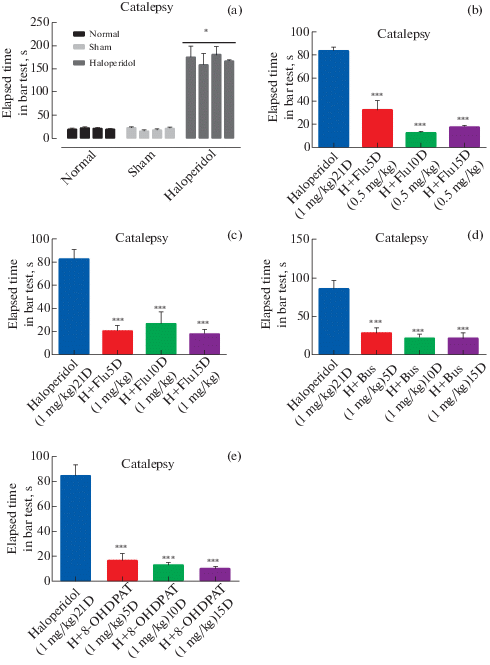
Table 1.
ANOVA Analysis Report Таблица 1. Анализ ANOVA
| Groups | Statistical Parameter | Catalepsy | Rotarod | Open field | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test1 | Test2 | Test3 | Test1 | Test2 | Test3 | Crossing | Rearing | ||||||||||||||||||
| 5D | 10D | 15D | 5D | 10D | 15D | 5D | 10D | 15D | 5D | 10D | 15D | 5D | 10D | 15D | 5D | 10D | 15D | 5D | 10D | 15D | 5D | 10D | 15D | ||
| Control Vs Haloperidol | Mean ± SEM | 13.20 ± 0.802 | 15.660 ± 0.6 | 11.960 ± 0.7 | 14.50 ± 0.82 | 13.50 ± 0.80 | 15.10 ± 0.51 | 14.96 ± 0.42 | 12.80 ± 0.1.1 | 14.2 ± 0.9 | 698 ± 26.940 | 692 ± 16.9 | 660 ± 26.940 | 702 ± 16.5 | 685 ± 26 | 705 ± 15 | 679 ± 11 | 670 ± 21 | 683 ± 28 | 27.33 ± 4.5400 | 26.53 ± 1.5 | 27.7 ± 2.4 | 11.960 ± 0.5 | 12.50 ± 0.7 | 10.90 ± 0.4 |
| P value | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P > 0.05 | P > 0.05 | P > 0.05 | P < 0.05 | P < 0.05 | P < 0.05 | |
| F | 22. 89 | 21.10 | 23. 45 | 21.818 | 22.186 | 21.089 | 22.103 | 19.246 | 21.118 | 24.786 | 20.089 | 18.103 | 25.246 | 21.118 | 25.786 | 20.089 | 19.103 | 21.246 | 1.12 | 0.9 | 1.05 | 3.5 | 2.8 | 3.3 | |
| df | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | |
| Haloperidol (1 mg/kg) | Mean ± SEM | 79.8300 ± 6.5200 | 78.8300 ± 1.8500 | 95.0000 ± 1.7100 | 15.4000 ± 5.1300 | 15.7300 ± 5.3000 | 15.4000 ± 5.1000 | 21.53 ± 1.5 | 22.66 ± 2.2 | 21.23 ± 1.8 | 8.5 ± 1.1 | 8 ± 0.8 | 7.5 ± 0.5 | ||||||||||||
| P value | P < 0.001 Vs control |
P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P > 0.05 | P < 0.05 | |||||||||||||||||
| F | 22. 89 | 21.10 | 23. 45 | 21.818 | 22.186 | 21.089 | 22.103 | 19.246 | 21.118 | 24.786 | 20.089 | 18.103 | 25.246 | 21.118 | 25.786 | 20.089 | 19.103 | 21.246 | 1.12 | 0.9 | 1.05 | 3.5 | 2.8 | 3.3 | |
| df | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | |
| Flu (0.5 mg/kg) | Mean ± SEM | 50.6600 ± 7.9200 | 14.3330 ± 2.5517 | 16.8333 ± 3.7180 | 22.8330 ± 5.7237 | 13.3330 ± 2.2459 | 17.33 ± 6.5400 | 22.8333 ± 5.7230 | 11.8300 ± 2.0560 | 23.6667 ± 6.6215 | 4.5100 ± 1.1600 | 15.000 ± 5.700 | 157.0000 ±25.2400 | 17.2300 ± 10.8900 | 19.960 ± 7.960 | 179.0000 ± 55.0700 | 4.5100 ± 1.1600 | 28.330 ± 7.740 | 185.0000 ± 56.5300 | Not Applicable (N.A) | |||||
| P value | P < 0.05 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P > 0.05 | P > 0.05 | P < 0.001 | P > 0.05 | P > 0.05 | P < 0.001 | P > 0.05 | P > 0.05 | P < 0.001 | |||||||
| F | 5.169 | 22.4 | 21.873 | 16.05 | 23.8 | 14.6 | 11.03 | 23.92 | 16.889 | 1.33 | 1.548 | 15.35 | .89 | 1.08 | 17.66 | .66 | 1.131 | 18.989 | |||||||
| df | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | |||||||
| Flu (1 mg/kg) | Mean ± SEM | 23.5000 ± 4.9580 | 16.660 ± 1.764 | 21.160 ± 2.482 | 150000 ± 1.9100 | 35.160 ± 11.860 | 16.330 ± 1.874 | 23.5000 ± 4.9500 | 27.160 ± 5.290 | 16.333 ± 2.431 | 393.000 ± 109.000 | 431.0000 ±105.0000 | 437.000 ± 103.000 | 371.000 ± 92.700 | 440.0000 ± 101.0000 | 432.000 ± 104.000 | 393.000 ± 109.000 | 363.0000 ±84.1400 | 439.000 ±94.960 | 27 ± 1.1 | 26.3 ± 0.8 | 21.6 ± 1.4 | 10.6 ± 1.2 | 12.1 ± 1.1 | 8.16 ± 0.9 |
| P value | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P < 0.05 | P > 0.05 | |
| F | 11.2 | 23.33 | 14.46 | 22.078 | 7.66 | 22.11 | 11.709 | 9.34 | 21.55 | 26.69 | 27.51 | 22.89 | 16.45 | 20.089 | 22.103 | 19.246 | 21.118 | 24.786 | 1.05 | 1.2 | 2.1 | 2.6 | 4.12 | 1.75 | |
| df | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | |
| Bus(1 mg/kg) | Mean ± SEM | 25.3300 ± 6.3000 | 17.6600 ± 1.6400 | 13.660 ± 0.802 | 33.5000 ± 5.3210 | 29.0000 ± 4.2300 | 25.83 ± 6.2 | 30.5000 ± 4.7800 | 21.0000 ± 5.4650 | 25.833 ± 6.769 | 115.0000 ± 12.5500 | 654.000 ± 16.590 | 510.000 ± 76.170 | 91.3300 ± 7.9500 | 586.000 ± 36.650 | 603.000 ± 64.970 | 106.0000 ± 9.9600 | 660.000 ± 26.940 | 641.000 ± 39.200 | 28.33 ± 2.7 | 23 ± 4.6 | 25.33 ± 3.6 | 12 ± 1.8 | 10.33 ± 1.5 | 12.66 ± 2.65 |
| P value | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P > 0.05 | P < 0.001 | P < 0.001 | P > 0.05 | P < 0.001 | P < 0.001 | P > 0.05 | P < 0.001 | P < 0.001 | P < 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P < 0.05 | |
| F | 8.53 | 9.66 | 10.82 | 7.25 | 7.83 | 8.712 | 10.06 | 7.617 | 8.38 | 1.27 | 22.378 | 24.63 | 1.78 | 20.89 | 25.57 | 1.65 | 24.92 | 23.19 | 5.29 | 1.22 | 1.43 | 1.18 | 1.72 | 5.23 | |
| df | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | |
| 8-OHDPAT(1 mg/kg) | Mean ± SEM | 15.833 ± 1.887 | 11.500 ± 0.870 | 8.1667 ± 1.1377 | 11.833 ± 1.013 | 11.160 ± 1.565 | 9.0000 ± 0.8563 | 25.833 ± 7.268 | 16.667 ± 2.060 | 13.0000 ± 1.9000 | 44.3300 ± 9.0800 | 28.0000 ± 8.2900 | 43.000 ± 8.080 | 52.5000 ± 8.0500 | 35.1600 ± 7.8400 | 40.660 ± 8.170 | 54.3300 ± 8.1100 | 36.3300 ± 10.2000 | 41.330 ± 7800 | 26.3 ± 7.1 | 25.3 ± 6.28 | 27.2 ± 8.1 | 11.3 ± 0.8 | 10.6 ± 1.2 | 12.7 ± 1.9 |
| P value | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.05 | P > 0.05 | P < 0.01 | P < 0.01 | P < 0.05 | P < 0.01 | P < 0.01 | P < 0.05 | P < 0.01 | P > 0.05 | P > 0.05 | P < 0.05 | P > 0.05 | P > 0.05 | P < 0.05 | |
| F | 15.64 | 19.33 | 20.538 | 19.86 | 18.92 | 23.647 | 8.93 | 13.76 | 14.88 | 4.23 | 1.38 | 6.76 | 7.22 | 6.12 | 6.52 | 7.61 | 3.87 | 6.68 | 0.48 | 1.419 | 4.28 | 0.95 | 0.78 | 5.12 | |
| df | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 11.71 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | |
3.2. The Effect of fluoxetine 0.5 mg/kg on catalepsy
To investigate the effects of fluoxetine on chronic Haloperidol-induced catalepsy, fluoxetine 0.5 mg/kg was administrated for 15 days after receiving haloperidol for 21 days and examining catalepsy. The effects of treatment were evaluated on the 5th, 10th and 15th day after the initiation of treatment by bar test. Comparison of the results of the fluoxetine receiving group (0.5 mg/kg) (32.61 ± 8.124: p < 0.001, 13.08 ± 0.9868: p < 0.001, 17.81 ± 1.599: p < 0.001 on the 5th, 10th and 15th day, respectively) with the results obtained after 21 days of administration of haloperidol (83.61 ± 3.277) suggests a strong positive effect of fluoxetine in the treatment of haloperidol induced catalepsy, which significantly reduce catalepsy in all days (Fig. 2 (b)). Details of the data are listed in Table 1.
3.3. The Effect of fluoxetine 1 mg/kg on catalepsy
To investigate the effects of fluoxetine on chronic Haloperidol-induced catalepsy, fluoxetine 1 mg/kg was administrated for 15 days after receiving haloperidol for 21 days and examining catalepsy. The effects of treatment were evaluated on the 5th, 10th and 15th day after the initiation of treatment by bar test. Comparison of the results of the fluoxetine receiving group (1 mg/kg) (20.83 ± 1.865: p < 0.001, 26.97 ± 4.127: p < 0.001, 18.3 ± 1.494: p < 0.001 on the 5th, 10th and 15th day, respectively) with the results obtained after 21 days of administration of haloperidol (82.78 ± 3.189) suggests a powerful restoring effect of haloperidol induced catalepsy by fluoxetine treatment in all days of test (p < 0.001) (Fig. 2 (c)). Details of the data are listed in Table 1.
3.4. The effect of buspirone on catalepsy
In order to reveal the effects of buspirone on chronic haloperidol-induced catalepsy, after 21 days of administration of haloperidol, the rats were treated with buspirone (1 mg/kg) for 15 days. The muscle rigidity of the rats was measured 5, 10 and 15 days after starting treatment by bar test. Our results indicated that injections of buspirone) (28.39 ± 2.567: p < 0.001, 21.78 ± 2.062: p < 0.001, 21.05 ± 3.069: p < 0.001 on the 5th, 10th and 15th day, respectively) can diminish the induced catalepsy(85.94 ± 4.459) significantly (p < 0.001) in all days of tests (Fig. 2 (d)). Details of the data are listed in Table 1.
3.5. The effect of 8-OHDPAT on catalepsy
To demonstrate the effects of 8-OHDPAT on catalepsy caused by prolonged use of neuroleptic drugs, a 15-day course of 8-OHDPAT treatment was initiated in rats infused with haloperidol for 21 days. Catalepsy was measured by bar test. This assessment was performed after 5, 10 and 15 days from the start of treatment. The results indicate the significant healing effects of 8-OHDPAT) (16.58 ± 2.22: p < 0.001, 12.55 ± 0.9814: p < 0.001, 10.03 ± 0.7155: p < 0.001 on the 5th, 10th and 15th day, respectively) on haloperidol induced catalepsy (84.28 ± 3.59) in all days of measurement (Fig. 2 (e)). Details of the data are listed in Table 1.
3.6. The Effect of Haloperidol on motor imbalance
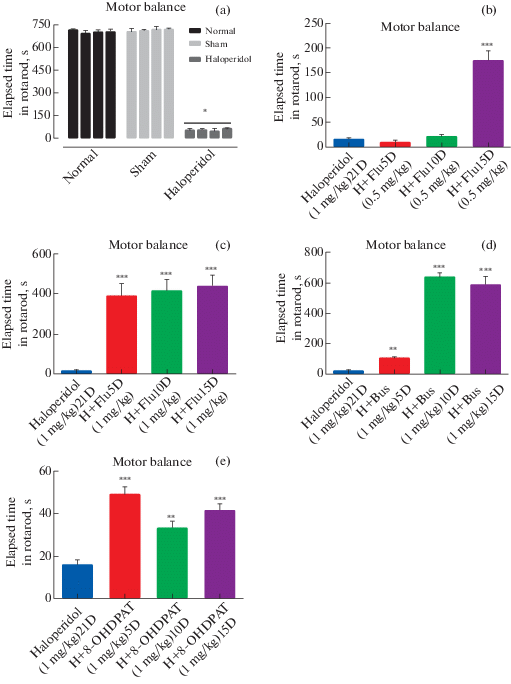
Comparison of the results of these groups demonstrated that haloperidol (1mg/kg, i.p.) as a neuroleptic drug was able to induce significantly (p < 0.001, dF: 11, 71) motor imbalance in receiving haloperidol group than the other two groups. So that, the duration of walking on the rotating rod was decreased in a significant manner (Fig. 3 (a)). Details of the data are listed in Table 1.
Fig. 3.
(a) Effect of haloperidol administration (1 mg/kg) on motor imbalance. Each bar represents the mean ± SEM of elapsed time (s) in rotarod. Tests have been repeated 4 times with 1 hour interval for approving of repeatability of each behavior. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following one-way ANOVA with repeated measure. †: p < 0.001. (b) Effect of chronic fluoxetine administration (0.5 mg/kg) on motor imbalance, which was treated by 5, 10, 15 days injection of fluoxetine i.p. (0.5 mg/kg) after 21 days injection of haloperidol. Each bar represents the mean ± SEM of elapsed time (s) in rotarod. Tests have been repeated 3 times with 1 hour interval for approving of repeatability of each behavior. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following one-way ANOVA with repeated measure. ***: p < 0.001 vs Haloperidol group. (c) Effect of chronic fluoxetine administration (1 mg/kg) on motor imbalance, which was treated by 5, 10, 15 days injection of fluoxetine i.p. (1 mg/kg) after 21 days injection of haloperidol. Each bar represents the mean ± SEM of elapsed time (s) in rotarod. Tests have been repeated 3 times with 1hour interval for approving of repeatability of each behavior. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following one-way ANOVA with repeated measure. ***: p < 0.001 vs Haloperidol group. (d) Effect of chronic buspirone administration (1 mg/kg) on motor imbalance, which was treated by 5, 10, 15 days injection of buspirone i.p. (1 mg/kg) after 21 days injection of haloperidol. Each bar represents the mean ± SEM of elapsed time (s) in rotarod. Tests have been repeated 3 times with 1 hour interval for approving of repeatability of each behavior. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following one-way ANOVA with repeated measure. **: p < 0.01, ***: p < 0.001 vs Haloperidol group. (e) Effect of chronic 8-OHDPAT administration (1 mg/kg) on motor imbalance, which was treated by 5, 10, 15 days injection of 8-OHDPAT i.p. (1 mg/kg) after 21 days injection of haloperidol. Each bar represents the mean ± SEM of elapsed time (s) in rotarod. Tests have been repeated 3 times with 1 hour interval for approving of repeatability of each behavior. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following one-way ANOVA with repeated measure. **: p < 0.01, **: p < 0.01 vs Haloperidol group.
Рис. 3. (а) Влияние введения галоперидола (1 мг/кг) на моторный дисбаланс. Каждый столбец представляет среднее значение ± SEM времени (с) в ротароде. Тесты были повторены 4 раза с интервалом в 1 ч. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. †: p < 0.001. (b) Влияние хронического введения флуоксетина (0.5 мг/ кг) на двигательный дисбаланс после введения галоперидола. Каждый столбец представляет среднее значение ± SEM времени (с) в ротароде. Тесты были повторены 3 раза с интервалом в 1 ч. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. ***: р < 0.001 в сравнении с группой галоперидол. (c) Влияние хронического введения флуоксетина (1 мг/кг) на двигательный дисбаланс после введения галоперидола. Каждый столбец представляет среднее значение ± SEM времени (с) в ротароде. Тесты были повторены 3 раза с интервалом в 1 ч. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. ***: р < 0.001 в сравнении с группой галоперидол. (d) Влияние хронического введения буспирона (1 мг/ кг) на двигательный дисбаланс после инъекции галоперидола. Каждый столбец представляет среднее значение ± SEM времени (с) в ротароде. Тесты были повторены 3 раза с 1-часовым. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. **: р < 0.01, ***: р < 0.001 в сравнении с группой галоперидол. (e) Влияние введения хронического 8-OHDPAT (1 мг/кг) на двигательный дисбаланс после инъекции галоперидола. Каждый столбец представляет среднее значение ± SEM времени (с) в ротароде. Тесты были повторены 3 раза с 1-часовым интервалом. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. **: р < 0.01, ***: р < 0.001 в сравнении с группой галоперидол. (e) Влияние введения хронического 8-OHDPAT (1 мг/кг) на двигательный дисбаланс после инъекции галоперидола. Каждый столбец представляет среднее значение ± SEM времени (с) в ротароде. Тесты были повторены 3 раза с 1-часовым интервалом. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. **: р < 0.01, **: р < 0.01 в сравнении с группой галоперидол.
3.7. The Effect of fluoxetine 0.5 mg/kg on the motor imbalance
To evaluate the effects of fluoxetine on chronic Haloperidol-induced motor impairment, fluoxetine 0.5 mg/kg was administrated for 15 days after receiving haloperidol for 21 days and examining motor imbalance. The effects of treatment were evaluated on the 5th, 10th and 15th day after the initiation of treatment by rotarod test. Comparison of the results of the fluoxetine receiving group (0.5 mg/kg) (8.542 ± 1.939, 20.05 ± 2.124, 174.5 ± 8.02: p < 0.001, on the 5th, 10th and 15th day, respectively) with the results obtained after 21 days of administration of haloperidol (14.92 ± 1.352) suggests a positive effect of fluoxetine in the treatment of haloperidol induced motor imbalance on 15th day after the initiation of treatment which is statistically significant (Fig. 3 (b)). Details of the data are listed in Table 1.
3.8. The effect of fluoxetine 1 mg/kg on the motor imbalance
To evaluate the effects of fluoxetine on chronic Haloperidol-induced motor impairment, fluoxetine 1 mg/kg was administrated for 15 days after receiving haloperidol for 21 days and examining motor imbalance. The effects of treatment were evaluated on the 5th, 10th and 15th day after the initiation of treatment by rotarod test. Comparison of the results of the fluoxetine receiving group (1 mg/kg) (386.8 ± 25.66: p < 0.001, 413 ± 23.57 : p < 0.001, 435.2 ± 24.02 : p < 0.001on the 5th, 10th and 15th day, respectively) with the results obtained after 21 days of administration of haloperidol (14.92 ± 1.352) suggests a returning motor imbalance by fluoxetine treatment which induce by haloperidol. (Fig. 3 (c)). Details of the data are listed in Table 1.
3.9. The effect of buspirone on the motor imbalance
In order to reveal the effects of buspirone on chronic haloperidol-induced motor imbalance, after 21 days of administration of haloperidol, the rats were treated with buspirone (1 mg/kg) for 15 days. The motor impairment of the rats was measured by rotarod test 5, 10 and 15 days after injection of buspirone. Our results indicated that injections of buspironecan diminish the induced motor imbalance. Statistical analysis revealed that in buspirone treated group (103.7 ± 3.878: p < < 0.01, 636.7 ± 11.45: p < 0.001, 582.5 ± 23.34: p < 0.001 on the 5th, 10th and 15th day, respectively) significant (p < 0.001) improvements in motor balance was seen in comparison with haloperidol group (15.92 ± 1.061). (Fig. 3 (d)). Details of the data are listed in Table 1.
3.10. The effect of 8-OHDPAT on the motor imbalance
To demonstrate the effects of 8-OHDPAT on motor imbalance caused by prolonged use of neuroleptic drugs, a 15-day course of 8-OHDPAT treatment was initiated in rats infused with haloperidol for 21 days. Motor imbalance was measured by rotarod test. This assessment was performed after 5, 10 and 15 days from the start of treatment. The results indicate the healing effects of 8-OHDPAT) (48.97 ± 1.603: p < 0.001, 33.25 ± 1.334: p < 0.01, 41.33 ± 1.385: p < 0.001 on the 5th, 10th and 15th day, respectively) on haloperidol induced motor impairment (15.76 ± 0.9387) which is statistically significant (Fig. 3 (e)). Details of the data are listed in Table 1.
3.11. The Effect of fluoxetine on general locomotor activity
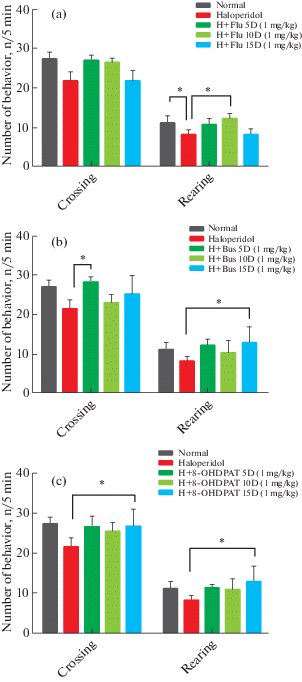
To evaluate the effects of fluoxetine on chronic Haloperidol-induced locomotor impairment, fluoxetine 1mg/kg was administrated for 15 days after receiving haloperidol for 21 days and examining locomotor activity. The effects of treatment were evaluated on the 5th, 10th and 15th day after the initiation of treatment by open field test. Comparison of the results of the fluoxetine receiving group (1 mg/kg) (10.6 ± 1.2, 12.1 ± 1.1: p < 0.05, 8.16 ± 0.8 on the 5th, 10th and 15th day, respectively) with the results obtained after 21 days of administration of haloperidol (8.5 ± 1.1: p < 0.05, 8 ± 0.8: p < 0.05, 7.5 ± 0.5: p < 0.05 on the 5th, 10th and 15th day, respectively) (that decrease statistically significant compare with normal group) in rearing and Comparison of the results of the fluoxetine receiving group (1 mg/kg) (27 ± 1.1, 26.3 ± 0.8, 21.6 ± 1.4 on the 5th, 10th and 15th day, respectively) with the results obtained after 21 days of administration of haloperidol (21.53 ± 1.5, 22.66 ± 2.2, 21.23 ± 1.8 on the 5th, 10th and 15th day, respectively) in crossing, suggests a statistically significant (p < 0.05) returning of locomotor activity just in rearing (Fig. 4 (a)). Details of the data are listed in Table 1.
Fig. 4.
(a) Effect of chronic Fluoxetine administration (1 mg/kg) on locomotor activity, which was treated by 5, 10, 15 days injection of Fluoxetine i.p. (1 mg/kg) after 21 days injection of haloperidol. Each bar represents the mean ± SEM of behavior number’s (n/5min) in open field. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following two-way ANOVA with repeated measure. *: p < 0.05 vs Haloperidol group. (b) Effect of chronic Buspirone administration (1 mg/kg) on locomotor activity, which was treated by 5, 10, 15 days injection of Buspirone i.p. (1 mg/kg) after 21 days injection of haloperidol. Each bar represents the mean ± SEM of behavior number’s (n/5min) in open field. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following two-way ANOVA with repeated measure. *: p < 0.05 vs Haloperidol group. (c) Effect of chronic 8-OHDPAT administration (1 mg/kg) on locomotor activity, which was treated by 5, 10, 15 days injection of 8-OHDPATi.p. (1 mg/kg) after 21 days injection of haloperidol. Each bar represents the mean ± SEM of behavior number’s (n/5min) in open field. n = 6 rats for each group. Significance refers to Tukey post-hoc multiple comparison tests following two-way ANOVA with repeated measure. *: p < 0.05 vs Haloperidol group.
Рис. 4. (а) Влияние хронического введения флуоксетина (1 мг/кг) на двигательную активность после инъекции галоперидола. Каждый столбец представляет среднее значение ± SEM числа поведенческих актов за 5 мин в открытом поле. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. *: р < 0.05 в сравнении с группой галоперидол. (b) Влияние хронического введения буспирона (1 мг/кг) на двигательную активность после инъекции галоперидола. Каждый столбец представляет среднее значение ± SEM в открытом поле. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. *: р < 0.05 в сравнении с группой галоперидол. (c) Влияние хронического введения 8-OHDPAT (1 мг/ кг) на двигательную активность после инъекции галоперидола. Каждый столбец представляет среднее значение ± SEM числа поведенческих актов за 5 мин в открытом поле. n = 6 крыс для каждой группы. Значимость различий оценивали тестом Tukey. *: р < 0.05 в сравнении с группой галоперидол.
3.12. The Effect of buspirone on general locomotor activity
To demonstrate the effects of buspirone on locomotor activity caused by prolonged use of neuroleptic drugs, a 15-day course of buspirone treatment was initiated in rats infused with haloperidol for 21 days. Locomotor activity was measured by open field test. This assessment was performed after 5, 10 and 15 days from the start of treatment. Comparison of the results of the buspirone receiving group (12 ± 1.8, 10.33 ± 1.5, 12.66 ± 2.65: p < 0.05 on the 5th, 10th and 15th day, respectively) with the results obtained after 21 days of administration of haloperidol (8.5 ± 1.1: p < 0.05, 8 ± 0.8: p < 0.05, 7.5 ± 0.5 : p < 0.05 on the 5th, 10th and 15th day, respectively) (that decrease statistically significant compare with normal group) in rearing and Comparison of the results of the buspirone receiving group (28.33 ± 2.7: p < < 0.05, 23 ± 4.6, 25.33 ± 3.6 on the 5th, 10th and 15th day, respectively) with the results obtained after 21 days of administration of haloperidol (21.53 ± 1.5, 22.66 ± 2.2, 21.23 ± 1.8 on the 5th, 10th and 15th day, respectively) in crossing, revealed that in day of 5 and 15 significant (p < 0.05) improvements in crossing and rearing was seen respectively (Fig. 4 (b)). Details of the data are listed in Table 1.
3.13. The Effect of 8-OHDPAT on general locomotor activity
To demonstrate the effects of 8-OHDPAT on locomotor activity caused by prolonged use of neuroleptic drugs, a 15-day course of 8-OHDPAT treatment was initiated in rats infused with haloperidol for 21 days. Locomotor activity was measured by open field test. This assessment was performed after 5, 10 and 15 days from the start of treatment. Comparison of the results of the 8-OHDPAT receiving group (11.3 ± 0.8, 10.6 ± ± 1.2, 12.7 ± 1.9: p < 0.05 on the 5th, 10th and 15th day, respectively) with the results obtained after 21 days of administration of haloperidol (8.5 ± 1.1: p < 0.05, 8 ± 0.8: p < 0.05, 7.5 ± 0.5 : p < 0.05 on the 5th, 10th and 15th day, respectively) (that decrease statistically significant compare with normal group) in rearing and Comparison of the results of the 8-OHDPAT receiving group (26.3 ± 7.1, 25.3 ± 6.28, 27.2 ± 8.1: p < 0.05 on the 5th, 10th and 15th day, respectively) with the results obtained after 21 days of administration of haloperidol (21.53 ± 1.5, 22.66 ± 2.2, 21.23 ± 1.8 on the 5th, 10th and 15th day, respectively) in crossing, indicate that 15 days after injection of 8-OHDPAT, haloperidol induced locomotor impairment showed statistically significant (p < 0.05) improvement (Fig. 4 (c)). Details of the data are listed in Table 1.
DISCUSSION
The results demonstrated that haloperidol injection induced obvious extrapyramidal disorder in rats. In order to actualize this efficacy, we investigated the effects of serotoninergic drugs Buspirone, different doses of Fluoxetine and 8-OHDPAT in rats receiving haloperidol for 21 days. Measurement of extrapyramidal disorder such as catalepsy and motor imbalance were conducted by bar test and rotarod test, respectively. According to our result, all the drugs above could significantly restore the catalepsy and motor imbalance induced by chronic administration of haloperidol.
All of these mechanisms and neuronal connections suggest that serotonergic drugs potentially can relieve neuroleptic drugs induced extrapyramidal disorders.
According to our results, chronic injection of buspirone (10 mg/Kg) as one of the 5HT1A serotonin receptor agonists has been able to reduce extrapyramidal disorders caused by prolonged use of haloperidol. This confirms the probable effects of buspirone on the extrapyramidal neural network which found in previous findings [McMillen, 1985; Sharifi et al., 2015; Ahmadi et al., 2019]. However, the precise mechanism of operation and effect of the buspirone is still not fully known and can be investigated in the future.
8-OHDPAT is another 5HT1A receptor agonist that was studied. 8-OHDPAT as well as buspirone have a diminished catalepsy effect and restore motor imbalance in the rotarod test. In previous studies, 8-OHDPAT’s ability to reduce the 6-hydroxy dopamine-induced catalepsy was obtained [Sharifi et al., 2015]. It has also been observed that this drug can reduce the haloperidol-induced catalepsy in monkeys through the effect on the serotonin receptors and not on dopamine receptors [Christoffersen, Meltzer, 1998]. Hence, our results emphasize the effect of 8-OHDPAT on the extrapyramidal neuronal network and its modulating effects on the dopaminergic system of the brain and its beneficial effects against chronic complications of haloperidol.
Effects of fluoxetine as a selective serotonin reuptake inhibitor were evaluated in two different doses. Our results demonstrated that fluoxetine (1 mg/Kg) could significantly treat catalepsy and motor imbalance induced by haloperidol. Fluoxetine (0.5 mg/Kg) also had a significant therapeutic efficacy on catalepsy; whereas motor imbalance significantly restores just after 15 day of injection. However, in previous human and animal studies, fluoxetine has been identified as the cause of extrapyramidal disorders, not a therapeutic factor [Halman, Goldbloom, 1990]. Some other studies also acknowledged that acute and chronic injection of fluoxetine has led to a cataleptic effect [Tatara et al., 2012; Aristieta et al., 2014]. That is completely in contradiction with our results. In another study, chronic administration of fluoxetine in low doses has not only failed to induce catalepsy but has been able to treat 6-hydroxy dopamine-induced catalepsy [Sharifi et al., 2013]. This is in line with the results of our study. The observed differences in fluoxetine function can be attributed to drug-dose dependence, which, at low doses, has anti-cataleptic effects and induce catalepsy at high doses.
All of the neuroleptic drugs inhibit the normal signaling of dopamine D2 receptors in the brain and thus exert their effects by blocking dopaminergic receptors on the Nigro striatal pathway, these drugs can lead to extrapyramidal motor disorders [Farde et al., 1992; Shireen, 2016; Moore, Furberg, 2017]. Our results demonstrated that haloperidol injection as a neuroleptic drug induced obvious extrapyramidal disorder in rats. Both serotonin (5-HT) and dopamine (DA) neurotransmitters play a key role in modulating synaptic transmission in the central nervous system. Among the various types of serotonergic receptors, 5HT1 receptors play a vital role in modulating the effects of extrapyramidal symptoms [Meltzer et al., 2003; Ohno et al., 2011; Shimizu et al., 2013]. As it shown in our results, 8-OHDPAT as well as buspirone have a diminished catalepsy effect and restore motor imbalance in the rotarod test. 8-OHDPAT and buspirone are both 5HT1A receptor agonist and create their effects by acting on these receptors. Therefore, stimulation of 5HT1A receptors is associated with improved extrapyramidal effects of neuroleptic drugs. Additionally, in a study conducted by Nayebi, the effect of buspirone and 8-OHDPAT on 6hydroxydopamine-induced catalepsy were investigated. They demonstrated that buspirone and 8-OHDPAT can improve the 6hydroxydopamine-induced catalepsy as well as our study showed. They also proved that the effects of buspirone and 8-OHDPAT were abolished by 1(2methoxyphenyl) 4[4(2phthalimido) butyl] piperazine hydrobromide, a 5HT1A receptor antagonist [Nayebi et al., 2010]. These results confirm our study findings. Also in this study we used several drugs, all of them were 5HT1A agonists. Similar results were obtained in Bartest and Rotarod test, all of the results confirm the effects of 5HT1A receptors on dopaminergic receptor block reduction. Therefore, it can be concluded that stimulation of 5HT1A receptors, in this study done by buspirone and 8-OHDPAT could reduce the effects of dopaminergic receptor blockade.
According to our results, buspirone, fluoxetine, and 8-OHDPAT consumption could improve extrapyramidal disturbances caused by neuroleptic drugs. Therefore, in order to prevent the aspectual extrapyramidal disorders, it is recommended to use these drugs after neuroleptic consume. Although evidence of this requires clinical studies.
In addition to the effects of serotonergic drugs on the extrapyramidal system, it appears that these medications also contribute to anxiety adjustment [Gardner, 1988]. Therefore, we examined the efficacy of these drugs on locomotor activity. Our results demonstrated that fluoxetine, buspirone, and 8-OHDPAT could restore the locomotor impairment and reversed the haloperidol effect. Buspirone and 8-OHDPAT improve both rearing and crossing which was statistically significant but fluoxetine just heals rearing behavior in animals. These positive effects were detected in different days of treatment and showed no relation between the period of treatment and improvement effects. Therefore, the prolonged treatment showed no preferences. Also, in a study conducted by Carey R. and colleagues, 8-OHDPAT wasn’t able to restore the progressive decline in locomotion caused by haloperidol. That is in contradiction to our results. However, in the same study, it was proved that 8-OHDPAT can restore the locomotor stimulant effects of cocaine blocked by haloperidol and reverse the blocking effect of haloperidol on cocaine [Carey et al., 2000; De la Casa et al., 2018]. In another study, injection of 5HT1A receptor agonists such as buspirone and 8-OHDPAT increased the locomotor activity in the animal models of reduced dopamine transmission [Mignon, Wolf, 2002]. This is in line with the results of our study. Some other studies indicated that fluoxetine produced negative side effects in the open field test, which is not in line with our study [Kulikova et al., 2015].
CONCLUSION
According to our findings, chronic administration of fluoxetine, buspirone, and 8-OHDPAT improve extrapyramidal disorders and locomotor impairment in haloperidol-induced Parkinsonism model through activation of nigral 5HT1A receptors. As a conclusion, lower doses of fluoxetine were not effective in decrease of extrapyramidal symptoms while higher doses are effective. Also, both lower and higher doses of buspirone and 8-OHDPAT could be efficient for extrapyramidal symptoms therapy then in order to reduce the side effects we could administrate lower doses. However, more clinical and pre-clinical studies are needed to address this issue.
Список литературы
Ahmadi S., Sabahi M., Haddadi R. The preventive/protective effect of testosterone on haloperidol-induced extrapyramidal disorders in male rats. Journal of Babol University of Medical Sciences. 2018. 20 (7): 55–62.
Ahmadi S.A., Sabahi M., Haddadi R. The effect of acute and repeated administration of buspirone, 8-OHDPAT and fluoxetine on haloperidol-induced extrapyramidal symptoms. Neuropsychopharmacologia Hungarica: a Magyar Pszichofarmakologiai Egyesulet lapja – official journal of the Hungarian Association of Psychopharmacology. 2019. 21 (2): 59–68.
Aristieta A., Morera-Herreras T., Ruiz-Ortega J., Miguelez C., Vidaurrazaga I., Arrue A., Zumarraga M., Ugedo L. Modulation of the subthalamic nucleus activity by serotonergic agents and fluoxetine administration. Psychopharmacology. 2014. 231 (9): 1913–1924.
Association A.P. (2006). American Psychiatric Association Practice Guidelines for the treatment of psychiatric disorders: compendium 2006, American Psychiatric Pub.
Bantick R.A., De Vries M.H., Grasby P.M. The effect of a 5-HT1A receptor agonist on striatal dopamine release. Synapse. 2005. 57 (2): 67–75.
Carey R., Damianopoulos E., De Palma G. 8-OHDPAT can restore the locomotor stimulant effects of cocaine blocked by haloperidol. Pharmacology Biochemistry and Behavior. 2000. 66 (4): 863–872.
Christoffersen C.L., Meltzer L.T. Reversal of haloperidol-induced extrapyramidal side effects in cebus monkeys by 8-hydroxy-2-(di-n-propylamino) tetralin and its enantiomers. Neuropsychopharmacology. 1998. 18 (5): 399–402.
De la Casa L.G., Carcel L., Ruiz-Salas J.C., Vicente L., Mena A. Conditioned increase of locomotor activity induced by haloperidol. PloS one. 2018. 13 (10): e0200178.
Dunstan R., Broekkamp C.L., Lloyd K.G. Involvement of caudate nucleus, amygdala or reticular formation in neuroleptic and narcotic catalepsy. Pharmacology Biochemistry and Behavior. 1981. 14 (2): 169–174.
Esposito E., Di Matteo V., Di Giovanni G. Serotonin–dopamine interaction: an overview. Progress in Brain Research. 2008. 172: 3–6.
Farde L., Nordström A.-L., Wiesel F.-A., Pauli S., Halldin C., Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine: relation to extrapyramidal side effects. Archives of general psychiatry. 1992. 49 (7): 538–544.
Gardner C.R. Potential use of drugs modulating 5HT activity in the treatment of anxiety. General Pharmacology: The Vascular System. 1988. 19 (3): 347–356.
Haddadi R., Brooshghalan S.E., Farajniya S., Nayebi A.M., Sharifi H. Short-Term Treatment with Silymarin Improved 6-OHDA-Induced Catalepsy andMotor Imbalance in Hemi-Parkisonian Rats. Advanced Pharmaceutical Bulletin. 2015. 5 (4): 463–469.
Haddadi R., Nayebi A.M., Brooshghalan S.E. Silymarin prevents apoptosis through inhibiting the Bax/caspase-3 expression and suppresses toll like receptor-4 pathway in the SNc of 6-OHDA intoxicated rats. Biomedicine & Pharmacotherapy. 2018a. 104: 127–136.
Haddadi R., Poursina M., Zeraati F., Nadi F. Gastrodin microinjection suppresses 6-OHDA-induced motor impairments in parkinsonian rats: insights into oxidative balance and microglial activation in SNc. Inflammopharmacology. 2018b. 1–12.
Halman M., Goldbloom D.S. Fluoxetine and neuroleptic malignant syndrome. Biological psychiatry. 1990. 28 (6): 518–521.
Hicks P.B. The effect of serotonergic agents on haloperidol-induced catalepsy. Life sciences. 1990. 47 (18): 1609–1615.
Kulikova E., Tikhonova M., Volcho K., Khomenko T., Salakhutdinov N., Kulikov A., Popova N. Comparison of behavioral effects of fluoxetine, imipramine and new psychotropic drug TC-2153 on mice with hereditary predisposition to catalepsy. Zhurnal vysshei nervnoi deiatelnosti imeni I.P. Pavlova. 2015. 65 (1): 105–112.
McMillen B. Comparative chronic effects of buspirone or neuroleptics on rat brain dopaminergic neurotransmission. Journal of neural transmission. 1985. 64 (1): 1–12.
Meltzer H. Y., Li Z., Kaneda Y., Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003. 27 (7): 1159–1172.
Mignon L., Wolf W.A. Postsynaptic 5-HT 1A receptors mediate an increase in locomotor activity in the monoamine-depleted rat. Psychopharmacology. 2002. 163 (1): 85–94.
Moore T.J., Furberg C.D. The harms of antipsychotic drugs: evidence from key studies. Drug safety. 2017. 40 (1): 3–14.
Nayebi A.M., Rad S.R., Saberian M., Azimzadeh S., Samini M. Buspirone improves 6-hydroxydopamine-induced catalepsy through stimulation of nigral 5-HT 1A receptors in rats. Pharmacological reports. 2010. 62 (2): 258–264.
Ohno Y., Imaki J., Mae Y., Takahashi T., Tatara A. Serotonergic modulation of extrapyramidal motor disorders in mice and rats: role of striatal 5-HT 3 and 5-HT 6 receptors. Neuropharmacology. 2011. 60 (2): 201–208.
Ossowska K., Karcz M., Wardas J., Wolfarth S. Striatal and nucleus accumbens D 1/D 2 dopamine receptors in neuroleptic catalepsy. European journal of pharmacology. 1990. 182 (2): 327–334.
Perälä J., Suvisaari J., Saarni S.I., Kuoppasalmi K., Isometsä E., Pirkola S., Partonen T., Tuulio-Henriksson A., Hintikka J., Kieseppä T. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Archives of general psychiatry. 2007. 64 (1): 19–28.
Pires J., Silva S., Futuro-Neto H. Dorsal raphe nucleus lesions have no effect on neuroleptic-induced catalepsy and on the anticataleptic activity of buspirone. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica. 1990. 24 (6): 615–617.
Rao S., Hrishikeshavan H., Guruswami M. Effect of serotonergic agents on neuroleptic induced catalepsy in rats. Functional neurology. 1989. 5 (4): 353–360.
Sabahi M., Amiahmadi S., Haddadi R. Effects of Estrogen and Progesterone on Catalepsy and Motor and Balance Impairment Classified as Haloperidol-induced Extrapyramidal Disorders. Journal of Obstetrics, Gynecology and Cancer Research (JOGCR). 2018. 3 (1): 1–7.
Sandyk R., Fisher H. Serotonin in involuntary movement disorders. International journal of neuroscience. 1988. 42 (3-4): 185-208.
Sharifi H., Mohajjel Nayebia A., Farajnia S. Dose-dependent effect of flouxetine on 6-OHDA-induced catalepsy in male rats: a possible involvement of 5-HT1A receptors. Advanced pharmaceutical bulletin. 2013. 3 (1): 203–206.
Sharifi H., Nayebi A., Farajnia S., Haddadi R. Effect of chronic administration of buspirone and fluoxetine on inflammatory cytokines in 6-hydroxydopamine-lesioned rats. Drug research. 2015a. 65 (08): 393–397.
Sharifi H., Nayebi A.M., Farajnia S., Haddadi R. Effect of Buspirone, Fluoxetine and 8-OHDPAT on Striatal Expression of Bax, Caspase-3 and Bcl-2 Proteins in 6-Hydroxydopamine-Induced Hemi-Parkinsonian Rats. Advanced pharmaceutical bulletin. 2015b. 5 (4): 491.
Shimizu S., Mizuguchi Y., Ohno Y. Improving the treatment of schizophrenia: role of 5-HT receptors in modulating cognitive and extrapyramidal motor functions. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders). 2013. 12 (6): 861–869.
Shireen E. Experimental treatment of antipsychotic-induced movement disorders. Journal of Experimental Pharmacology. 2016. 8: 1.
Tatara A., Shimizu S., Shin N., Sato M., Sugiuchi T., Imaki J., Ohno Y. Modulation of antipsychotic-induced extrapyramidal side effects by medications for mood disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012. 38 (2): 252–259.
Üçok A., Gaebel W. Side effects of atypical antipsychotics: a brief overview. World Psychiatry. 2008. 7 (1): 58–62.
van Os J., Hanssen M., Bijl R.V., Vollebergh W. Prevalence of psychotic disorder and community level of psychotic symptoms: an urban-rural comparison. Archives of General Psychiatry. 2001. 58 (7): 663–668.
Wade M., Tai S., Awenat Y., Haddock G. A systematic review of service-user reasons for adherence and nonadherence to neuroleptic medication in psychosis. Clinical Psychology Review. 2017. 51: 75–95.
Wei L., Chen L. Effects of 5-HT in globus pallidus on haloperidol-induced catalepsy in rats. Neuroscience letters. 2009. 454 (1): 49–52.
Zubkov E., Kulikov A., Naumenko V., Popova N. Chronic Actions of Thyroxine on Behavior and Serotonin Receptors in Mouse Strains with Contrasting Predispositions to Catalepsy. Neuroscience and behavioral physiology. 2009. 39 (9): 909–914.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


