Журнал высшей нервной деятельности им. И.П. Павлова, 2023, T. 73, № 6, стр. 819-832
Нарушения двигательной активности на модели расстройств аутистического спектра
А. Е. Хайруллин 1, 2, *, Д. В. Ефимова 1, Д. В. Иванова 1, Т. В. Балтина 2, М. Э. Балтин 2, С. Н. Гришин 1, А. У. Зиганшин 1
1 Казанский государственный медицинский университет
Казань, Россия
2 Казанский Федеральный Университет
Казань, Россия
* E-mail: khajrulli@yandex.ru
Поступила в редакцию 13.07.2023
После доработки 27.08.2023
Принята к публикации 31.08.2023
- EDN: SLNBWY
- DOI: 10.31857/S0044467723060047
Аннотация
Аутизм, или расстройство аутистического спектра (РАС), является многофакторным заболеванием, которое характеризуется не только нарушениями психоэмоционального состояния и социального взаимодействия, но и соматическими дисфункциями. В ряде исследований также сообщалось об изменениях со стороны опорно-двигательной системы у пациентов с РАС. В данной работе методом видеоанализа движений мы продемонстрировали снижение горизонтальной и вертикальной двигательной активности, кроме этого, были зарегистрированы девиантные движения, что говорит о нарушении в локомоторной активности и повышенной тревожности крыс с вальпроатной моделью аутизма. Однако механо-миографическое исследование не выявило достоверных изменений в параметрах сократимости изолированных скелетных мышц крыс с моделью РАС. Таким образом, можно заключить, что общие различия в движении могут быть независимым фактором диагностики аутизма. Более тщательное исследование с использованием большей выборки и подробного кинематического анализа может помочь в дальнейшей оценке вариабельности двигательных функций, как потенциального диагностического и прогностического маркера РАС.
ВВЕДЕНИЕ
По оценкам мониторинга аутизма и нарушений развития США, на 2020 год один из 36 детей в возрасте 8 лет (примерно 4% мальчиков и 1% девочек) страдал расстройством аутистического спектра (РАС) (Maenner et al., 2023). Расстройство аутистического спектра (РАС) — это нарушение развития нервной системы, характеризующееся нарушениями социального взаимодействия, преобладанием стереотипных моделей поведения и сужением круга интересов (Widiger, Hines, 2022). Несмотря на растущую распространенность, РАС по-прежнему остается расстройством с плохо изученной патофизиологией и медленным поиском лекарств. До сих пор этиология РАС неизвестна, при этом некоторые авторы возникновение и прогрессирование РАС связывают с влиянием генетической предрасположенности и факторов окружающей среды (Taylor et al., 2020). Среди основных патофизиологических механизмов развития РАС можно выделить окислительный стресс, нейровоспаление, различные иммунные нарушения и митохондриальную дисфункцию (Saffari et al., 2019; Citrigno et al., 2020; Doi et al., 2022; Singh et al., 2023).
Значительный прогресс в представлении сложной патофизиологии РАС связывают с решающей ролью животных моделей (Qi et al., 2021). Полезная модель, подтвержденная исчерпывающими исследованиями на животных, путем воздействия вальпроевой кислоты (ВПК) на грызунов показывает поразительное сходство с поведением, анатомией, клеточными и молекулярными изменениями, наблюдаемыми у пациентов с аутизмом. Многочисленные анатомические исследования также доказали, что модель ВПК может хорошо воспроизводить дисплазию центральной нервной системы при РАС, предоставляя ценный инструмент для изучения основного механизма РАС (Mabunga et al., 2015).
Существуют исследования, доказывающие вовлеченность пуринергического сигнального пути в развитие нервной системы, за счет влияния на такие механизмы, как клеточная пролиферация, дифференцировка и формирование нейронно-глиальных клеточных взаимодействий, миграция предшественников нейронов и рост нейритов (Burnstock et al., 2011), но связь между аномальным метаболизмом пуринов и этиологией РАС в настоящее время до конца не ясна. Но, согласно теории онтогенеза, дефекты ранних процессов развития способствуют возникновению различных психических заболеваний на более поздних этапах жизни (Ren et al., 2016; Williams et al., 2018; Courchesne et al., 2019). Появились дополнительные доказательства того, что пурины, метаболизирующие пурины эктоферменты и пуринорецепторы также участвуют в патофизиологических процессах развития нейронов (Fumagalli et al., 2017) и психических расстройствах (Cieslak et al., 2016).
В зависимости от лиганда пуринергические сигнальные рецепторы делятся на два основных класса: P1 (рецепторы аденозина) и P2 (рецепторы АТФ/АДФ и УТР/УДФ) (Burnstock, 2007). Последний включает P2X и P2Y, которые опосредуют гиперактивацию глиальных клеток и начало воспалительных реакций в центральной нервной системе (ЦНС) (Abbracchio, Ceruti, 2006; Inoue, 2008; Huang et al., 2019). Кроме того, было показано снижение экспрессии P2X7-рецепторов, играющих ключевую роль в патофизиологии нарушений ЦНС и опосредующих самые сильные признаки нейровоспаления, у детей с РАС (Lister et al., 2007; Naviaux et al., 2013).
Нарушение функционирования пуринергической сигнальной системы связывают с инициацей РАС, что в свою очередь позволяет рассматривать данную сигнальную систему в качестве потенциальной терапевтической мишени. Существуют данные о том, что лечение сурамином (20 мг/кг, внутрибрюшинно) крыс с моделью РАС восстанавливает их коммуникативные способности и уменьшает тревожность, измеряемую при помощи приподнятого крестообразного лабиринта (Hirsch et al., 2020).
Лечение сурамином не влияет на индуцированную вальпроевой кислотой активацию P2X4- и P2Y2-рецепторов в гиппокампе и экспрессию рецептора P2X4 в медиальной префронтальной коре, но нормализует повышенный уровень интерлейкина 6 (IL-6) (Smith et al., 2007).
Коморбидность уже давно признана у детей с нарушениями развития, такими как аутистическое расстройство, синдром дефицита внимания и гиперактивности (Gillberg et al., 1995; Watson et al., 2003). И хотя РАС считается психическим расстройством, с ним могут быть связаны и расстройства функций других внутренних органов и систем. К таким особенностям относятся, в частности, сенсорные аномалии (Kern et al., 2006), сенсорно-моторные дефициты (Piek, Dyck, 2004), проблемы с мелкой и грубой моторикой (Provost et al., 2007), нарушения в движении/моторных навыках (Green et al., 2009), проблемы с равновесием (Minshew et al., 2004), мышечная слабость (Hardan et al., 2003) и гипотония (Ming et al., 2007).
Двигательные аномалии были признаны неотъемлемой частью расстройств аутистического спектра (Ghaziuddin, Butler, 1988). Причем наблюдения показывают, что аномалии движений при аутизме разнообразны и могут быть причиной нарушений в различных частях центральной нервной системы. Вовлечение нескольких структур вполне ожидаемо из-за сложного распределения двигательной системы на уровне спинного мозга, ствола мозга, мозжечка, подкорковых и корковых отделов нервной системы (Kingsley, 2000). Мы предположили, что в дополнение к основным поведенческим симптомам РАС у крыс могут также проявляться другие проблемы развития нервной системы, связанные с двигательной дисфункцией.
На основании вышеизложенного, целью данного исследования было оценить общий характер изменений в двигательной активности скелетных мышц крыс с моделью РАС.
МЕТОДИКА
Объект исследования. В ходе экспериментов были обследованы нелинейные лабораторные крысы-самцы, массой 160–240 г. Животные содержались при свободном доступе к пище и воде, а также естественном чередовании суточной освещенности.
Экспериментальные группы:
1) контрольная группа (n = 12);
2) группа с воспроизведением модели аутизма (ВМА, n = 12).
Моделирование РАС на крысах. Вальпроевая кислота в высоких дозах блокирует ферменты, участвующие в деацетилировании гистоновых белков, что влияет на экспрессию определенных генов и, соответственно, модифицирует их функционирование. При введении беременным самкам у потомства лабораторных животных развивается фетальный вальпроатный синдром, который по своим проявлениям сходен с РАС (Zheng et al., 2019). Для выработки у потомства фетального вальпроатного синдрома самкам крыс на 12–13-й день беременности однократно подкожно в область холки вводят натриевую соль вальпроевой кислоты в дозе 500 мг/кг. Не было зафиксировано увеличение числа эмбриональных смертей, увеличение постимплантационных потерь, уменьшение размера помета и массы тела плода. Рожденных от такого воздействия крыс в возрасте 6 мес. использовали для экспериментов. В качестве контроля использовали крыс этого же возраста, рожденных от крыс, не подвергавшихся лекарственному воздействию.
Тест “Открытое поле”. Установка “открытое поле” представляет собой белую квадратную арену. Пол разделен на 25 прямоугольников одинаковой площади для удобства визуальной регистрации горизонтальной двигательной активности животных на периферии, в 2/3 арены и ее центра.
Методика “Открытое поле” (ОП) позволяет изучить врожденные особенности ориентировочного и исследовательского поведения и сопротивляемость к стрессу (Козловский, Кенунен, 1992). С помощью данного метода оценивают ориентировочно-исследовательскую реакцию (ОИР) по количеству пройденных квадратов в арене, по количеству стоек на задних лапах (вертикальная двигательная активность, ВДА) при искусственном освещении.
Животное, которое помещено на незнакомую открытую площадку, демонстрирует ориентировочно-исследовательские реакции, например, характерное замирание, которое необходимо для оценки степени риска (Blume et al., 2018).
Ход эксперимента. Животное помещали всеми 4 лапами в центральный квадрат арены и с помощью видеосистемы фиксировали его передвижения в установке в течение 3 мин. После прохождения тестирования каждого животного арену обрабатывали водой для удаления запаха.
Регистрировали следующие показатели:
1) горизонтальную двигательную активность (ГДА). Основным критерием ГДА является участие в перемещении животного всех четырех лап. За единицу перемещения считали один пересеченный квадрат всеми лапами. Регистрируют ГДА на периферии, в 2/3 и в центре арены;
2) вертикальную двигательную активность (ВДА). Представлена двумя видами стоек: задние лапы животного остаются на полу арены, а передние упираются в стенку поля (пристеночная стойка); если лапы остаются на весу (свободная стойка). Раздельно считали число открытых и пристеночных стоек.
Метод видеоанализа движения на платформе Vicon
Для оценки и сравнения походки крыс в контрольной группе с группой крыс ВМА был использован метод видеоанализа движения. Трехмерные данные были получены с использованием шести камер Vicon MX (Vicon Motion Systems, Оксфорд, Великобритания), размещенных на специальных креплениях полукругом. Для калибровки и синхронизации камер был использован калибровочный маркер Active Wand (Vicon Motion Systems, Оксфорд, Великобритания). Для получения стандартного видеоизображения была использована видеокамера Sony. 10 пассивных светоотражающих маркеров были размещены на мышцах спины, крестцовой кости, на коленных суставах, голеностопных суставах, как это было показано на рис. 1.
Рис. 1.
Схема расположения маркеров для видеоанализа движения. Fig. 1. Layout of markers for motion video analysis.
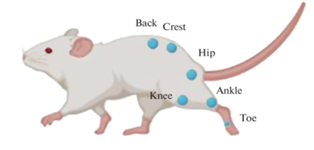
Во время видеозахвата крысы начинали свободно двигаться в “открытом поле” при искусственном освещении. Использовали сплайн-интерполяцию для повторной выборки данных Vicon до 30 Гц перед анализом. Определяли фазы цикла походки с временными метками событий походки – отрыва стопы и возобновления контакта с поверхностью. Кинематический анализ был выполнен для полного цикла походки каждой испытуемой крысы.
Для обработки полученных данных использовали программное обеспечение Vicon Nexus 2.9 для ручной достройки 3D-модели движения и удаления артефактов с записи.
Полученные Vicon Nexus 2.9 данные с помощью модуля ASCII конвертировались в текстовый формат, после чего обрабатывались с помощью программного обеспечения MATLAB; методика обсчета подробно описана в статье (Smirnova et al., 2022). Для каждой группы получали кривые из усреднения 30 шагов по углам в фазах одного шага. Были получены данные в виде ангулограмм: кинематический профиль коленного сустава крыс, нормированный по фазе шага. С помощью ангулограмм высчитывались углы сгибания коленных суставов как разность показателя угла в начале фазы переноса и показателя угла в начале фазы толчка. Были построены траектории перемещений стопы с целью определения объема движения конечности и максимальной точки подъема стопы, а также длины шага.
Статистическую обработку данных проводили с помощью программы SPSS Statistics. Проверку соответствия полученных данных нормальному распределению проводили с помощью критерия Колмогорова. Данные параметров двигательной активности в “открытом поле” и характеристики шага при выполнении движения представлены в виде среднего и среднеквадратичного отклонения М ± SD. Данные параметров кинематического анализа представлены в виде медианы и нижнего и верхнего квартиля (Ме; Q1; Q3). Для сравнения независимых выборок использовался U-критерий Манна–Уитни. Рассчитанные значения U-критерия Манна–Уитни сравнивались с критическими при уровне значимости p < 0.05: в том случае, если рассчитанное значение U было равно или меньше критического, признавалась статистическая значимость различий.
Механо-миографиический метод регистрации сократительных ответов мышц
Перед проведением экспериментов животных наркотизировали внутрибрюшинным введением раствора этаминала натрия в дозе 40 мг/кг, обескровливали и выделяли m. soleus и m. extensor digitorium longus (EDL) с культями нервов, которые были помещены во всасывающий электрод оригинальной конструкции (Гришин и др., 2023). Мионевральные препараты фиксировали за сухожильные концы, после погружали в резервуары объемом 10 мл, заполненные раствором Кребса (состав в мМ: NaCl – 118.0, KCl – 4.75, CaCl2 – 2.5, NaHCO3 – 24.8, KH2PO4 – 1.18, MgSO4 ⋅ 7H2O – 1.18, глюкоза – 11), рН 7.4, t – 37°C.
Для вызова сокращений мышечных образцов применялся электростимулятор Digitimer MultiStimul D330 (Великобритания), производилась подача прямоугольных импульсов амплитудой̆ 10 В и продолжительностью 0.5 мс при частоте 0.1 Гц в течение 2 мин. Силу сокращений мышц регистрировали с помощью датчика двигательной активности Linton FСG-01 (Великобритания), аналоговый сигнал преобразовывали системой сбора данных Biopack MP100МSW (США).
Изначальная нагрузка на мионевральные препараты составляла 1 г на m. soleus и 0.5 г на m. EDL. После получасовой адаптации мышечных препаратов к среде дважды с интервалом в 5 мин производили оценку стабильности сократительных ответов.
Для оценки эффектов пуринергических агонистов и антагонистов в емкость добавляли 100 мкМ АТФ и через 7 мин оценивали механические ответы мышц. Затем мышцу промывали раствором Кребса и инкубировали с раствором сурамина в концентрации 100 мкМ в течение 20 мин с последующим добавлением 100 мкМ АТФ и вновь регистрировали механические ответы мышц.
Все полученные в течение двух минут ответы (12 сократительных ответов) усредняли и обрабатывали как один результат. Рассчитывали их в % относительно исходных результатов, полученных в начале эксперимента.
Статистическую обработку данных проводили с помощью программы SPSS Statistics. Проверку соответствия полученных данных нормальному распределению проводили с помощью критерия Колмогорова. Рассчитывали средние арифметические анализируемых параметров и стандартную ошибку. Статистическую значимость наблюдаемых изменений оценивали с помощью критерия Стьюдента для независимых и попарно сопряженных выборок. Различия рассматривали как значимые при p < 0.05.
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЙ
Анализ ориентировочно-исследовательской активности в тесте “Открытое поле” показал, что у животных группы ВМА горизонтальная двигательная активность (ГДА) была снижена по сравнению с контрольной группой (рис. 2).
Рис. 2.
Показатели горизонтальной (ГДА) и вертикальной (ВДА) двигательной активности животных контрольной группы и группы с воспроизведением модели аутизма (ВМА) в тесте “Открытое поле”; Данные представлены в виде среднего, планки погрешностей – в виде стандартного отклонения. * – p < 0.05 достоверность отличий по сравнению с контрольной группой. Fig. 2. Indicators of horizontal (GDA) and vertical (VDA) motor activity of animals of the control group and the group with the reproduction of the autism model (VMA) in the “Open Field” test; the data are presented in the form of an average; the error bars are in the form of a standard deviation. * – p < 0.05 reliability of differences compared to the control group.
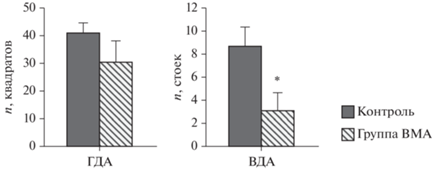
Количество пройденных квадратов составляло 30.5 ± 7.5, а в контрольной группе 41 ± 3.6. После выхода из центральной зоны поля возвращение в него не наблюдалось, что свидетельствует о более высокой тревожности животных этой группы. Вертикальная двигательная активность (ВДА), отражающая как двигательные, так и исследовательские реакции, была ниже у животных группы ВМА и составляла 3.2 ± 1.6 вертикальных стоек (р < < 0.05) (рис. 2). У группы ВМА прослеживалась тенденция к пониженной двигательной активности, а также повышенной тревожности.
Были построены ангулограммы суставов задней конечности по записям видеофиксации движения, которые приведены на рис. 3 (а, б). По ангулограммам видно увеличение угла в фазе толчка у крыс группы ВМА по сравнению с контрольной группой.
Рис. 3.
Ангулограмма коленного сустава крыс группы с воспроизведением модели аутизма (ВМА) (а) и контрольной группы (б). Репрезентативное изображение положения задней конечности крыс на протяженности фазы шага у крыс в группе ВМА (в) и контрольной группы (г). Фиолетовой линией представлена траектория движения стопы, голубым треугольником показан объем движений задней конечности. Стрелками показан момент окончания фазы толчка. Fig. 3. Angulogram of the knee joint of the autistic group of rats (a) and the control group (б). A representative image of the position of the hindlimb of rats over the length of the step phase in the BMA group of rats (в) and the control group (г). The purple line represents the trajectory of the foot movement, the blue triangle shows the volume of movements of the hindlimb. The arrows show the moment of the end of the push phase.
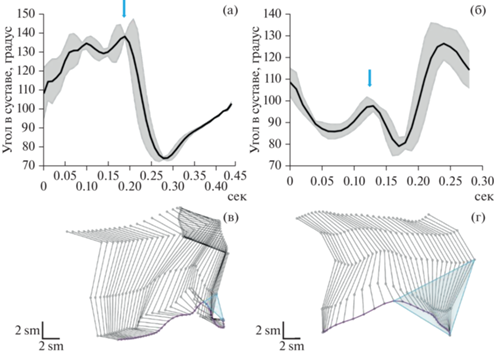
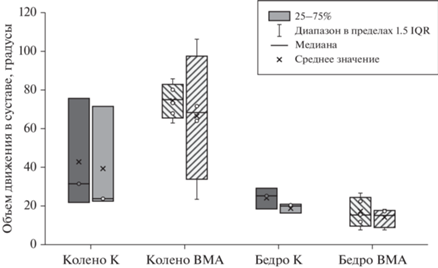
В первой трети цикла при окончании фазы толчка, при инициации движения наблюдались неадекватные движения у животных группы ВМА: крыса сжимала заднюю лапу, после чего следовал низкоамплитудный толчок (рис. 3 (в, г)). Фаза переноса тела была короче во времени в группе ВМА. Такое движение может говорить о характерной тревожности данной группы и пониженной двигательной функции. Однако изменения объема движения в коленном и тазобедренном суставах были статистически не достоверны (рис. 4).
Рис. 4.
Объем движения в суставах: коленном (колено) и тазобедренном (бедро) слева, справа в контрольной группе (К) и группы с воспроизведением модели аутизма (ВМА), данные представлены в виде медианы, разброс в группах – в виде интерквартильного размаха. Fig. 4. The volume of movement in the joints: knee (колено) and hip (бедро) on the left, on the right in the control group (K) and the group with the reproduction of the autism model (BMA), the data are presented as a median, the spread in the groups is in the form of an interquartile span.
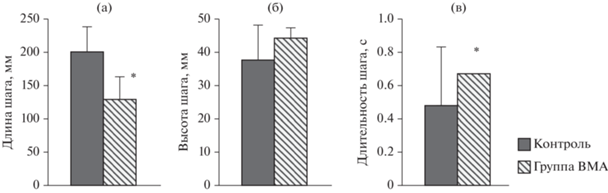
У крысы группы ВМА было значительное снижение длины шага и увеличение длительности шага по сравнению с контрольной группой: 131 ± 38 мм (р < 0.05) и 0.63 с (р < 0.05) соответственно. Высота подъема ноги в обеих группах была одинаковая (рис. 5).
Рис. 5.
Характеристики шага при выполнении движения в “открытом поле”, определяемые с помощью системы видеозахвата движения (Vicon) у крыс двух групп – контроля и группы с воспроизведением модели аутизма (ВМА): (а) длина шага, мм, (б) высота шага, мм и (в) длительность шага. Данные представлены в виде среднего и стандартного отклонения. * – p < 0.05, статистически значимое различие между группами. Fig. 5. Characteristics of the step when performing movement in an open field determined by the motion capture system (Vicon) in rats of two groups of control and groups with reproduction of the autism model (BMA): (a) step length, mm, (б) step height, mm and (в) step duration. The data is presented in the form of mean and standard deviation. * – p < 0.05 statistically significant difference between the groups.
Описанная выше девиация локомоторной активности животных с РАС подтолкнула нас к следующей серии экспериментов. Существуют доказательства (Khairullin et al., 2023a, 2023b), подтверждающие вовлеченность синаптической компоненты периферических отделов нервной системы на различных моделях расстройств. Ключевым звеном пластичности в нервно-мышечном переходе является пуринергическая трансдукция (Ziganshin et al., 2020). Поэтому мы решили сравнить механическую активность мышц голени у крыс из контрольной группы и с моделью ВМА в условиях пуринергической модуляции.
Полученные данные демонстрируют отсутствие значимых изменений в параметрах сокращений исследуемых мышц (табл. 1). АТФ сохраняла значимую модулирующую способность, различий между сравниваемыми группами мы не выявили.
Таблица 1.
Зависимость сократительных параметров исследованных мышц крыс, вызванных электрической стимуляцией, от экспериментальных условий Table 1. Dependence of contractile parameters of the studied rat muscles caused by electrical stimulation on experimental conditions
| Экспериментальные условия | Параметр | Фоновый уровень | АТФ (100 мкМ) |
Сурамин (100 мкМ) |
Сурамин + АТФ (100 мкМ) |
|---|---|---|---|---|---|
| m. soleus | |||||
| Контроль (n = 12) | СС | 100.0 ± 3.8 | 73.1 ± 6.4* | 102.7 ± 4.4 | 96.4 ± 6.5 |
| ВС | 0.083 ± 0.005 | 0.082 ± 0.004 | 0.081 ± 0.005 | 0.080 ± 0.003 | |
| ВП/2 | 0.090 ± 0.006 | 0.104 ± 0.010 | 0.092 ± 0.004 | 0.094 ± 0.011 | |
| ВМА (n = 12) | СС | 98.6 ± 5.1 | 74.8 ± 5.9* | 103.2 ± 6.3 | 98.7 ± 5.3 |
| ВС | 0.081 ± 0.004 | 0.079 ± 0.006 | 0.080 ± 0.004 | 0.078 ± 0.006 | |
| ВП/2 | 0.091 ± 0.011 | 0.110 ± 0.013 | 0.093 ± 0.009 | 0.095 ± 0.009 | |
| m. EDL | |||||
| Контроль (n = 12) | СС | 100.0 ± 4.8 | 85.9 ± 3.7* | 101.9 ± 4.6 | 99.2 ± 4.5 |
| ВС | 0.055 ± 0.006 | 0.058 ± 0.007 | 0.060 ± 0.007 | 0.059 ± 0.005 | |
| ВП/2 | 0.068 ± 0.004 | 0.070 ± 0.006 | 0.066 ± 0.005 | 0.069 ± 0.004 | |
| ВМА (n = 12) | СС | 98.5 ± 3.6 | 84.2 ± 7.1* | 97.9 ± 6.3 | 96.1 ± 5.2 |
| ВС | 0.059 ± 0.006 | 0.060 ± 0.005 | 0.061 ± 0.005 | 0.061 ± 0.004 | |
| ВП/2 | 0.070 ± 0.005 | 0.072 ± 0.008 | 0.071 ± 0.007 | 0.070 ± 0.005 | |
Примечание: * – р < 0.05 от фонового уровня, # – р < 0.05 от контроля. СС – сила сокращения, ВС – время сокращения, ВП/2 – время полурасслабления. Note: * – p < 0.05 from the background level, # – p < 0.05 from the control. СС is the contraction force, ВС is the contraction time, ВП/2 is the half-relaxation time.
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
В качестве подхода к разработке новых терапевтических средств было создано несколько моделей РАС на грызунах, которые повторяют многие поведенческие фенотипы, наблюдаемые у людей с РАС (Pardo, Meffert, 2018; Chaliha et al., 2020). В настоящее время применение вальпроевой кислоты (ВПК) является наиболее релевантным фармакологическим моделированием РАС на животных моделях, так как было доказано, что введение ВПК на ранних сроках беременности приводит к различным нарушениям в развитии мозга, гиперактивности, дефициту внимания и РАС (Wood, 2014; Christensen et al., 2019).
Еще в 1996 г. Rodier и соавт. обнаружили морфологические изменения головного мозга, которые происходят вследствие введения ВПК беременным крысам: снижение числа нейронов в ядрах черепно-мозговых нервов и аномалии развития мозжечка (Rodier et al., 1996). Впоследствии были доказаны и нарушения в поведении крыс с вальпроатной моделью РАС, которые проявляются в повышении порога болевой чувствительности, снижении социально-исследовательской активности и повышении двигательной активности и гиперактивности, проявляющихся в стереотипном поведении (Schneider, Przewłocki, 2005).
Модели нервно-психических расстройств и нарушений развития нервной системы, в том числе и аутизма, на животных предоставили соответствующие знания о нейронных схемах и мишенях-рецепторах, вовлеченных в этиологию и патофизиологию измененного поведения (Gandhi, Lee, 2021). Одной из нейротрансмиттерных систем, вовлеченных в патофизиологию психических расстройств, является пуринергическая (Cheffer et al., 2018). Известно, что при патологии пуринового и пиримидинового обмена повреждается нервная система (задержка развития, эпилептические приступы, аутизм).
P2-рецепторы тесно связаны с эмбриональным развитием нервной системы, и любое нарушение пуринергической передачи сигналов может быть основным процессом, приводящим к психическим заболеваниям в целом (Oliveira et al., 2016).
Известно, что P2X- и P2Y-рецепторы контролируют широкий спектр биологических характеристик, которые имеют отношение к аутизму; например, пуринергическая сигнализация модулирует нормальный синаптогенез и развитие мозга (Pan et al., 2020), врожденные и адаптивные иммунные реакции, а также хроническое воспаление (Lee et al., 2015), нейровоспаление, противовирусную сигнализацию (Mitchell et al., 2017), активацию микроглии, хемотаксис нейтрофилов, аутофагию, моторику кишечника (Talos et al., 2012), проницаемость кишечника (Amiet et al., 2008), хемосенсорную трансдукцию вкуса (Besag, 2018), хронический болевой синдром (Lamb et al., 2019). Несомненно, причины большинства подобных нарушений лежат в области ЦНС, но, помимо этого, не отрицаются нарушения в функционировании периферической нервной системы (в том числе и в функционировании нервно-мышечного синапса).
Хотя аутизм диагностируется по трем основным характеристикам: социальному дефициту, коммуникативным нарушениям и повторяющемуся или стереотипному поведению, – другие поведенческие особенности, такие как сенсорные и двигательные нарушения, присутствуют более чем у 70% людей с РАС (Bhat, 2021). Связанные с аутизмом характеристики, такие как нарушения сенсорной обработки и дефицит координации движений, широко распространены, но им уделяется меньше внимания со стороны исследовательского сообщества. Например, существует ряд качественных и количественных отчетов по РАС, описывающих нарушения зрительно-моторной и мануальной ловкости, координации конечностей во время задач, требующих баланса, ловкости и скорости, а также нарушения походки и атаксии (Fatemi et al., 2012). Кроме того, двигательные нарушения могут быть одними из самых ранних признаков некоторых форм РАС (Ozonoff et al., 2008). Соответственно, оценка двигательных расстройств может помочь в ранней и количественной диагностике патологии и выявлении дисфункциональных областей и цепей мозга при РАС. Мы показали, что у животных группы ВМА наблюдалось снижение вертикальной и горизонтальной двигательной активности в “открытом поле”. Это согласуется с предыдущими результатами, полученными у животных, демонстрирующих снижение двигательной активности после пре- и постнатального введения ВПК (Гедзун и др., 2020; Kataoka et al., 2013; Mabunga et al., 2015), а также с клиническими исследованиями, показывающими, что дети с РАС тратят меньше времени на активное изучение окружающей среды (Elandaloussi et al., 2023). РАС также связано с тревожными расстройствами, и оценки распространенности тревожности у людей с РАС широко варьируют от 22 до 84% (Nimmo-Smith et al., 2020). Мы также выявили повышенную тревожность у грызунов, подвергшихся пренатальному воздействию ВПК, на что указывает уменьшение ВДА, изменение структуры шага и отсутствие исследования центральной области в тестах в “открытом поле”, что согласуется и с другими исследованиями (Kataoka et al., 2013; Cartocci et al., 2018; Servadio et al., 2018). Изменения в исследовательском поведении могут отражать нарушения развития ЦНС. Одним из возможных объяснений снижения исследовательского поведения у крыс c ВПК может быть уменьшение количества клеток Пуркинье в мозжечковых дольках червя (Fatemi et al., 2012). Точно так же уменьшенные дольки мозжечкового червя, которые коррелируют со снижением исследовательской активности, наблюдались у детей, страдающих аутизмом (Pierce, Courchesne, 2001). Кроме того, на нарушение активности мозжечка также может указывать значительное снижение длины шага и увеличение длительности шага у крыс с ВМА по сравнению с контрольной группой (Main, Kulesza, 2017). Вторым возможным объяснением могут быть изменения в нейронных структурах, участвующих в регуляции страха. Сюда входят медиальная префронтальная кора и миндалевидное тело. Аномалии в этих структурах наблюдались как у крыс в модели с ВПК (Sui, Chen, 2012), так и у аутичных людей (Bachevalier, Loveland, 2006; Arutiunian et al., 2023). Согласно (T. Schneider и R. Przewłocki, 2005), сниженное исследовательское поведение у взрослых крыс в модели с ВПК, скорее всего, может быть опосредовано связанным со страхом торможением исследовательского поведения.
Наблюдаемые групповые различия в двигательной активности демонстрируют, что крысы группы ВМА выполняли шагание менее эффективно. Крысам группы ВМА требовалось больше движений лап, и у них проявлялось более нетипичное сенсомоторное поведение (например, резкий подъем лапы, короткая фаза переноса); по-видимому, эти крысы испытывали больше трудностей в более тонких аспектах моторного контроля и/или трудности с координацией функций конечностей. Подобные сенсомоторные изменения связывают с нарушением баланса синаптического возбуждения и торможения в ЦНС, вызванных дисфункцией ГАМКергической передачи сигналов, обширными изменениями в морфологии нейронов и локальными нарушениями микроцепей неокортекса (Banerjee et al., 2013; Lee et al., 2017; Jiang et al., 2022). Поскольку оптимальная двигательная активность определяется точным и эффективным приемом и обработкой сенсорной информации, нарушение соматосенсорной обработки является одним из объяснений наблюдаемого моторного дефицита. Как у детей, так и у взрослых с РАС часто сообщалось о грубой моторной неуклюжести и нарушениях походки и равновесия (Fournier et al., 2010). В дополнение к отсроченному началу ходьбы, у малышей с аутизмом также отсутствует зрелый паттерн “пятка-носок” и они имеют более переваливающуюся походку по сравнению с контрольной группой того же возраста (Esposito, Venuti, 2008).
В данной работе было показано отсутствие значимых изменений в силе сокращений мышц при наличии тенденции к пониженной двигательной активности у группы с воспроизведением модели аутизма, в отличие от контрольных животных, а также повышенной тревожности, что соотносится с результатами другого исследования периферической холинергической нейротрансмиссии у крыс с моделью РАС (Архипов и др., 2021). Интересно, что изменения механической активности гладкомышечных органов и повышение парасимпатических влияний у крыс с РАС были достоверно показаны (Зиганшин, Иванова, 2021).
В нашем исследовании было несколько ограничений. Во-первых, наши исследования были проведены на самцах крыс. В некоторых исследованиях были обнаружены доказательства различий в социально-коммуникативных навыках и ограниченном повторяющемся поведении животных в моделях с РАС между полами. Во-вторых, ограниченная выборка. Кроме того, изначально при постановке задач основное внимание было уделено эффектам нервно-мышечной передачи скелетной мышцы, поэтому использовался ограниченный набор поведенческих тестов. В дальнейшем мы можем расширить поведенческую оценку и добавить подробный анализ кинематики.
ЗАКЛЮЧЕНИЕ
Основываясь на результатах настоящего исследования, можно предположить, что общие различия в движении могут быть независимым фактором диагностики аутизма. Более тщательное исследование с использованием большей выборки и подробного кинематического анализа может помочь в дальнейшей оценке вариабельности двигательных функций, как потенциального диагностического и прогностического маркера РАС. Нарушение моторики – крайне недооцененная область для оценки и вмешательства при РАС. Двигательные навыки должны регулярно включаться в комплексный скрининг, оценку и планирование лечения РАС, и особенно после периода раннего развития, когда внимание родителей часто смещается на другие проявления РАС.
ФИНАНСИРОВАНИЕ
Исследование выполнено при финансовой поддержке гранта Международного научного совета Казанского государственного медицинского университета для молодых ученых 2023 в рамках Программы развития Университета, а также в рамках программы “Стратегическое академическое лидерство Казанского федерального университета” (ПРИОРИТЕТ-2030).
FUNDING
The study was carried out with the financial support of a grant from the International Scientific Council of Kazan State Medical University for Young Scientists 2023 as part of the University Development Program, as well as under the program “Strategic Academic Leadership of Kazan Federal University” (PRIORITY-2030).
Список литературы
Архипов А.Ю., Самигуллин Д.В., Семина И.И., Маломуж А.И. Функциональная оценка периферической холинергической нейротрансмиссии у крыс с фетальным вальпроатным синдромом. Российский физиологический журнал им. И.М. Сеченова, 2021. 107 (4–5): 605–615.
Гедзун В.Р., Свинов М.М., Сарычева Н.Ю., Шлапакова П.С., Довбнюк К.О., Дубынин В.А. Влияние пренатального и раннего постнатального введения вальпроата на поведение и цитологические характеристики крыс линии Wistar. Журн. высш. нерв. деят. им. И.П. Павлова. 2020. 70 (5): 682–695.
Зиганшин А.У., Иванова Д.В. Вызванные карбахолном сокращения изолированной тонкой кишки возрастают у крыс с экспериментальным аутизмом, вызванным вальпроевой кислотой. Экспериментальная и клиническая фармакология, 2021. 84 (2): 99–103.
Козловский В.Л., Кенунен О.Г. Структура двигательного поведения лабораторных животных – новые возможности методики “открытого поля”. Физиологический журн. им. И.М. Сеченова, 1992. 78 (1): 120–123.
Патент на полезную модель № 216564 U1 Российская Федерация, МПК A61N 1/04, G09B 23/28. Всасывающий культю нерва электрод для электрической стимуляции: № 2022131919: заявл. 07.12.2022: опубл. 14.02.2023 / С.Н. Гришин, А.Е. Хайруллин, А.У. Зиганшин, Д.В. Ефимова; заявитель Федеральное государственное бюджетное образовательное учреждение высшего образования “Казанский государственный медицинский университет” Министерства здравоохранения Российской Федерации. – EDN GPHYYZ.
Abbracchio M.P., Ceruti S. Roles of P2 receptors in glial cells: Focus on astrocytes. Purinergic Signal, 2006. 2: 595–604. https://doi.org/10.1007/s11302-006-9016-0
Amiet C., Gourfinkel-An I., Bouzamondo A., Tordjman S., Baulac M., Lechat P., Mottron L., Cohen D. Epilepsy in autism is associated with intellectual disability and gender: evidence from a meta-analysis. Biol Psychiatry, 2008. 64: 577–582.
Arutiunian V., Davydova E., Pereverzeva D., Sorokin A., Tyushkevich S., Mamokhina U., Danilina K., Dragoy O. Reduced grey matter volume of amygdala and hippocampus is associated with the severity of autistic symptoms and language abilities in school-aged children with Autism Spectrum Disorder: an exploratory study. Brain structure & function. 2023. 228 (6): 1573–1579.
Bachevalier J., Loveland K.A. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neuroscience and biobehavioral reviews. 2006. 30 (1): 97–117.
Banerjee A., García-Oscos F., Roychowdhury S., Galindo L.C., Hall S., Kilgard M.P., Atzori M. Impairment of cortical GABAergic synaptic transmission in an environmental rat model of autism. The international journal of neuropsychopharmacology. 2013. 16 (6): 1309–1318.
Besag F.M. Epilepsy in patients with autism: links, risks and treatment challenges. Neuropsychiatr Dis. Treat., 2018. 14: 1–10.
Bhat A.N. Motor impairment increases in children with autism spectrum disorder as a function of social communication, cognitive and functional impairment, repetitive behavior severity, and comorbid diagnoses: a SPARK study report. Autism Res. 2021. 14: 202–219.
Blume S.R., Nam H., Luz S., Bangasser D.A., Bhatnagar S. Sex- and Age-dependent Effects of Orexin 1 Receptor Blockade on Open-Field Behavior and Neuronal Activity. Neuroscience, 2018. 381: 11–21. https://doi.org/10.1016/j.neuroscience.2018.04.005
Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci., 2007 64 (12):1471–83. https://doi.org/10.1007/s00018-007-6497-0
Burnstock G., Krugel U., Abbracchio M.P., Illes P. Purinergic signalling: From normal behaviour to pathological brain function. Prog. Neurobiol., 2011. 95: 229–274. https://doi.org/10.1016/j.pneurobio.2011.08.006
Cartocci V., Catallo M., Tempestilli M., Segatto M., Pfrieger F.W., Bronzuoli M.R., Scuderi C., Servadio M., Trezza V., Pallottini V. Altered brain cholesterol/isoprenoid metabolism in a rat model of autism spectrum disorders. Neuroscience. 2018. 372: 27–37.
Chaliha D., Albrecht M., Vaccarezza M., Takechi R., Lam V., Al-Salami H., Mamo J. A Systematic Review of the Valproic-Acid-Induced Rodent Model of Autism. Developmental neuroscience. 2020. 42 (1): 12–48.
Cheffer A., Castillo A., Corrêa-Velloso J., Gonçal-ves M.C.B., Naaldijk Y., Nascimento I.C., Burnstock G., Ulrich H. Purinergic system in psychiatric diseases. Mol. Psychiatry, 2018. 23: 94–106.
Christensen J., Pedersen L., Sun Y., Dreier J.W., Brikell I., Dalsgaard S. Association of Prenatal Exposure to Valproate and Other Antiepileptic Drugs With Risk for Attention-Deficit/Hyperactivity Disorder in Offspring. JAMA network open. 2019. 2 (1): e186606. https://doi.org/10.1001/jamanetworkopen.2018. 6606
Cieslak M., Czarnecka J., Roszek K. The roles of purinergic signaling in psychiatric disorders. Acta Biochim. Pol., 2016. 63 (1): 1–9.
Citrigno L., Muglia M., Qualtieri A., Spadafora P., Cavalcanti F., Pioggia G., Cerasa A. The mitochondrial dysfunction hypothesis in autism spectrum disorders: Current status and future perspectives. Int. J. Mol. Sci., 21: 5785. https://doi.org/10.3390/ijms21165785
Courchesne E., Pramparo T., Gazestani V.H., Lombardo M.V., Pierce K., Lewis N.E. The ASD living Biology: from cell proliferation to clinical phenotype. Mol. Psychiatry, 2019. 24 (1): 88–107. https://doi.org/10.1038/s41380-018-0056-y
Doi M., Li M., Usui N., Shimada S. Genomic Strategies for Understanding the Pathophysiology of Autism Spectrum Disorder. Frontiers in molecular neuroscience. 2022. 15: 930941. https://doi.org/10.3389/fnmol.2022.930941
Elandaloussi Y., Floris D.L., Coupé P., Duchesnay E., Mihailov A., Grigis A., Bègue I., Victor J., Frouin V., Leboyer M., Houenou J., Laidi C. Understanding the relationship between cerebellar structure and social abilities. Molecular autism. 2023. 14 (1): 18. https://doi.org/10.1186/s13229-023-00551-8
Esposito G., Venuti P. Analysis of toddlers' gait after six months of independent walking to identify autism: a preliminary study. Perceptual and motor skills. 2008. 106 (1): 259–269.
Fatemi S.H., Aldinger K.A., Ashwood P., Bauman M.L., Blaha C.D., Blatt G.J., Chauhan A., Chauhan V., Dager S.R., Dickson P.E., Estes A.M., Goldowitz D., Heck D.H., Kemper T.L., King B.H., Martin L.A., Millen K.J., Mittleman G., Mosconi M.W., Persico A.M., Welsh J.P. Consensus paper: pathological role of the cerebellum in autism. Cerebellum (London, England). 2012. 11 (3): 777–807.
Fournier K.A., Hass C.J., Naik S.K., Lodha N., Cauraugh J.H. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. Journal of autism and developmental disorders 2010. 40 (10): 1227–1240.
Fumagalli M., Lecca D., Abbracchio M.P., Ceruti S. Pathophysiological role of purines and pyrimidines in neurodevelopment: unveiling new pharmacological approaches to congenital brain diseases. Front. Pharmacol., 2017. 8: 941.
Gandhi T., Lee C.C. Neural Mechanisms Underlying Repetitive Behaviors in Rodent Models of Autism Spectrum Disorders. Frontiers in cellular neuroscience. 2021. 14: 592710. https://doi.org/10.3389/fncel.2020.592710
Ghaziuddin M., Butler E. Clumsiness in autism and Asperger syndrome: A further report. J. Intellect Disabil. Res., 1988. 42: 43–48.
Gillberg C., Schaumann H., Gillberg I.C. Autism in immigrants: children born in Sweden to mothers born in Uganda. J Intellect Disabil Res., 1995. 39 (2): 141–144. https://doi.org/10.1111/j.1365-2788.1995.tb00482.x
Green D., Charman T., Pickles A., Chandler S., Loucas T., Simonoff E., Baird G. Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine & Child Neurology, 2009. 51: 311–316.
Hardan A.Y., Kilpatrick M., Keshavan M.S., Minshew N.J. Motor performance and anatomic magnetic resonance imaging (MRI) of the basal ganglia in autism. Journal of Child Neurology, 2003. 18: 317–324.
Hirsch M.M., Deckmann I., Santos-Terra J., Staevie G.Z., Fontes-Dutra M., Carello-Collar G., Körbes-Rockenbach M., Brum Schwingel G., Bauer-Negrini G., Rabelo B., Gonçalves M.C.B., Corrêa-Velloso J., Naaldijk Y., Castillo A.R.G., Schneider T., Bambini-Junior V., Ulrich H., Gottfried C. Effects of single-dose antipurinergic therapy on behavioral and molecular alterations in the valproic acid-induced animal model of autism. Neuropharmacology. 2020 167: 107930. https://doi.org/10.1016/j.neuropharm.2019.107930
Huang L., Otrokocsi L., Sperlagh B. Role of P2 receptors in normal brain development and in neurodevelopmental psychiatric disorders. Brain Res. Bull., 2019. 151: 55–64. https://doi.org/10.1016/j.brainresbull.2019.01.030
Inoue K. Purinergic systems in microglia. Cell. Mol. Life Sci., 2008. 65: 3074–3080. https://doi.org/10.1007/s00018-008-8210-3
Jiang S., He M., Xiao L., Sun Y., Ding J., Li W., Guo B., Wang L., Wang Y., Gao C., Sun T., Wang F. Prenatal GABAB Receptor Agonist Administration Corrects the Inheritance of Autism-Like Core Behaviors in Offspring of Mice Prenatally Exposed to Valproic Acid. Frontiers in psychiatry. 2022. 13: 835993. https://doi.org/10.3389/fpsyt.2022.835993
Kataoka S., Takuma K., Hara Y., Maeda Y., Ago Y., Matsuda T. Autism-like behaviours with transient histone hyperacetylation in mice treated prenatally with valproic acid. The international journal of neuropsychopharmacology. 2013. 16 (1): 91–103.
Kern J.K., Trivedi M.H., Garver C.R., Grannemann B.D., Andrews A.A., Savla J.S., Johnson D.G., Mehta J.A., Schroeder J.L. The pattern of sensory processing abnormalities in autism. Autism, 2006. 10: 480–494.
Khairullin A.E., Grishin S.N., Ziganshin A.U. P2 Receptor Signaling in Motor Units in Muscular Dystrophy. International Journal of Molecular Sciences. 2023b. 24 (2): 1587.
Khairullin A.E., Mukhamedyarov M.A., Grishin S.N., Teplov A.Yu., Nagiev K.K., Ziganshin A.U. Synaptic Aspects of the Pathogenesis of Autism, Amyotrophic Lateral Sclerosis, and Alzheimer’s Disease. Biophysics, 2023a. 68 (1): 137–145.
Kingsley R.E. Motor systems, in Kingsley RE (ed): Concise Text of Neuroscience. Baltimore, Lippincott Williams & Wilkins, 2000. 209–336.
Lamb G.V., Green R.J., Olorunju S. Tracking epilepsy and autism. Egypt J. Neurol. Psychiatry Neurosurg., 2019. 55: 55. https://doi.org/10.1186/s41983-019-0103-x
Lee B.H., Smith T., Paciorkowski A.R. Autism spectrum disorder and epilepsy: Disorders with a shared biology. Epilepsy Behav., 2015. 47: 191–201.
Lee E., Lee J., Kim E. Excitation/Inhibition Imbalance in Animal Models of Autism Spectrum Disorders. Biological psychiatry. 2017. 81 (10): 838–847.
Lister M.F., Sharkey J., Sawatzky D.A., Hodgkiss J.P., Davidson D.J., Rossi A.G., Finlayson K. The role of the purinergic P2X7 receptor in inflammation. J. Inflamm. (Lond), 2007. 4: 5. https://doi.org/10.1186/1476-9255-4-5
Mabunga D.F., Gonzales E.L., Kim J.W., Kim K.C., Shin C.Y. Exploring the Validity of Valproic Acid Animal Model of Autism. Experimental neurobiology. 2015. 24 (4): 285–300.
Maenner M.J., Warren Z., Williams A.R., Amoakohene E., Bakian A.V., Bilder D.A., Durkin M.S., Fitzgerald R.T., Furnier S.M., Hughes M.M., Ladd-Acosta C.M., McArthur D., Pas E.T., Salinas A., Vehorn A., Williams S., Esler A., Grzybowski A., Hall-Lande J., Nguyen R.H.N., Pierce K., Zahorodny W., Hudson A., Hallas L., Mancilla K.C., Patrick M., Shenouda J., Sidwell K., DiRienzo M., Gutierrez J., Spivey M.H., Lopez M., Pettygrove S., Schwenk Y.D., Washington A., Shaw K.A. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years – Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C: 2002), 2023. 72 (2): 1–14.
Main S.L., Kulesza R.J. Repeated prenatal exposure to valproic acid results in cerebellar hypoplasia and ataxia. Neuroscience. 2017. 6 (340): 34–47.
Ming X., Brimacombe M., Wagner G.C. Prevalence of motor impairment in autism spectrum disorders. Brain & Development, 2007. 29: 565–570.
Minshew N.J., Sung K., Jones B.L., Furman J.M. Underdevelopment of the postural control system in autism. Neurology, 2004. 63: 2056–2061.
Mitchell R., Barton S., Harvey A.S., Williams K. Risk factors for the development of autism spectrum disorder in children with tuberous sclerosis complex: protocol for a systematic review. Systematic reviews. 2017. 6: 49. https://doi.org/10.1186/s13643-017-0448-0
Naviaux R.K., Zolkipli Z., Wang L., Nakayama T., Naviaux J.C., Le T.P., Schuchbauer M.A., Rogac M., Tang Q., Dugan L.L., Powell S.B. Antipurinergic therapy corrects the autism-like features in the poly (IC) mouse model. PLoS One, 2013. 8: 57380. https://doi.org/10.1371/journal.pone.0057380
Nimmo-Smith V., Heuvelman H., Dalman C., Lundberg M., Idring S., Carpenter P., Magnusson C., Rai D. Anxiety Disorders in Adults with Autism Spectrum Disorder: A Population-Based Study. Journal of autism and developmental disorders. 2020. 50 (1): 308–318.
Oliveira Á., Illes P., Ulrich H. Purinergic receptors in embryonic and adult neurogenesis. Neuropharmacology, 2016. 104: 272–281.
Ozonoff S., Young G.S., Goldring S., Greiss-Hess L., Herrera A.M., Steele J., Macari S., Hepburn S., Rogers S.J. Gross motor development, movement abnormalities, and early identification of autism. Journal of autism and developmental disorders. 2008. 38 (4): 644–656.
Pan P.Y., Bölte S., Kaur P., Jamil S., Jonsson U. Neurological disorders in autism: A systematic review and meta-analysis. Autism. 2021. 25 (3): 812–830.
Pardo C.A., Meffert M.K. Animal models in autism research: The legacy of Paul H. Patterson. Experimental neurology. 2018. 299 (Pt A): 197–198.
Piek J.P., Dyck M.J. Sensory-motor deficits in children with developmental coordination disorder, attention deficit hyperactivity disorder and autistic disorder. Hum. Mov. Sci., 2004. 23 (3–4): 475–488.
Pierce K., Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biological psychiatry. 2001. 49 (8): 655–664.
Provost B., Heimerl S., Lopez B.R. Levels of gross and fine motor development in young children with autism spectrum disorder. Physical & Occupational Therapy in Pediatrics, 2007. 27: 21–36.
Qi Z., Lyu M., Yang L., Yuan H., Cao Y., Zhai L., Dang W., Liu J., Yang F., Li Y. A Novel and Reliable Rat Model of Autism. Front Psychiatry. 2021. 12: 549810. https://doi.org/10.3389/fpsyt.2021.549810
Rodier P.M., Ingram J.L., Tisdale B., Nelson S., Romano J. Embryological origin for autism: Developmental anomalies of the cranial nerve motor nuclei. Journal of Comparative Neurology. 1996. 370 (2): 247–261.
Ren J., Zhao T., Xu Y., Ye H. Interaction between DISC1 and CHL1 in regulation of neurite outgrowth. Brain Res., 2016. 1648 (Pt A): 290–297.
Saffari A., Arno M., Nasser E., Ronald A., Wong C.C.Y., Schalkwyk L.C., Mill J., Dudbridge F., Meaburn E.L. RNA sequencing of identical twins discordant for autismreveals blood-based signatures implicating immune and transcriptional dysregulation. Mol. Autism, 2019. 10: 38. https://doi.org/10.1186/s13229-019-0285-1
Schneider T., Przewłocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2005. 30 (1): 80–89.
Servadio M., Manduca A., Melancia F., Leboffe L., Schiavi S., Campolongo P., Palmery M., Ascenzi P., di Masi A., Trezza V. Impaired repair of DNA damage is associated with autistic-like traits in rats prenatally exposed to valproic acid. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2018. 28 (1): 85–96.
Singh R., Kisku A., Kungumaraj H., Nagaraj V., Pal A., Kuma S., Sulakhiya K. Autism Spectrum Disorders: A Recent Update on Targeting Inflammatory Pathways with Natural Anti-Inflammatory Agents. Biomedicines. 2023. 11 (1): 115. https://doi.org/10.3390/biomedicines11010115
Smirnova V., Yaikova E., Baltin M., Kharin N., Baltina T., Sachenkov O. Movement estimation methods based on the motion capture system. 2022 Fourth International Conference Neurotechnologies and Neurointerfaces (CNN), 2022. 158–161. https://doi.org/10.1109/CNN56452.2022.9912543
Smith S.E.P., Li J., Garbett K., Mirnics K., Patterson P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007. 27 (40): 10695–10702.
Sui L., Chen M. Prenatal exposure to valproic acid enhances synaptic plasticity in the medial prefrontal cortex and fear memories. Brain research bulletin. 2012. 87 (6): 556–563.
Talos D.M., Sun H., Zhou X., Fitzgerald E.C., Jackson M.C., Klein P.M., Lan V.J., Joseph A., Jensen F.E. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mtor) pathway. Plos One, 2012. 7: 35885.
Taylor M.J., Rosenqvist M.A., Larsson H., Gillberg C., D’Onofrio B.M., Lichtenstein P., Lundström S. Etiology of autism spectrum disorders and autistic traits over time. JAMA Psychiatry, 2020. 77: 936–943. https://doi.org/10.1001/jamapsychiatry.2020.0680
Watson L.R., Baranek G.T., DiLavore P.C. Toddlers with autism: Developmental perspectives. Infants and Young Children, 2003. 16: 201–214.
Widiger T.A., Hines A. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition alternative model of personality disorder. Personality disorders. 2022. 13 (4): 347–355.
Williams M., Prem S., Zhou X., Matteson P., Yeung P.L., Lu C.W., Pang Z., Brzustowicz L., Millonig J.H., Dicicco-Bloom E. Rapid detection of neuro-developmental phenotypes in human neural precursor cells (NPCs). J. Vis. Exp., 2018. 133: 56628.
Wood A. Prenatal exposure to sodium valproate is associated with increased risk of childhood autism and autistic spectrum disorder. Evidence-based nursing. 2014. 17 (3): 84. https://doi.org/10.1136/eb-2013-101422
Zheng W., Hu Y., Chen D., Li Y., Wang S. Improvement of a mouse model of valproic acid-induced autism. Nan Fang Yi Ke Da Xue Xue Bao, 2019. 39 (6): 718–723. https://doi.org/10.12122/j.issn.1673-4254.2019.06.14
Ziganshin A.U., Khairullin A.E., Hoyle C.H.V., Grishin S.N. Modulatory roles of ATP and adenosine in cholinergic neuromuscular transmission. International Journal of Molecular Sciences, 2020. 21 (17): 1–15.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


