Журнал общей биологии, 2023, T. 84, № 4, стр. 263-278
Филлоплана как местообитание грибов
А. А. Царелунга 1, *, Е. Ю. Благовещенская 1, **
1 Московский государственный университет им. М.В. Ломоносова, биологический факультет
119234 Москва, Ленинские горы, 1, стр. 12, Россия
* E-mail: alexcar333@mail.ru
** E-mail: kathryn@yandex.ru
Поступила в редакцию 24.11.2022
После доработки 18.04.2023
Принята к публикации 24.07.2023
- EDN: YZMKTY
- DOI: 10.31857/S0044459623040073
Аннотация
Как показано в настоящее время, филлоплана растений активно заселяется различными дрожжевыми и мицелиальными грибами разных таксономических групп. Особенностями листа как микроместообитания являются низкая влажность, подверженность механическим воздействиям дождя и ветра, бедность питательных веществ на поверхности и высокая инсоляция, что обуславливает выделение грибов-эпифитов как отдельной экологической группы. Несмотря на то, что данные по разным растениям отличаются, в целом можно сказать, что на поверхности растений наиболее часто встречаются дрожжи базидиального аффинитета и такие мицелиальные грибы, как Alternaria, Epicoccum, Cladosporium, Phoma и Trichoderma. Биологический цикл эпифитных грибов в настоящее время не исследован, но предполагается, что он начинается со специфического закрепления споры на поверхности, далее следует формирование биопленок или так называемых агрегатов, объединяющих бактерии, дрожжи и мицелиальные грибы, и завершается формированием спор либо на поверхности живого растения, либо на отмерших и разлагающихся листьях.
И надземные, и подземные органы растения активно заселяются различными микроорганизмами. Для обозначения поверхности надземной части растений многими авторами используется термин “филлосфера”, который охватывает все надземные органы: почки, листья, стебли, цветки, плоды и семена, – хотя в трактовке термина могут возникать различия (Благовещенская, 2015). Основной частью филлосферы является филлоплана или строго поверхность листа. Листья растений суммарно составляют огромную площадь, выполняя множество важных функций, среди которых осуществление фотосинтеза и участие в круговороте биогенных элементов. Лист также является тем органом, который часто поражается фитопатогенными грибами, в связи с чем долгое время интерес исследователей был прикован исключительно к паразитическим видам филлопланы, в то время как работ, посвященных микромицетным сапротрофным сообществам, обитающим на поверхности листьев, довольно мало. Несмотря на то, что данные о биоразнообразии, физиологии и экологии эпифитных микроорганизмов остаются крайне разрозненными, уже сейчас известно, что обитатели филлопланы выполняют множество важных экологических функций, среди которых влияние на заселение поверхности листа патогенами и первые этапы разложения листовой пластинки (Kirschner, 2015; Sivakumar et al., 2020). Кроме того, филлосфера в целом и филлоплана в частности в настоящее время привлекают внимание как местообитание с большим потенциалом для поиска организмов, способных продуцировать различные биологически активные вещества (Waill, Ghoson, 2018; Sivakumar et al., 2020).
Целью данной работы является описание особенностей строения листа с точки зрения возможности заселения его грибами и обобщение имеющихся данных о развитии грибов на поверхности листа.
ЛИСТ КАК МЕСТООБИТАНИЕ
Лист – один из важнейших вегетативных органов растения, строение которого подробно разбирается в любом учебнике по ботанике (напр., Лотова, 2001). Как и практически любая поверхность, он может служить субстратом для поселения различных организмов. При этом филлосфера в целом является достаточно экстремальным местообитанием, так как здесь возможны сильные перепады температур, особенно по сравнению с ризосферой; здесь сравнительно небольшая влажность, что очень критично для развития грибов; существует проблема питательных веществ; наконец, при развитии на поверхности листа организмы подвергаются воздействию инсоляции, что тоже следует рассматривать как серьезный стрессирующий фактор (Kirschner, 2015).
Микроклиматические условия будут заметно различаться не только для грибов, заселяющих растения разных регионов, но и для грибов, поселяющихся на соседних растениях, так как виды растений отличаются друг от друга по общему габитусу, включая, например, наклон листовой пластинки, и по особенностям строения листа, что приведет к различным уже микроклиматическим условиям, существенным для развития грибов (Багирова и др., 2012). В течение сезона условия жизни грибов также будут меняться, что связано, с одной стороны, с погодными изменениями, а с другой – со старением самого листа (Copeland et al., 2015).
С точки зрения микроусловий следует выделить две важных позиции: форму самого листа (рельеф) и химический состав поверхности. Эти два фактора обуславливают смачиваемость листа, удержание влаги, накопление пыли и пр.
Листья большинства растений не абсолютно плоские. Если рассматривать виды, живущие в сравнительно засушливых условиях, то, например, многие овсяницы (Festuca spp.) имеют сложенную вдоль листовую пластинку, так что добраться до адаксиальной ее стороны довольно затруднительно (напр., Dabrowska, 2012). Лист может иметь выраженные углубления или, наоборот, “ребра” в тех местах, где находятся проводящие пучки (рис. 1). Поверхность листа часто покрыта различными трихомами, которые могут иметь довольно сложное строение (рис. 1).
Рис. 1.
Примеры рельефа поверхности листьев, сканирующая электронная микроскопия: а – выступающая жилка на листе Trifolium pratense L., б – щитковидные волоски Hippophae rhamnoides L.
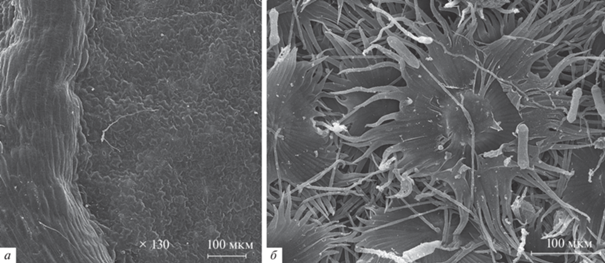
Функционально выделяют трихомы двух типов: кроющие и железистые (Лотова, 2001). Кроющие волоски участвуют в процессах транспирации, защищают от перегрева и поедания листа насекомыми, влияют на смачиваемость листа и устойчивость к засухе. Железистые волоски способны выделять различные вещества, например, полисахариды, липиды, летучие соединения и белки, участвующие во взаимодействии с микроорганизмами или насекомыми (Sivakumar et al., 2020). В целом, наиболее часто эпифиты проявляют рост в виде биопленок, преимущественно около трихом, жилок и в углублениях между эпидермальными клетками (Sivakumar et al., 2020). Опушенность листьев оказывает значимое влияние на состав комплексов выявляемых грибов (Pajares-Murgó et al., 2022).
Если смотреть на поверхность при большем увеличении, то мы столкнемся с микрорельефом листа, который формируется, исходя из четырех признаков (Dickison, 2000): 1) взаимное расположение клеток или паттерн; 2) форма эпидермальных клеток или первичная скульптура; 3) рельеф клеточных стенок с их утолщениями, а также рельеф кутикулы или вторичная скульптура; 4) выделения эпикутикулярного воска или третичная скульптура.
Топография поверхности листа, таким образом, напрямую влияет на доступность инфицирования патогенными организмами (Mechaber et al., 1996). Например, мандарин оказывается более устойчивым, по сравнению с грейпфрутом, к поражению бактерией Xanthomonas citri из-за того, что наружные стенки устьиц имеют выступы, которые не дают проникнуть в устьице каплям жидкости. Именно через капли данный паразит проникает в ткани растения (Багирова и др., 2012). Кроме того, что поверхность листа может оказаться непригодной для ее заселения некоторыми видами, общая топография поверхности может иметь очень большое значение в распознавании растения-хозяина специфичным патогеном (Agrios, 2005). Но и для сапротрофных видов микрорельеф чрезвычайно важен, так как он имеет очень большое значение для гидрофобности/гидрофильности поверхности листа (Burton, Bhushan, 2006; Kolyva et al., 2012).
Эпидермальные клетки листа, как правило, покрыты кутикулой. По структуре кутикула очень вариабельна, разные растения имеют различную толщину, ультраструктуру и химический состав. Между кутикулой и эпидермисом часто имеется слой пектиновых веществ, прикрепляющих кутикулу к клеточным стенкам (Dickison, 2000). Существует несколько моделей строения кутикулярного слоя. По самой распространенной теории кутикула отделена от клеточной стенки и не включает в себя пектины, однако есть и модели, в которых кутикула рассматривается как часть клеточной стенки эпидермальных клеток (Fernández et al., 2016). Кутикула является важным барьером на пути проникновения вредоносных микроорганизмов внутрь растения (Dickison, 2000). Сама по себе кутикула проницаема для многих соединений, причем способность абаксиальной и адаксиальной сторон листа пропускать различные химические вещества отличается (Norms, Bukovac, 1968; Popp et al., 2005). Дополнительным барьером являются растительные воски, которые могут откладываться или непосредственно на поверхности кутикулы, или интегрированно. Воск так же, как и кутикула, ограничивает потерю растением воды и выполняет барьерные функции. Воск может образовывать сплошной слой, отдельные корки или одиночные кристаллоиды различной толщины. Такие кристаллоиды бывают самой разной формы, причем форма может характеризовать отдельное семейство или род растений (Barthlott et al., 1998; Tomasi et al., 2018). Воск на поверхности растений может и отсутствовать, как, например, у семейств Pontederiaceae и Amaranthaceae (Neinhuis, Barthlott, 1997). Во многом именно воск определяет гидрофобность поверхности листа (Neinhuis, Barthlott, 1997; Burton, Bhushan, 2006; Kolyva et al., 2012), а кроме того, воск может способствовать отражению солнечных лучей, что снижает температуру поверхности (Kolyva et al., 2012).
Таким образом, можно ожидать, что строение поверхности листа, его рельеф и гидрофобность играют большую роль в возможности заселения растения и прикрепления к его поверхности как для патогенов, так и для сапротрофных организмов.
МЕТОДЫ ВЫЯВЛЕНИЯ ГРИБОВ ФИЛЛОПЛАНЫ
Известно достаточно много работ, посвященных грибам, постоянно или временно присутствующим на поверхности листьев, т.е. “грибам филлопланы”. При этом часто используется термин “эпифитные грибы”, который по-разному трактуется разными авторами. В данном случае термины “эпифитные грибы” или “грибы-эпифиты” мы будем использовать в узком смысле, подразумевая сапротрофные грибы, обитающие на поверхности листьев и других зеленых частей растения.
Используемые методы изучения можно разделить на прямые и косвенные, и те, и другие обладают своими достоинствами и недостатками.
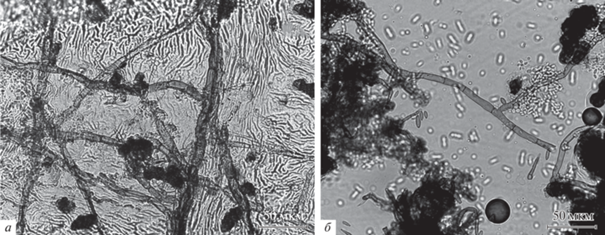
Прямые методы заключаются в том, чтобы увидеть эпифитный организм непосредственно на поверхности листа, таким образом – это световая микроскопия (СМ) и сканирующая электронная микроскопия (СЭМ). Эти методы позволяют оценить обилие мицелия и его распределение по поверхности листа, а также изучить сезонную динамику роста эпифитных грибов (Lee, Hyde, 2002). С помощью СМ можно исследовать либо срыв эпидермиса, либо слепок поверхности листа (рис. 2) (Dickinson et al., 1974; Hallett et al., 2010). При работе со срывами эпидермиса сложно различить структуры, находящиеся на поверхности эпидермиса и под ним (Благовещенская, 2015). Метод слепков в этом случае более надежен, но и он имеет ряд недостатков. Если мицелий не имеет структур размножения, исследователь не может понять, с чем имеет дело, более того, часто мицелий неразличим без дополнительного окрашивания. Также необходимо отметить, что метод слепков будет крайне затруднителен при работе с сильно опушенными объектами. СЭМ, в свою очередь, требует большого количества времени, притом что на образцах может и не оказаться мицелия (Благовещенская, 2015).
Рис. 2.
Обнаружение эпифитных грибов с помощью светового микроскопа: a – отпечаток поверхности листа Padus avium Mill., сделанный с помощью клея БФ-6; б – отпечаток поверхности листа Tilia cordata Mill., сделанный с использованием скотча и жидкости Шира.
Косвенные методы включают выделение грибов в культуру и молекулярные методы. Два основных метода выделения в чистую культуру, которые применяются практически во всех работах, – это метод смывов и метод отпечатков. Метод смывов заключается в том, что листовые пластинки исследуемого растения активно взбалтываются в дистиллированной воде, после чего полученной суспензией засевают питательную среду (Last, Deighton, 1965; Возняковская, 1969; Ruscoe, 1971; Bopaih et al., 1978; Falconi, Mendgen, 1994; Osono, Mori, 2005; Osono, 2008; Mukhtar et al., 2010, 2012; Глазунова и др., 2011; Yusifova et al., 2017). Главный недостаток – большая вероятность того, что на листовой пластинке могли оказаться случайно занесенные споры, которые прорастут на питательной среде. Метод отпечатков исключает такой вариант развития событий, так как сначала лист тщательно промывают, а после этого прикладывают к среде на некоторое время (Lamb, Brown, 1970; Diem, 1974; Fiss et al., 2000; Santamaría, Bayman, 2005; Воронин, 2010; Ерина, Коптева, 2015a, б; Благовещенская, 2017). Один лист можно прикладывать несколько раз, что может улучшить результаты (Благовещенская, 2017). Оба метода не позволяют выявить виды, крепко прикрепляющиеся к поверхности листа, а также некультивируемые виды.
Описан интересный метод, фокусирующийся на спороносящих видах, который можно назвать методом улавливания спор (spore-fall method). Листовая пластинка закрепляется в чашке Петри над питательной средой, куда падают споры (Lamb, Brown, 1970; Ruscoe, 1971; Dickinson, 1973; Pennycook, Newhook, 1978). Этот метод некоторые исследователи используют для количественной оценки заселенности листа, однако он дает неоднозначные результаты.
При выделении эпифитных грибов обычно используют достаточно “богатые” среды на основе растительного сырья – сусловый агар, картофельно-глюкозный агар, картофельно-морковный агар. Для того, чтобы избежать выделения бактерий на питательные среды, их часто подкисляют или используют антибиотики, например, сульфат стрептомицина, хлортетрациклин, хлорамфеникол и др. (Diem, 1974; Bills, Polishook, 1991; Благовещенская, 2015; Duarte et al., 2016).
С появлением методов секвенирования нового поколения, таких как 454 пиросеквенирование или Illumina (напр., Janakiev et al., 2019; Dong et al., 2021), стал популярен метагеномный анализ сообществ эпифитов, хотя он преимущественно применяется для изучения бактериальных сообществ (Sivakumar et al., 2020). Молекулярные методы обычно используют для выявления определенных патогенов или для нахождения всей грибной ДНК, содержащейся в листовой пластинке (Santamaría, Bayman, 2005; Duarte et al., 2016; Yao et al., 2019; Lazarević, Menkis, 2020; Dong et al., 2021). Если обрабатывать лишь смыв с поверхности (напр., Castro et al., 2022), появляются те же проблемы, что и в случае метода смывов: занос не эпифитных видов и пропуск видов, которые закреплены на листе. Кроме того, эти методы не позволяют отличить живые структуры от умерших, покоящиеся от активно растущих, хотя в этом случае могут применяться подходы, основанные на анализе РНК (Kemler et al., 2017). К сожалению, по отношению к грибам молекулярные методы имеют ряд ограничений, связанных в том числе с тем, что, например, к 2004 г. только 5–10% от общего числа грибов оценивались как известные, и далеко не для всех из них, а лишь для приблизительно 16% отсеквенированы последовательности ДНК (Hawksworth, 2004). В настоящий момент возможно, что процент известных грибов даже упал в связи с новыми оценками суммарного числа видов, основанными на молекулярных методах (Blackwell, 2011). Кроме того, основная последовательность, которая используется в молекулярном анализе грибов, ITS, также отсеквенирована лишь для ограниченного числа таксонов (Nilsson et al., 2006; Schoch et al., 2012). Большой проблемой является наличие ошибок в базах данных, число неправильно аннотированных последовательностей доходило до 20% (Nilsson et al., 2006). К ошибкам идентификации добавляются ошибки считывания и прочтения последовательностей и прочие возможные причины (Kirschner, 2015). Дополнительным ограничением является некоторая избирательность выбранных праймеров, что может приводить к смещению той картины разнообразия, которая наблюдается при применении молекулярных методов (Kemler et al., 2017).
Различные методы в итоге дают довольно сильно различающиеся результаты. Выделение в культуру выявляет лишь наиболее легко культивируемые виды, в то время как результаты молекулярных исследований сильно зависят от подбираемых праймеров, и зачастую группы грибов, которые ими выявляются, невозможно выявить другими методами (Kirschner, 2015). С другой стороны, среди выделяемых в культуру обычно есть гетерогенные виды и роды, для правильной идентификации которых необходимы молекулярные методы. Поэтому наилучшим подходом было бы использование нескольких методов, как прямых, так и косвенных, причем и культуральных, и молекулярных.
В целом, в большинстве опубликованных работ используют методы отпечатков, а также метод смывов. Методы прямых наблюдений, метод улавливания спор, а также молекулярные методы используются гораздо реже. Также стоит отметить, что, по-видимому, пока не разработано надежных методов количественной оценки, подходящих для грибов. Хотя можно попытаться оценить степень покрытия гифами поверхности листа, возникают большие сложности при изучении и сравнении обилия отдельных групп или видов.
РАЗНООБРАЗИЕ ГРИБОВ ФИЛЛОПЛАНЫ
Предполагается, что эпифитные и эндофитные грибы сопровождают растение с самого его выхода на сушу (Taylor et al., 2014). Так, задокументировано наличие по крайней мере двух находок ископаемых эпифитных грибов (Srivastava, 1993; Hübers et al., 2011). Судя по ископаемым находкам и данным молекулярных часов, построенных на основе последовательностей ДНК ряда семейств эпифитных грибов, оценивается, что эпифиты из совершенно разных групп появились на листьях по крайней мере в пермском периоде, около 298–252 млн лет назад (Hongsanan et al., 2016).
В настоящее время среди организмов филлопланы отмечено очень большое разнообразие грибов, среди которых лихенизированные грибы, фитопатогенные грибы и сапротрофные грибы филлопланы, которые и являются грибами-эпифитами sensu stricto. Лишайники или лихенизированные грибы, населяющие поверхность листьев, достаточно распространены в тропиках, где высокая влажность и длительный срок жизни зеленых листьев вкупе с высокой конкуренцией за субстрат создают благоприятные условия для развития эпифильной лихенобиоты (Pinokiyo et al., 2006). Среди паразитических грибов следует обратить внимание на возбудителей мучнистой росы (Erysiphales, Leotiomycetes, Ascomycota), для которых в типе характерен именно эктофитный мицелий, покрывающий густой сетью поверхность листа. Но с поверхности могут выделяться и многие паразитические грибы, развивающиеся в тканях и формирующие структуры размножения, выходящие наружу (Agrios, 2005). Последние, разумеется, было бы неверно рассматривать как эпифитные sensu stricto, но они наглядно демонстрируют проблему отнесения к той или иной группе. Можно легко выявить еще несколько спорных групп. Например, это гиперпаразитические грибы, такие как Ampelomyces quisqualis Ces., паразитирующий на мучнисторосяных грибах (Kiss et al., 2004), и Sphaerellopsis filum (Biv.) B. Sutton, развивающийся на ржавчинных (Płachecka, 2005).
Неоднозначное положение занимают так называемые сажистые грибы или грибы, вызывающие чернь листьев. Это различные представители отдела Ascomycota (преимущественно из порядков Capnodiales и Chaetothyriales), которые вызывают появление черного налета на листьях, который портит их внешний вид и закрывает доступ света к внутренним тканям. Эта группа приурочена к жарким странам тропиков и субтропиков, но встречается и севернее (Chomnunti et al., 2014). Считается, что данные грибы состоят в биотических связях с сосущими насекомыми, которые выделяют избытки сахаров в виде нектара, и, по-видимому, отсутствует какая-либо специфика в том, на каких видах растений их обнаруживают, т.е. они появляются там, где имеется достаточное количество питательных выделений тлей и других сосущих насекомых (Dixon, 1971; Jouraeva et al., 2006; Chomnunti et al., 2014). Поскольку при рассмотрении экологических групп грибов приоритет отдается их трофическому статусу, то, таким образом, сажистые грибы хотя и являются эпифитными по факту своего местообитания, но не являются специфическими грибами филлопланы, приспособленными существовать в условиях низкой влажности и нехватки питательных веществ, т.е. не являются грибами-эпифитами в строгом смысле этого слова. Зато данная группа наглядно демонстрирует реакцию на один из важнейших стрессирующих факторов грибов филлопланы – инсоляцию. Все сажистые грибы, что и отражено в их названии, имеют меланизированные клеточные стенки и гиф, и структур размножения.
Если рассматривать грибы-эпифиты sensu stricto, то в первую очередь это будут грибы с дрожжевой формой роста (Glushakova, Chernov, 2007, 2010; Schreiber et al., 2008, и др.). Дрожжи, подобно бактериям, способны формировать микроколонии, развиваясь в углублениях рельефа листа. Среди эпифитов выявляются преимущественно базидиальные дрожжи, которые характеризуются олиготрофностью и наличием баллистоспор и фотопротекторных пигментов, таких как каротиноиды и микоспорины (Kirschner, 2015). Наиболее часто отмечаются такие роды базидиальных дрожжей, как Cryptococcus и Sporobolomyces (Last, Deighton, 1965; Lamb, Brown, 1970; Schreiber et al., 2008; Воронин, 2010, и др.), но отмечаются и многие другие роды как сумчатых, так и базидиальных дрожжей, особенно в работах последних лет (Limtong, Kaewwichian, 2015; Sukmawati et al., 2015; Limtong, Nasanit, 2017; Srisuk et al., 2019). Работы методом смывов часто дают преобладание аскомицетных дрожжей (Castro et al., 2022), но детальные исследования подтверждают преобладание дрожжей именно базидиального аффинитета, что, в частности, связывают с большей распространенностью в этой группе фотопротекторных пигментов (Limtong, Nasanit, 2017; Srisuk et al., 2019).
В качестве эпифитов отмечены и некоторые мицелиальные грибы. Так как исследователи работали разными методами, с разными видами растений, в разных странах и в разные сезоны, то проводить обобщения довольно сложно. Но в целом можно сказать, что видовой состав эпифитных грибов достаточно слабо зависит от вида растения, хотя отдельные исключения здесь встречаются (Kemler et al., 2017; Yao et al., 2019; Pajares-Murgó et al., 2022). Большинство работ проводилось на покрытосеменных растениях, характерных для региона, где осуществлялось исследование. Работы, посвященные растениям других групп, немногочисленны (Abdel-Hafez, 1984; Legault et al., 1989; Lazarević, Menkis, 2020). Что касается покрытосеменных, то в поле зрения ученых попадали в первую очередь сельскохозяйственные растения, в основном злаки и бобовые. Работы по дикорастущим растениям крайне фрагментарны. Однако, несмотря на имеющиеся разнообразие и разноплановость работ, можно выявить несколько общих особенностей.
Изучение разнообразия эпифитных грибов молекулярными методами основано на анализе смывов с поверхности и обычно позволяет получить представление об основных классах грибов, среди которых ожидаемо преобладают классы отдела Ascomycota. Идентификация до вида в подобных работах обычно возможна только для некоторых фитопатогенных грибов, так как, как уже было сказано выше, в основном проводится анализ ITS (Dong et al., 2021; Pajares-Murgó et al., 2022). Облигатные фитопатогены выявляются и при работе культуральными методами, например, Ascochyta, Phomopsis, Phyllosticta (Osono, Mori, 2005; Воронин, 2010). В таком случае можно с высокой вероятностью предполагать, что на поверхности листьев споры или мицелий этих грибов присутствовали временно, в связи либо с активным периодом размножения патогена, находящегося в тканях, либо, наоборот, в связи с тем, что патоген только что попал на лист и еще не успел внедриться внутрь.
С другой стороны, иногда в списках отмечаются достаточно обычные сапротрофные грибы, такие как Aspergillus, Mucor, Penicillium, Rhizopus (Last, Deighton, 1965; Ruscoe, 1971; Santamaría, Bayman, 2005; Yusifova et al., 2017). Так как подобные виды обнаруживаются преимущественно в работах методами смывов или при работе молекулярно-генетическими методами, то можно предположить, что выявление этих обычных сапротрофных видов может быть связано не с их эпифитным образом жизни, а со случайным заносом спор на поверхность листа.
И все же можно выделить несколько родов, которые достаточно стабильно появляются в разных работах, независимо от вида растения и региона произрастания. Это Alternaria, Aureobasidium, Cladosporium, Epicoccum, Phoma, Trichoderma (Ruscoe, 1971; Santamaría, Bayman, 2005; Osono, 2008; Glenn et al., 2015; Yusifova et al., 2017; Janakiev et al., 2019; Pajares-Murgó et al., 2022, и др.). Все эти роды характеризуются широкой экологической амплитудой, часто отмечаются как почвенные или как грибы растительных остатков, хотя в ряде случаев могут переходить и к паразитизму на растениях. Важно отметить, что все эти грибы в той или иной степени характеризуются наличием фотопротекторных пигментов.
Aureobasidium pullulans (de Bary) G. Arnaud (Dothideales, Dothideomycetidae, Dothideomycetes, Pezizomycotina, Ascomycota) характеризуется способностью расти и в дрожжевой, и в мицелиальной форме, и что касается мицелиального роста, у этого гриба известны меланизированные формы. Кроме синтеза меланина, данный вид также известен как производитель полимера, названного “пуллулан”, который также обладает защитными свойствами по отношению к различным стрессирующим факторам и в настоящее время активно используется в промышленности (Liu et al., 2021).
Alternaria spp. (Pleosporales, Pleosporomycetidae, Dothideomycetes, Pezizomycotina, Ascomycota) и Cladosporium spp. (Capnodiales, Dothideomycetidae, Dothideomycetes, Pezizomycotina, Ascomycota) имеют темную окраску и мицелия, и конидий. Epicoccum nigrum Link формирует темноокрашенные многоклеточные споры. У видов р. Phoma темноокрашенный мицелий, а конидии, хотя и бесцветные, располагаются внутри специальных темноокрашенных споровместилищ – пикнид.
Более сложная ситуация наблюдается с р. Trichoderma. С одной стороны, конидии видов этого рода почти всегда имеют яркую окраску, обычно различных тонов зеленого, что обусловлено присутствием кроме меланина других фенольных соединений (Benítez et al., 1976), которые, возможно, тоже имеют защитные свойства. С другой стороны, хотя эти грибы способны развиваться сапротрофно, очень часто они обнаруживаются как антагонисты других грибов, в том числе как антагонисты р. Cladosporium (Barbosa et al., 2001). Таким образом, возможно, что выявление видов р. Trichoderma вместе с прочими эпифитными грибами носит вторичный характер.
АДГЕЗИЯ К ПОВЕРХНОСТИ ЛИСТА
Биологический цикл грибов, как правило, составляет последовательность стадий развития от споры до споры. Что касается эпифитных грибов, спора должна достигнуть субстрата – поверхности растения – и прорасти, далее последует стадия питания и роста и, наконец, формирование новых спор. Несмотря на кажущуюся простоту такого цикла, даже применительно к хорошо изученным группам грибов возникает ряд сложных и неоднозначных вопросов. Что же касается эпифитных грибов, то на настоящий момент исследований их биологического цикла не проводилось, хотя определенные представления о процессе имеются.
Начнем с момента попадания пропагулы гриба на субстрат – лист. Грибы способны производить огромное количество инокулюма, который распространяется с ветром, водой или насекомыми, но лишь малая его часть достигает нужного растения. Даже после того, как инокулюм достигнет поверхности листа или стебля, для спор существует риск быть удаленными с этой поверхности дождем или ветром, поэтому прежде, чем проникнуть в растение, спора должна прикрепиться. Прикрепление происходит за счет адгезии спор, при помощи специальных веществ, различных по составу. Адгезия пропагул к поверхности распространена во всех таксономических классах грибов, и особенно важную роль она выполняет у фитопатогенных грибов (Kuo, Hoch, 1996; Stanley et al., 2002), которым и посвящена значительная часть работ по адгезии спор. К сожалению, работ по специфике адгезии именно эпифитных грибов на настоящее время нет, поэтому далее мы кратко очертим некоторые особенности адгезии на примере фитопатогенных грибов как наиболее близкой и хорошо изученной экологической группы.
Пропагулы грибов на своей поверхности часто имеют слизистые вещества или выделяют их при намокании. Эти вещества представлены смесью нерастворимых полисахаридов, гликопротеидов, липидов, фибриллярных компонентов, которые при увлажнении становятся липкими, что способствует адгезии на поверхности растения-хозяина. Для обозначения этих веществ разные авторы используют такие слова, как “mucilage” (слизь) и “glue” (клей), не оговаривая особенности данных терминов, тем более что во многих случаях провести границу между этими понятиями довольно сложно. Эти клейкие вещества приклеивают споры к поверхности листа, что предотвращает их смывание. Не все пропагулы способны мгновенно прикрепиться, многие синтезируют клейкие вещества только после попадания на поверхность листа или после иного сигнала (Agrios, 2005).
Один и тот же организм может быть способен к адгезии в разные периоды своего жизненного цикла, а разные стадии могут иметь разные механизмы адгезии. Важно отметить, что клей лишь один из компонентов внеклеточного матрикса многих спор, а прикрепление лишь одна из функций этого матрикса (Epstein, Nicholson, 2016). В целом спора может прикрепляться к поверхности неспецифично при помощи различного состава клеев, либо специфично посредством рецепторов к поверхности или специальных взаимодействий с поверхностью растения-хозяина.
Как было сказано выше, поверхность органов растения покрыта гидрофобными кутикулой и воском, поэтому грибы чаще приспособлены к прикреплению на гидрофобные поверхности. Несмотря на это, часть грибов изменяет поверхность листа, выделяя кутиназы и неспецифические эстеразы, благодаря действию которых поверхность становится более гидрофильной, и в силу вступают ионные взаимодействия (Epstein, Nicholson, 1997).
Что касается механизма действия клея, видимо, многие клеи выделяются в жидкую среду на поверхность, там они полимеризуются и вытесняют воду, становясь нерастворимым материалом. Помимо собственно клеящих компонентов, в состав клея могут входить регулирующие соединения. Среди веществ, которые контролируют процесс полимеризации можно выделить пероксидазы и трансглутаминазы, которые, в свою очередь, могут зависеть от ионов Ca2+ (Epstein, Nicholson, 1997).
Можно выделить два признака, по которым различают грибные клеи, это состав приклеивающих веществ и необходимые для приклеивания условия. Преимущественно клеи представляют собой нерастворимые гликопротеины (Tucker, Talbot, 2001). Об этом говорит большое число косвенных методов (взаимодействие с антителами, лектинами, блокирование специфических метаболических путей и др.) (Epstein, Nicholson, 2016). Также в клеточной стенке спор находится множество белков, отличных от гликопротеинов, но способных участвовать в адгезии и узнавании поверхности. Наиболее интересные – низкомолекулярные белки гидрофобины, которые тоже претендуют на компонент адгезивного клея у некоторого количества грибов. Однако, например, для Magnaporthe oryzae B.C. Couch, вызывающего увядание риса, показано, что споры, выделяющие гидрофобины, прикрепляются так же успешно, как и мутанты, не синтезирующие эти соединения (Epstein, Nicholson, 1997). Гликозилфосфатидилинозитол-зависимые белки клеточной стенки (glycosylphosphatidylinositol cell-wall protein, GPI-CWP), участвующие в приклеивании, являются еще одним примером протеинов, опосредующих адгезию у грибов. В этом случае, видимо, запускается сигнальный каскад при контакте с поверхностью, что оказывается необходимым для адгезии пропагулы (Epstein, Nicholson, 1997).
По способу прикрепления к поверхности растения можно выделить две основные группы. Первая группа грибов имеет уже готовые соединения, которые после необходимого стимула тут же экскретируются и полимеризуются, приклеивая пропагулу к поверхности листа растения-хозяина, причем иногда довольно специфично. Например, подобная картина наблюдается у Venturia inaequalis (Cooke) G. Winter (Braun, Howard, 1994; Schumacher et al., 2008) и у Magnaporthe grisea (T.T. Hebert) M.E. Barr (Hamer et al., 1988; Tucker, Talbot, 2001). Вторая группа способна к адгезии к различным поверхностям, включая синтетические (стекло, полистерол), однако в этом случае необходимо время и энергетические затраты для синтеза клея, уже после касания с поверхностью. Здесь можно привести в пример возбудителя южной болезни листа кукурузы Cochliobolus heterostrophus (Drechsler) Drechsler и Nectria haematococca Berk. et Broome, споры которых способны прикрепляться практически к любым поверхностям, но только по прошествии достаточного времени контакта (Kwon, Epstein, 1993; Braun, Howard, 1994).
РАЗВИТИЕ НА ПОВЕРХНОСТИ ЛИСТА
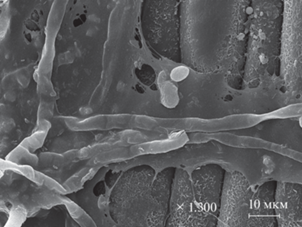
В тропических условиях эпифиты способны формировать настоящие биопленки (Andrews, Harris, 2000), но в отсутствие высокой влажности воздуха, по всей видимости, эпифитные грибы живут в виде микроскопических колоний, зачастую приуроченных к различным углублениям на поверхности листьев (Sivakumar et al., 2020). Тем не менее, несмотря на меньший масштаб, не достигающий формата настоящей биопленки, в комплексе с мицелиальными грибами происходит развитие и дрожжей, и бактерий, формируя тем самым своеобразное микросообщество (рис. 3). Для подобных скоплений было предложено использовать термин “агрегат” (“aggregate”); предполагается, что аналогично с биопленками способ роста в агрегатах (особенно при наличии слизистого матрикса) может способствовать защите отдельных клеток от высыхания (Andrews, Harris, 2000).
Рис. 3.
Мицелий и дрожжевые клетки на поверхности листа Dactylis glomerata L., сканирующая электронная микроскопия.
Предполагается, что в таких условиях проявляется так называемое чувство кворума, которое способствует совместной приспособленности эпифитных организмов (Andrews, Harris, 2000). “Чувство кворума” (“quorum sensing”) как термин было введено в 1994 г. (Fuqua et al., 1994), а как явление было описано еще раньше (Nealson et al., 1970) и заключается в способности бактерий обнаруживать плотность популяции клеток и реагировать на нее посредством изменения экспрессии ряда генов. В более широком значении чувство кворума – это механизм, основанный на плотности микробных клеток, который регулирует жизнедеятельность клеток, включая производство и секрецию факторов вирулентности, развитие защитных мембран или биопленок, темпы роста, морфологию, подвижность, споруляцию и пр. (Bacon, White, 2016). В то время как первые исследования были посвящены исключительно прокариотам, в настоящее время данное явление известно также и для многих грибов (Bacon, White, 2016; Mehmood et al., 2019). При развитии эпифитного агрегата, состоящего из комплекса разных организмов, чувство кворума позволяет регулировать сложные физиологические функции и обуславливать развитие симбиотических или паразитических отношений (Bacon, White, 2016). Важным составляющим чувства кворума в данном случае является “сигналинг” или “сигнализирование” (“signaling”), который может происходить и между разными видами, и сейчас имеется ряд публикаций, где указываются вещества, которые могут служить сигнальными молекулами для грибов, особенно в случае дрожжевого роста (Albuquerque, Casadevall, 2012; Bacon, White, 2016). Любопытно, что сигнальные вещества в одних случаях могут обеспечивать чувство кворума в сообществе для одних видов, но одновременно подавлять его для других видов. Например, эффектом подавления чувства кворума обладают пенициллиновые кислоты и патулин, выделяемые грибами р. Penicillium, и фузаровые кислоты, выделяемые грибами р. Fusarium. Фумонизин, выделяемый Fusarium verticillioides (Sacc.) Nirenberg, может как являться сигнальной молекулой, так и подавлять чувство кворума. Некоторые растения так же, как и микроорганизмы, способны выделять вещества, подавляющие чувство кворума или способствующие ему (Bacon, White, 2016).
Принципиальным вопросом развития грибов на листе растения будет источник питательных веществ. Здесь существуют два возможных варианта, не являющихся взаимоисключающими: грибы могут использовать выделения самого растения (см., напр., Sivakumar et al., 2020) или же частицы пыли, попадающие на растения. Второй вариант особенно вероятен при выраженном сложном рельефе поверхности и существовании углублений и волосков, способствующих задержке пыли и одновременно обеспечивающих микролокусы с повышенной влажностью, что также очень важно для развития микроорганизмов. Обе возможности вполне правдоподобны, и, вероятнее всего, оба варианта в дальнейшем будут подтверждены. Также необходимо добавить, что дополнительным источником могут служить выделения насекомых, что при массовом их развитии как раз может и привести к соответствующему массовому развитию грибов, формируя уже упомянутую выше чернь листьев (Chomnunti et al., 2014).
Хотя эпифиты развиваются преимущественно в виде микроколоний и вполне могут рассматриваться как комменсалы, тем не менее их развитие способно менять структуру поверхности листа (Epstein, Nicholson, 1997). Кроме того, эпифиты оказывают воздействие и на метаболизм растений, изменяя ферментативную активность (Goswami et al., 2019; Mitra et al., 2019) и выделение биогенных летучих органических соединений растением (Saunier et al., 2020). Но данное направление исследований сейчас находится только на начальном этапе, и здесь, вероятно, еще предстоит узнать много нового о взаимодействии растения и эпифитов.
Также остается открытым вопрос о формировании структур спороношения грибов филлопланы. Можно предположить, что в некоторых случаях происходит микроциклическое развитие грибов прямо на поверхности листьев, тем более что для многих видов грибов показана способность к так называемому “репетивному” прорастанию конидий, когда конидия формирует не вегетативный мицелий, а сразу конидиеносец со спорами. Также возможна и ситуация, когда развитие продолжается на отмерших листьях, и именно на растительных остатках происходит завершение цикла. Тем не менее, как уже было сказано, изучением биологического цикла эпифитных грибов “от споры до споры” на настоящий момент практически не занимались.
СЕЗОННАЯ ДИНАМИКА
Сообщество, которое присутствует на листе того или иного растения, не является постоянным, и можно наблюдать сукцессию и динамику эпифитных организмов в течение сезона. В целом исследователи обычно отмечают, что пик численности эпифитных организмов отмечается осенью (в теплых регионах – и зимой), а самые низкие значения – летом (Cabral, 1985; Osono, Mori, 2005; Glushakova, Chernov, 2007, 2010). Хотя, например, при изучении филлопланы Camellia japonica L. наибольшее число выделяемых грибов наблюдалось в мае (Osono, 2008), а при изучении эпифитных грибов филлопланы эвкалиптов Гонконга пик численности наблюдался в середине лета (Lee, Hyde, 2002). Так что особенности вида растения и климатические условия в месте его произрастания, несомненно, также имеют значение (Osono, 2008). Более подробно этот вопрос изучен для эпифитных дрожжей, и в зависимости от характерной сезонной динамики Глушаковой и Черновым было предложено выделять три группы растений (Glushakova, Chernov, 2007).
Первая группа показывает рост численности в течение вегетационного сезона с пиком ранней зимой и спадом к весне. Такой характер изменений был отмечен для эпифитов гигрофитных и мезофитных растений, листья которых остаются зелеными в течение зимы. Авторы связывают это с тем, что весной появляются новые листья с толстой кутикулой и малым количеством питательных экссудатов на их поверхности, затем к осени листья стареют, и кутикула становится менее целостной. Это приводит к повышению количества доступных для дрожжей сахаров, и их количество резко возрастает, но затем, по мере истощения питательных компонентов, к весне их количество снова снижается. Вторая группа характеризуется отсутствием значимых различий в численности дрожжей в течение года, что отмечено для филлопланы ксерофитных вечнозеленых трав. Это может быть связано с особенностями строения кутикулы таких ксерофитных растений. Наконец, однолетние и эфемерные растения, листья которых завершали свой цикл развития до наступления зимы, формируют третью группу. Для них характерно резкое повышение числа дрожжей в филлоплане в конце вегетации. В этом случае в конце вегетативного периода листья уже подлежат разложению, что сопровождается увеличением числа дрожжей (Glushakova, Chernov, 2007).
Что касается видового состава, то он часто остается постоянным на протяжении всего периода исследований, хотя относительная представленность различных групп может меняться. В целом, на молодых листьях бактерии являются доминирующей группой, однако по мере роста и старения листа главными доминантами становятся сначала дрожжи, а потом и мицелиальные грибы (Kirschner, 2015). Показатели видового разнообразия, а именно индекс Шеннона, указывают на постепенное увеличение разнообразия грибов с пиком в осенний период (Glushakova, Chernov, 2010). Также, помимо разнообразия грибного сообщества, к концу сезона обычно растет и его выровненность, т.е. растет не просто количество редко встречаемых видов, а усложняется структура сообщества с увеличением числа таксонов со сравнимой представленностью (Gobbi et al., 2020). Отдельные таксоны грибов при этом могут иметь собственную динамику встречаемости, например, р. Alternaria показывал пик развития в середине сезона (Jumpponen, Jones, 2010), а для Aureobasidium pullulans максимум был отмечен в августе (Osono, 2008).
Помимо сезонных изменений, ряд авторов уделяли внимание возрасту листа и его влиянию на состав эпифитной микобиоты. У некоторых грибов наблюдалась зависимость от возраста листа, например, частота встречаемости Pestalotiopsis sp. и Trichoderma viride Pers. была выше на молодых листьях (Osono, Mori, 2005). Зависимость от возраста листьев также показал Cladosporium cladosporioides – чем старше был лист, тем выше была частота встречаемости (Osono, 2008). Такой гриб, как Alternaria alternata (Fr.) Keissl., показал минимальную зависимость от сезонных изменений, однако его встречаемость на старых листьях была значительно выше, чем на молодых (Cabral, 1985). В целом, наибольшее разнообразие наблюдается на зрелых листьях, по сравнению с молодыми и стареющими листьями (Osono, 2006; Mulka et al., 2019).
БИОТЕХНОЛОГИЧЕСКИЙ ПОТЕНЦИАЛ ЭПИФИТНЫХ ГРИБОВ
В связи с тем, что филлоплана является экстремальным местообитанием, она является средой, имеющей высокий потенциал для поиска продуцентов веществ, полезных в биотехнологии, а также биологических агентов контроля других, патогенных организмов (Kirschner, 2015), что в настоящее время уже подтверждено для эпифитных бактерий (Helfrich et al., 2018). Обитатели филлосферы могут иметь потенциал в производстве веществ, стимулирующих рост растений, включая растительные гормоны, ингибирующих рост патогенов или индуцирующих иммунитет растения-хозяина, а также имеющих полезные свойства, для применения в медицине и косметологии (Sivakumar et al., 2020).
Как было сказано выше, эпифитные грибы способны влиять на состояние листа, на котором они развиваются. Более того, показано, что они могут индуцировать его устойчивость к другим, уже патогенным организмам. Например, показано, что присутствие в филлоплане Ipomoea cairica (Convolvulaceae) непатогенных изолятов Fusarium oxysporum и F. fujikuroi приводит к повышению продукции салициловой и жасминовой кислот, являющихся важными сигнальными молекулами, опосредующими экспрессию многих генов устойчивости, в том числе PR-генов, кодирующих PR-белки (pathogenesis-related proteins), предназначенные для борьбы с инфекцией (Xu et al., 2020). Для многих эпифитных грибов показана способность синтезировать растительные гормоны. Такие аскомицетные дрожжи, как Debaryomyces hansenii и Metschnikowia pulcherrima, а также базидиальные дрожжи Cystofilobasidium capitatum, Rhodotorula mucilaginosa и другие, способны производить индол-3-уксусную кислоту (Kemler et al., 2017), некоторые штаммы Sporobolomyces roseus продуцируют фитогормон зеатин (Streletskii et al., 2019), а также многие изоляты способны к продукции ауксинов (Pirog et al., 2018).
Многие культуры грибов, выделенных с филлопланы, проявляют антагонистические свойства по отношению к возбудителям болезней растений (Kharwar et al., 2010; Берестецкий и др., 2014). Например, представители базидиальных дрожжей, Pseudozyma flocculosa и P. aphidis, подавляют развитие мучнисторосяных грибов, а P. churashimaensis подавляет развитие патогенной бактерии Xanthomonas sp. (Kitamoto, 2019). При изучении комплексов эпифитных дрожжей, выделяемых с поверхности различных растений, всегда обнаруживается хотя бы несколько изолятов, обладающих антагонистической активностью против патогенов соответствующего вида растения (Indratmi, 2018; Muhibuddin et al., 2018; Into et al., 2020). Для одного из наиболее часто встречаемых в филлоплане грибов, Aureobasidium spp., включая A. pullulans, была показана способность подавлять развитие гриба Aspergillus flavus (Sukmawati et al., 2020). Такой типичный представитель филлопланы, как Cladosporium cladosporioides, а также и некоторые другие эпифитные грибы, выделенные с поверхности листьев риса, способны подавлять рост целого ряда фитопатогенных грибов, поражающих рис (Mardani, Hadiwiyono, 2018; Chaibub et al., 2020). Штаммы Trichoderma hamatum и Aspergillus sp., изолированные с листьев Stevia rebaudiana, которая является натуральным низкокалорийным подсластителем, отлично проявили себя против гриба Rhizoctonia solani, часто поражающего это растение (Chauhan, Gautam, 2019). Среди эпифитов, выделенных с листьев Rauwolfia serpentina, был выявлен штамм Trichoderma harzianum, обладающий антагонистическим действием по отношению к патогену данного кустарника (Thakur, Harsh, 2014). Способность к защите растения от болезней обнаруживается и у представителей Epicoccum sp. (Wittig et al., 1997; Waill, Ghoson, 2018). Что важно с точки зрения перспектив, среди изолятов эпифитных грибов часто можно выявить штаммы, обладающие антагонистической активностью по отношению и к патогенам других растений, и к патогенам человека (Берестецкий и др., 2014; Shara et al., 2023).
Какие именно вещества могут обеспечивать антагонистическую активность различных эпифитов, практически неизвестно. Хотя имеются отдельные публикации, где описывается антимикробное действие различных соединений, полученных из культур эпифитных грибов, при более внимательном изучении материалов исследования часто оказывается, что работа проводилась не с эпифитами sensu stricto (Zhu et al., 2011; González-Montiel et al., 2020). Достаточно подробные исследования ведутся по эпифитам риса, и здесь действительно не только показан антагонизм эпифитов по отношению к патогенам, но и выявляется возможный спектр химических соединений, ответственных за подобное действие. Но на настоящий момент имеются данные преимущественно по литическим ферментам (протеазы, хитиназы и пр.), которые могут подавлять рост фитопатогенных грибов и бактерий (Chaibub et al., 2020; Into et al., 2020). Так что в этой области сейчас идет процесс накопления первичных данных, и, вероятно, в будущем можно ожидать описания новых соединений.
Что касается грибных ферментов, то они в целом имеют широкое применение в самых разных областях человеческой деятельности, так что работы по ферментативному комплексу различных грибов ведутся независимо от нацеленности на антифунгальный эффект. И исследования эпифитных грибов здесь тоже довольно перспективны. Например, для ряда дрожжей было показано наличие высокой продукции белков, разлагающих различные виды сахаров, что также может являться мишенью для биотехнологического производства (Camargo et al., 2018). Эпифитные дрожжи Pseudozyma antarctica показали способность к продукции большого количества липаз (Kitamoto, 2019).
Еще одна группа перспективных соединений – это биосурфактанты, поверхностно активные вещества биогенного происхождения, обладающие способностью влиять на поверхностное натяжение на разделе термодинамических фаз. Эти соединения нашли свое применение, например, в производстве моющих средств, косметики, а также в ряде промышленных отраслей и здравоохранении. Вещества данной группы, а именно маннозилэритрит-липиды, описаны для ряда эпифитных дрожжей р. Pseudozyma, а продукт P. tsukubaensis даже был коммерциализирован с целью производства косметических средств (Kitamoto, 2019).
Таким образом, спектр веществ, производимых эпифитными грибами, изучен весьма слабо, но даже предварительные данные показывают высокий потенциал данной группы.
ЗАКЛЮЧЕНИЕ
Подводя итоги – филлоплана растений представляет своеобразную экологическую нишу, активно осваиваемую различными мицелиальными и дрожжевыми грибами разных таксономических групп. Среди грибов, которые можно выявить на поверхности растений, имеются сапротрофные виды, которые способны развиваться в подобных сложных условиях среды – высокой инсоляции, низкой влажности и нехватке питательных веществ. Мы предлагаем использовать термин “эпифитные грибы” именно для этой своеобразной экологической группы, важность которой в настоящее время уже общепризнана. Эти эпифитные грибы могут играть важную роль в жизни растения-хозяина, влияя на его метаболизм и проявляя антагонистические действия по отношению к грибам паразитическим. Кроме того, эпифитные грибы, скорее всего, являются важным начальным этапом последующего разложения листового опада, участвуя в цикле круговорота веществ. С практической точки зрения грибы, обитающие в филлоплане и подвергающиеся комплексу стрессирующих факторов, могут являться потенциальным источником важных биологических веществ, тем самым имея значительный потенциал для биотехнологии.
Список литературы
Багирова С.Ф., Джавахия В.Г., Дьяков Ю.Т., Озерецковская О.Л., Проворов Н.А. и др., 2012. Фундаментальная фитопатология. М.: КРАСАНД. 508 с.
Берестецкий А.О., Гасич Е.Л., Полуэктова Е.В., Николаева Е.В., Сокорнова С.В., Хлопунова Л.Б., 2014. Биологическая активность грибов филлосферы сорных и дикорастущих травянистых растений // Микробиология. Т. 83. № 5. С. 534–542. https://doi.org/10.7868/S002636561405005X
Благовещенская Е.Ю., 2015. Методы выявления грибов филлопланы // Мат-лы VII Всеросс. микологической шк.-конф. с междунар. участием “Биотические связи грибов: мосты между царствами”. Сб. докл. и тез. М.: МГУ. С. 5–9.
Благовещенская Е.Ю., 2017. Влияние повторностей разных типов на выявление эпифитных микромицетов // Современная микология в России. Т. 6. М.: Национальная академия микологии. С. 361–363.
Возняковская Ю.М., 1969. Микрофлора растений и урожай. Л.: Колос. 240 с.
Воронин Л.В., 2010. Грибы филлопланы Nuphar lutea (L.) Smith в малых реках бассейна рыбинского водохранилища // Ярославский пед. вестн. Т. 3. С. 91–95.
Глазунова Н.Н., Романенко Е.С., Шипуля А.Н., 2011. Видовой состав микромицетов надземной части растений озимой пшеницы в разные фазы ее онтогенеза на Ставрополье // Науч. журн. КубГАУ. № 68. С. 1–13.
Ерина Н.В., Коптева Т.С., 2015a. Видовой состав эпифитной микрофлоры некоторый растений семейства Grossulariaceae и различные типы их взаимодействий // Науч. журн. КубГАУ. № 114. С. 1–9.
Ерина Н.В., Коптева Т.С., 2015б. Микробные сообщества некоторых растений семейства Grossulariaceae // Науч. журн. КубГАУ. № 110. С. 1–12.
Лотова Л.И., 2001. Ботаника. Морфология и анатомия высших растений. М.: УРСС. 528 с.
Abdel-Hafez S.I.I., 1984. Rhizosphere and phyllosphere fungi of four fern plants growing in Saudi Arabia // Mycopathologia. V. 85. № 1–2. P. 45–52. https://doi.org/10.1007/BF00436701
Agrios G.N., 2005. Plant Pathology. 5th eds. Amsterdam: Elsevier Academic Press. 952 p.
Albuquerque P., Casadevall A., 2012. Quorum sensing in fungi – a review // Med. Mycol. V. 50. P. 337–345. https://doi.org/10.3109/13693786.2011.652201
Andrews J.H., Harris R.F., 2000. The ecology and biogeography of microorganisms on plant surfaces // Annu. Rev. Phytopathol. V. 38. P. 145–180. https://doi.org/10.1146/annurev.phyto.38.1.145
Bacon C.W., White J.F., 2016. Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants // Symbiosis. V. 68. № 1–3. P. 87–98. https://doi.org/10.1007/s13199-015-0350-2
Barbosa M.A.G., Rehn K.G., Menezes M., Mariano R.L.R., 2001. Antagonism of Trichoderma species on Cladosporium herbarum and their enzimatic characterization // Braz. J. Microbiol. V. 32. P. 98–104. https://doi.org/10.1590/S1517-83822001000200005
Barthlott W., Neinhuis C., Cutler D., Ditsch F., Meusel I. et al., 1998. Classification and terminology of plant epicuticular waxes // Bot. J. Linn. Soc. V. 126. P. 237–260. https://doi.org/10.1111/j.1095-8339.1998.tb02529.x
Benítez T., Villa T.G., García Acha I., 1976. Some chemical and structural features of the conidial wall of Trichoderma viride // Can. J. Microbiol. V. 22. P. 318–321. https://doi.org/10.1139/m76-046
Bills G.F., Polishook J.D., 1991. Microfungi from Carpinus caroliniana // Can. J. Bot. V. 69. P. 1477–1482. https://doi.org/10.1139/b91-191
Blackwell M., 2011. The Fungi: 1, 2, 3… 5.1 million species? // Am. J. Bot. V. 98. № 3. P. 426–438. https://doi.org/10.3732/ajb.1000298
Bopaih B.M., Wani S.P., Rai P.V., 1978. Phyllosphere microflora of some common plants // Mysore J. Agric. Sci. V. 12. P. 398–403.
Braun E.J., Howard R.J., 1994. Adhesion of fungal spores and germlings to host plant surfaces // Protoplasma. V. 181. P. 202–212. https://doi.org/10.1007/BF01666396
Burton Z., Bhushan B., 2006. Surface characterization and adhesion and friction properties of hydrophobic leaf surfaces // Ultramicroscopy. V. 106. P. 709–719. https://doi.org/10.1016/j.ultramic.2005.10.007
Cabral D., 1985. Phyllosphere of Eucalyptus viminalis: Dynamics of fungal populations // Trans. Br. Mycol. Soc. V. 85. P. 501–511. https://doi.org/10.1016/S0007-1536(85)80047-4
Camargo J.Z., Nascimento V.M., Stefanello I., Andrade Silva C.A., de, Gonçalves F.A. et al., 2018. Biochemical evaluation, molecular characterization and identification of novel yeast strains isolated from Brazilian savannah fruits, chicken litter and a sugar and alcohol mill with biotechnological potential for biofuel and food industries // Biocatal. Agric. Biotechnol. V. 16. P. 390–399. https://doi.org/10.1016/j.bcab.2018.09.006
Castro J., Costa D., Tavares R.M., Baptista P., Lino-Neto T., 2022. Olive fungal epiphytic communities are affected by their maturation stage // Microorganisms. V. 10. Art. 376. https://doi.org/10.3390/microorganisms10020376
Chaibub A.A., Sousa T.P., de, Araújo L.G., de, Filippi M.C.C., de, 2020. Molecular and morphological characterization of rice phylloplane fungi and determination of the antagonistic activity against rice pathogens // Microbiol. Res. V. 231. Art. 126353. https://doi.org/10.1016/j.micres.2019.126353
Chauhan R., Gautam S.S., 2019. Potential antagonistic phylloplane fungi from Stevia rebaudiana Bert. as bio-control of aerial blight disease caused by Rhizoctonia solani // Indian Phytopathol. V. 72. № 1. P. 177–180. https://doi.org/10.1007/s42360-019-00116-x
Chomnunti P., Hongsanan S., Aguirre-Hudson B. et al., 2014. The sooty moulds // Fungal Divers. V. 66. P. 1–36. https://doi.org/10.1007/s13225-014-0278-5
Copeland J.K., Yuan L., Layeghifard M., Wang P.W., Guttman D.S., 2015. Seasonal community succession of the phyllosphere microbiome // Mol. Plant Microbe Interact. V. 28. № 3. P. 274–285. https://doi.org/10.1094/MPMI-10-14-0331-FI
Dabrowska A., 2012. Morpho-anatomical structure of the leaves of Festuca trachyphylla (Hack.) Krajina in the ecological aspect // Modern Phytomorphol. V. 1. P. 19–22. https://doi.org/10.5281/zenodo.162713
Dickinson C.H., 1973. Effects of ethirimol and zineb on phylloplane microflora of barley // Trans. Br. Mycol. Soc. V. 60. P. 423–431. https://doi.org/10.1016/S0007-1536(73)80027-0
Dickinson C.H., Watson J., Wallace B., 1974. An impression method for examining epiphytic micro-organisms and its application to phylloplane studies // Trans. Br. Mycol. Soc. V. 63. P. 616–619. https://doi.org/10.1016/S0007-1536(74)80118-X
Dickison W.C., 2000. Integrative Plant Anatomy. San Diego: Academic Press. 533 p.
Diem H.G., 1974. Micro-organisms of the leaf surface: Estimation of the mycoflora of the barley phyllosphere // Microbiology. V. 80. P. 77–83. https://doi.org/10.1099/00221287-80-1-77
Dixon A.F.G., 1971. The role of aphids in wood formation. II. The effect of the lime aphid, Eucallipterus tiliae L. (Aphididae), on the growth of lime, Tilia x vulgaris Hayne // J. Appl. Ecol. V. 8. P. 393–399. https://doi.org/10.2307/2402878
Dong C., Wang L., Li Q., Shang Q., 2021. Epiphytic and endophytic fungal communities of tomato plants // Hortic. Plant J. V. 7. № 1. P. 38–48. https://doi.org/10.1016/j.hpj.2020.09.002
Duarte L.L., Santos F.M.C., Barreto R.W., 2016. Mycobiota of the weed Conyza canadensis (Asteraceae) in Brazil // Fungal Biol. V. 120. P. 1118–1134. https://doi.org/10.1016/j.funbio.2016.05.015
Epstein L., Nicholson R.L., 1997. Adhesion of spores and hyphae to plant surfaces // Plant Relationships. The Mycota. V. 5 / Eds Carroll G.C., Tudzynski P. Berlin; Heidelberg: Springer. P. 11–25. https://doi.org/10.1007/978-3-662-10370-8_2
Epstein L., Nicholson R., 2016. Adhesion and adhesives of fungi and oomycetes // Biological Adhesives / Eds Smith A.M., Callow J.A. Berlin; Heidelberg: Springer. P. 25–55. https://doi.org/10.1007/978-3-540-31049-5_3
Falconi C.J., Mendgen K., 1994. Epiphytic fungi on apple leaves and their value for control of the postharvest pathogens Botrytis cinerea, Monilinia fructigena and Penicillium expansum // J. Plant Dis. Prot. V. 101. P. 38–47.
Fernández V., Guzmán-Delgado P., Graça J., Santos S., Gil L., 2016. Cuticle structure in relation to chemical composition: Re-assessing the prevailing model // Front. Plant Sci. V. 7. Art. 427. https://doi.org/10.3389/fpls.2016.00427
Fiss M., Kucheryava N., Schönherr J., Kollar A., Arnold G., Auling G., 2000. Isolation and characterization of epiphytic fungi from the phyllosphere of apple as potential biocontrol agents against apple scab (Venturia inaequalis) // J. Plant Dis. Prot. V. 107. № 1. P. 1–11.
Fuqua W.C., Winans S.C., Greenberg E.P., 1994. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators // J. Bacteriol. V. 176. P. 269–275. https://doi.org/10.1128/jb.176.2.269-275.1994
Glenn D.M., Bassett C., Dowd S.E., 2015. Effect of pest management system on ‘Empire’ apple leaf phyllosphere populations // Sci. Hortic. V. 183. P. 58–65. https://doi.org/10.1016/j.scienta.2014.12.009
Glushakova A.M., Chernov I.Y., 2007. Seasonal dynamic of the numbers of epiphytic yeasts // Microbiology. V. 76. № 5. P. 590–595. https://doi.org/10.1134/S0026261707050128
Glushakova A.M., Chernov I.Y., 2010. Seasonal dynamics of the structure of epiphytic yeast communities // Microbiology. V. 79. № 6. P. 830–839. https://doi.org/10.1134/S0026261710060160
Gobbi A., Kyrkou I., Filippi E., Ellegaard-Jensen L., Hansen L.H., 2020. Seasonal epiphytic microbial dynamics on grapevine leaves under biocontrol and copper fungicide treatments // Sci. Rep. V. 10. Art. 681. https://doi.org/10.1038/s41598-019-56741-z
González-Montiel G.A., Kaweesa E.N., Feau N., Hamelin R.C., Stone J.K., Loesgen S., 2020. Chemical, bioactivity, and biosynthetic screening of epiphytic fungus Zasmidium pseudotsugae // Molecules. V. 25. № 10. Art. 2358. https://doi.org/10.3390/molecules25102358
Goswami S., Paul P.K., Sharma P.D., 2019. Aspergillus niger, a dominant phylloplane coloniser, influences the activity of defense enzymes in Solanum lycopersicum // J. Plant Prot. Res. V. 59. P. 512–518. https://doi.org/10.24425/jppr.2019.131265
Hallett I.C., Boyd-Wilson K.S.H., Everett K.R., 2010. Microscope methods for observation of the phylloplane flora // N. Z. Plant Prot. V. 63. P. 15–23. https://doi.org/10.30843/nzpp.2010.63.6608
Hamer J.E., Howard R.J., Chumley F.G., Valent B., 1988. A mechanism for surface attachment in spores of a plant pathogenic fungus // Science. V. 239. № 4837. P. 288–290. https://doi.org/10.1126/science.239.4837.288
Hawksworth D.L., 2004. Fungal diversity and its implications for genetic resource collections // Stud. Mycol. V. 50. № 1. P. 9–18.
Helfrich E.J.N., Vogel C.M., Ueoka R., Schäfer M., Ryffel F. et al., 2018. Bipartite interactions, antibiotic production and biosynthetic potential of the Arabidopsis leaf microbiome // Nat. Microbiol. V. 3. № 8. P. 909–919. https://doi.org/10.1038/s41564-018-0200-0
Hongsanan S., Sánchez-Ramírez S., Crous P.W., Ariyawansa H.A., Zhao R.L., Hyde K.D., 2016. The evolution of fungal epiphytes // Mycosphere. V. 7. № 11. P. 1690–1712. https://doi.org/10.5943/mycosphere/7/11/6
Hübers M., Bomfleur B., Krings M., Kerp H., 2011. An early carboniferous leaf-colonizing fungus // N. Jb. Geol. Paläont. Abh. V. 261. № 1. P. 77–82. https://doi.org/10.1127/0077-7749/2011/0150
Indratmi D., 2018. Biological control of chili anthracnose disease with Rhodotorula spp. // 4th International Conference on Food, Agriculture and Natural Resources (FANRes 2018). Atlantis Press. P. 112–116. https://doi.org/10.2991/fanres-18.2018.22
Into P., Khunnamwong P., Jindamoragot S., Am-in S., Intanoo W., Limtong S., 2020. Yeast associated with rice phylloplane and their contribution to control of rice sheath blight disease // Microorganisms. V. 8. № 3. Art. 362. https://doi.org/10.3390/microorganisms8030362
Janakiev T., Dimkić I., Unković N., Ljaljević Grbić M., Opsenica D. et al., 2019. Phyllosphere fungal communities of plum and antifungal activity of indigenous phenazine-producing Pseudomonas synxantha against Monilinia laxa // Front. Microbiol. V. 10. Art. 2287. https://doi.org/10.3389/fmicb.2019.02287
Jouraeva V.A., Johnson D.L., Hassett J.P., Nowak D.J., Shipunova N.A., Barbarossa D., 2006. Role of sooty mold fungi in accumulation of fine-particle-associated PAHs and metals on deciduous leaves // Environ. Res. V. 102. № 3. P. 272–282. https://doi.org/10.1016/j.envres.2006.06.004
Jumpponen A., Jones K.L., 2010. Seasonally dynamic fungal communities in the Quercus macrocarpa phyllosphere differ between urban and nonurban environments // New Phytol. V. 186. P. 496–513. https://doi.org/10.1111/j.1469-8137.2010.03197.x
Kemler M., Witfeld F., Begerow D., Yurkov A., 2017. Phylloplane yeasts in temperate climates // Yeasts in Natural Ecosystems: Diversity / Eds Buzzini P., Lachance M.A., Yurkov A. Cham: Springer. P. 171–197. https://doi.org/10.1007/978-3-319-62683-3_6
Kharwar R.N., Gond S.K., Kumar A., Mishra A., 2010. A comparative study of endophytic and epiphytic fungal association with leaf of Eucalyptus citriodora Hook., and their antimicrobial activity // World J. Microbiol. Biotechnol. V. 26. P. 1941–1948. https://doi.org/10.1007/s11274-010-0374-y
Kirschner R., 2015. Fungi on the leaf – a contribution towards a review of phyllosphere microbiology from the mycological perspective // Biodiversity and Ecology of Fungi, Lichens, and Mosses. Kerner von Marilaun Workshop 2015 in memory of Josef Poelt. Wien: Austrian Academy of Sciences Press. P. 433–448.
Kiss L., Russell J.C., Szentiványi O., Xu X., Jeffries P., 2004. Biology and biocontrol potential of Ampelomyces mycoparasites, natural antagonists of powdery mildew fungi // Biocontrol Sci. Technol. V. 14. № 7. P. 635–651. https://doi.org/10.1080/09583150410001683600
Kitamoto H., 2019. The phylloplane yeast Pseudozyma: A rich potential for biotechnology // FEMS Yeast Res. V. 19. № 5. Art. foz053. https://doi.org/10.1093/femsyr/foz053
Kolyva F., Stratakis E., Rhizopoulou S., Chimona C., Fotakis C., 2012. Leaf surface characteristics and wetting in Ceratonia siliqua L. // Flora Morphol. Distrib. Funct. Ecol. Plants. V. 207. P. 551–556. https://doi.org/10.1016/j.flora.2012.06.001
Kuo K., Hoch H.C., 1996. Germination of Phyllosticta ampelicida pycnidiospores: Prerequisite of adhesion to the substratum and the relationship of substratum wettability // Fungal Genet. Biol. V. 20. P. 18–29. https://doi.org/10.1006/fgbi.1996.0005
Kwon Y.H., Epstein L., 1993. A 90-kDa glycoprotein associated with adhesion of Nectria haematococca macroconidia to substrata // Mol. Plant Microbe Interact. V. 6. № 4. P. 481–487.
Lamb R.J., Brown J.F., 1970. Non-parasitic microflora on leaf surfaces of Paspalum dilatatum, Salix babylonica and Eucalyptus stellulata // Trans. Br. Mycol. Soc. V. 55. P. 383–390. https://doi.org/10.1016/S0007-1536(70)80059-6
Last F.T., Deighton F.C., 1965. The non-parasitic microflora on the surfaces of living leaves // Trans. Br. Mycol. Soc. V. 48. P. 83–99. https://doi.org/10.1016/S0007-1536(65)80011-0
Lazarević J., Menkis A., 2020. Fungal diversity in the phyllosphere of Pinus heldreichii H. Christ – an endemic and high-altitude pine of the Mediterranean Region // Diversity. V. 12. № 5. Art. 172. https://doi.org/10.3390/d12050172
Lee O.H.K., Hyde K.D., 2002. Phylloplane fungi in Hong Kong mangroves: Evaluation of study methods // Mycologia. V. 94. № 4. P. 596–606.
Legault D., Dessureault M., Laflamme G., 1989. Mycoflora of Pinus banksiana and Pinus resinosa needles. II. Epiphytic fungi // Can. J. Bot. V. 67. P. 2061–2065. https://doi.org/10.1139/b89-260
Limtong S., Kaewwichian R., 2015. The diversity of culturable yeasts in the phylloplane of rice in Thailand // Ann. Microbiol. V. 65. P. 667–675. https://doi.org/10.1007/s13213-014-0905-0
Limtong S., Nasanit R., 2017. Phylloplane yeasts in tropical climates // Yeasts in Natural Ecosystems: Diversity / Eds Buzzini P., Lachance M.A., Yurkov A. Cham: Springer. P. 199–223. https://doi.org/10.1007/978-3-319-62683-3_7
Liu F., Zhang J., Zhang L., Diao M., Ling P., Wang F., 2021.Correlation between the synthesis of pullulan and melanin in Aureobasidium pullulans // Int. J. Biol. Macromol. V. 177. P. 252–260. https://doi.org/10.1016/j.ijbiomac.2021.02.108
Mardani A., Hadiwiyono, 2018. Antagonism of rice phylloplane fungi against Cercospora oryzae // IOP Conf. Ser. Earth Environ. Sci. V. 142. Art. 012064. https://doi.org/10.1088/1755-1315/142/1/012064
Mechaber W.L., Marshall D.B., Mechaber R.A., Jobe R.T., Chew F.S., 1996. Mapping leaf surface landscapes // Proc. Natl. Acad. Sci. V. 93. P. 4600–4603. https://doi.org/10.1073/pnas.93.10.4600
Mehmood A., Liu G., Wang X., Meng G., Wang C., Liu Y., 2019. Fungal quorum-sensing molecules and inhibitors with potential antifungal activity: A review // Molecules. V. 24. № 10. Art. 1950. https://doi.org/10.3390/molecules24101950
Mitra J., Sharma P.D., Paul P.K., 2019. Do phylloplane microfungi influence activity of Rubisco and Carbonic anhydrase? // S. Afr. J. Bot. V. 124. P. 118–126. https://doi.org/10.1016/j.sajb.2019.04.033
Muhibuddin A., Fadhilah S., Sektiono A.W., Qomariyah U.K.N., Faizah M. et al., 2018. Yeast from epiphyte of avocadoes to control Colletotrichum gloesporioides causing antrachnose disease // SAINTEKBU. V. 10. № 2. P. 52–60. https://doi.org/10.32764/saintekbu.v10i2.208
Mukhtar I., Mushtaq S., Ali A., Khokhar I., 2010. Epiphytic and endophytic phyllosphere microflora of Cassytha filiformis L. and its hosts // Ecoprint: Int. J. Ecol. V. 17. P. 1–8. https://doi.org/10.3126/eco.v17i0.4096
Mukhtar I., Mushtaq S., Ali A., Khokhar I., 2012. Phyllospheric microflora of Cuscuta pedicillata Ledeb and its host Trifolium alexandrium L. // Sarhad J. Agric. V. 28. P. 437–441.
Mulka N., Begum S.R., Surekha M., 2019. Succession of phylloplane mycoflora of transgenic bt cotton (JKCH 8836 BG II) // J. Pharmacogn. Phytochem. V. 8. P. 436–438.
Nealson K.H., Platt T., Hastings J.W., 1970. Cellular control of the synthesis and activity of the bacterial luminescent system // J. Bacteriol. V. 104. P. 313–322. https://doi.org/10.1128/jb.104.1.313-322.1970
Neinhuis C., Barthlott W., 1997. Characterization and distribution of water-repellent, self-cleaning plant surfaces // Ann. Bot. V. 79. P. 667–677. https://doi.org/10.1006/anbo.1997.0400
Nilsson R.H., Ryberg M., Kristiansson E., Abarenkov K., Larsson K.H., Kõljalg U., 2006. Taxonomic reliability of DNA sequences in public sequence databases: A fungal perspective // PloS One. V. 1. № 1. Art. e59. https://doi.org/10.1371/journal.pone.0000059
Norms R.F., Bukovac M.J., 1968. Structure of the pear leaf cuticle with special reference to cuticular penetration // Am. J. Bot. V. 55. P. 975–983. https://doi.org/10.1002/j.1537-2197.1968.tb07457.x
Osono T., 2006. Role of phyllosphere fungi of forest trees in the development of decomposer fungal communities and decomposition processes of leaf litter // Can. J. Microbiol. V. 52. P. 701–716. https://doi.org/10.1139/w06-023
Osono T., 2008. Endophytic and epiphytic phyllosphere fungi of Camellia japonica: Seasonal and leaf age-dependent variations // Mycologia. V. 100. P. 387–391. https://doi.org/10.3852/07-110R1
Osono T., Mori A., 2005. Seasonal and leaf age-dependent changes in occurrence of phyllosphere fungi of giant dogwood // Mycoscience. V. 46. P. 273–279. https://doi.org/10.1007/S10267-005-0246-8
Pajares-Murgó M., Garrido J.L., Perea A.J., López-García Á., Alcántara J.M., 2022. Biotic filters driving the differentiation of decomposer, epiphytic and pathogenic phyllosphere fungi across plant species // Oikos. V. 2023. № 5. Art. e09624. https://doi.org/10.1111/oik.09624
Pennycook S.R., Newhook F.J., 1978. Spore fall as a quantitative method in phylloplane studies // Trans. Br. Mycol. Soc. V. 71. P. 453–456. https://doi.org/10.1016/S0007-1536(78)80072-2
Pinokiyo A., Singh K.P., Singh J.S., 2006. Leaf-colonizing lichens: Their diversity, ecology and future prospects // Curr. Sci. V. 90. P. 509–518.
Pirog T.P., Iutynska G.O., Leonova N.O., Beregova K.A., Shevchuk T.A., 2018. Microbial synthesis of phytohormones // Biotechnol. Acta. V. 11. № 1. P. 5–24.
Płachecka A., 2005. Microscopical observations of Sphaerellopsis filum, a parasite of Puccinia recondita // Acta Agrobot. V. 58. № 1. P. 67–71. https://doi.org/10.5586/aa.2005.010
Popp C., Burghardt M., Friedmann A., Riederer M., 2005. Characterization of hydrophilic and lipophilic pathways of Hedera helix L. cuticular membranes: Permeation of water and uncharged organic compounds // J. Exp. Bot. V. 56. P. 2797–2806. https://doi.org/10.1093/jxb/eri272
Ruscoe Q.W., 1971. Mycoflora of living and dead leaves of Nothofagus truncata // Trans. Br. Mycol. Soc. V. 56. P. 463–474. https://doi.org/10.1016/S0007-1536(71)80138-9
Santamaría J., Bayman P., 2005. Fungal epiphytes and endophytes of coffee leaves (Coffea arabica) // Microb. Ecol. V. 50. P. 1–8. https://doi.org/10.1007/s00248-004-0002-1
Saunier A., Mpamah P., Biasi C., Blande J.D., 2020. Microorganisms in the phylloplane modulate the BVOC emissions of Brassica nigra leaves // Plant Signal. Behav. V. 15. Art. 1728468. https://doi.org/10.1080/15592324.2020.1728468
Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L. et al., 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi // Proc. Natl. Acad. Sci. V. 109. № 16. P. 6241–6246. https://doi.org/10.1073/pnas.1117018109
Schreiber L., Krimm U., Knoll D., 2008. Interactions between epiphyllic microorganisms and leaf cuticles // Plant Surface Microbiology. Berlin; Heidelberg: Springer. P. 145–156. https://doi.org/10.1007/978-3-540-74051-3_9
Schumacher C.F.A., Steiner U., Dehne H.W., Oerke E.C., 2008. Localized adhesion of nongerminated Venturia inaequalis conidia to leaves and artificial surfaces // Phytopathology. V. 98. P. 760–768. https://doi.org/10.1094/PHYTO-98-7-0760
Shara M., Basyuni M., Hasanuddin, 2023. Potential of phylloplane fungi from mangrove plant (Rhizophora apiculata Blume) as biological control agents against Fusarium oxysporum f. sp. cubense in Banana plant (Musa acuminata L.) // Forests. V. 14. Art. 167. https://doi.org/10.3390/f14020167
Sivakumar N., Sathishkumar R., Selvakumar G., Shyamkumar R., Arjunekumar K., 2020. Phyllospheric microbiomes: Diversity, ecological significance, and biotechnological applications // Plant Microbiomes for Sustainable Agriculture / Eds Yadav A., Singh J., Rastegari A., Yadav N. Cham: Springer. P. 113–172. https://doi.org/10.1007/978-3-030-38453-1_5
Srisuk N., Nutaratat P., Surussawadee J., Limtong S., 2019. Yeast communities in sugarcane phylloplane // Microbiology. V. 88. P. 353–369. https://doi.org/10.1134/S0026261719030135
Srivastava A.K., 1993. Evidence of fungal parasitism in the Glossopteris flora of India // Comptes Rendu XII ICC-P Buenos Aires. V. 2. P. 141–146.
Stanley M.S., Callow M.E., Perry R., Alberte R.S., Smith R., Callow J.A., 2002. Inhibition of fungal spore adhesion by zosteric acid as the basis for a novel, nontoxic crop protection technology // Phytopathology. V. 92. P. 378–383. https://doi.org/10.1094/PHYTO.2002.92.4.378
Streletskii R.A., Kachalkin A.V., Glushakova A.M., Yurkov A.M., Demin V.V., 2019. Yeasts producing zeatin // PeerJ. V. 7. Art. e6474. https://doi.org/10.7717/peerj.6474
Sukmawati D., Andrianto M.H., Arman Z., Ratnaningtyas N.I., Al Husna S.N. et al., 2020. Antagonistic activity of phylloplane yeasts from Moringa oleifera Lam. leaves against Aspergillus flavus UNJCC F-30 from chicken feed // Indian Phytopathol. V. 73. № 1. P. 79–88. https://doi.org/10.1007/s42360-020-00194-2
Sukmawati D., Oetari A., Hendrayanti D., Atria M., Sjamsuridzal W., 2015. Identification of phylloplane yeasts from paper mulberry (Broussonetia papyrifera (L.) L'Hér. ex Vent.) in Java, Indonesia // Malays. J. Microbiol. V. 11. № 4. P. 324–340. https://doi.org/10.21161/mjm.68114
Taylor T.N., Krings M., Taylor E.L., 2014. Fossil Fungi. San Diego: Academic Press. 398 p. https://doi.org/10.1016/C2010-0-68335-0
Thakur S., Harsh N.S.K., 2014. Efficacy of volatile metabolites of phylloplane fungi of Rauwolfia serpentina against Alternaria alternata // Curr. Res. Environ. Appl. Mycol. V. 4. № 2. P. 152–156. https://doi.org/10.5943/cream/4/2/2
Tomasi P., Dyer J.M., Jenks M.A., Abdel-Haleem H., 2018. Characterization of leaf cuticular wax classes and constituents in a spring Camelina sativa diversity panel // Ind. Crops Prod. V. 112. P. 247–251. https://doi.org/10.1016/j.indcrop.2017.11.054
Tucker S.L., Talbot N.J., 2001. Surface attachment and pre-penetration stage development by plant pathogenic fungi // Ann. Rev. Phytopathol. V. 39. P. 385–417. https://doi.org/10.1146/annurev.phyto.39.1.385
Waill A.E., Ghoson M.D., 2018. Where to find? A report for some terrestrial fungal isolates, and selected applications using fungal secondary metabolites // Biomed. J. Sci. Tech. Res. V. 4. № 4. https://doi.org/10.26717/BJSTR.2018.04.001070
Wittig H.P.P., Johnson K.B., Pscheidt J.W., 1997. Effect of epiphytic fungi on brown rot blossom blight and latent infections in sweet cherry // Plant Dis. V. 81. № 4. P. 383–387. https://doi.org/10.1094/PDIS.1997.81.4.383
Xu H., Zhu M., Li S., Ruan W., Xie C., 2020. Epiphytic fungi induced pathogen resistance of invasive plant Ipomoea cairica against Colletotrichum gloeosporioides // PeerJ. V. 8. Art. e8889. https://doi.org/10.7717/peerj.8889
Yao H., Sun X., He C., Maitra P., Li X.C., Guo L.D., 2019. Phyllosphere epiphytic and endophytic fungal community and network structures differ in a tropical mangrove ecosystem // Microbiome. V. 7. № 1. P. 1–15. https://doi.org/10.1186/s40168-019-0671-0
Yusifova A.A., Rzayeva A.A., Muradova S.M., Jabrailzadeh S.M., Ghahramanova F.X., Keyseruxskaya F.Sh., 2017. Diversity of micromycetes which found in phyllosphere and rhizosphere of leguminous plants // J. Basic. Appl. Sci. Res. V. 7. P. 13–17.
Zhu F., Chen G., Chen X., Huang M., Wan X., 2011. Aspergicin, a new antibacterial alkaloid produced by mixed fermentation of two marine-derived mangrove epiphytic fungi // Chem. Nat. Compd. V. 47. P. 767–769. https://doi.org/10.1007/s10600-011-0053-8
Дополнительные материалы отсутствуют.
Инструменты
Журнал общей биологии


