Журнал высшей нервной деятельности им. И.П. Павлова, 2022, T. 72, № 2, стр. 149-158
Применение оптических зондов для визуализации внутриклеточного кальция и регистрации потенциалов действия нейронов
Е. С. Никитин 1, *, М. В. Рощин 1, А. А. Бородинова 1, А. Б. Зюзина 1, П. М. Балабан 1
1 Федеральное государственное бюджетное учреждение науки
Институт высшей нервной деятельности и нейрофизиологии РАН
Москва, Россия
* E-mail: nikitin@ihna.ru
Поступила в редакцию 21.07.2021
После доработки 21.07.2021
Принята к публикации 05.10.2021
- EDN: YRYPOH
- DOI: 10.31857/S0044467722010075
Аннотация
На сегодняшний день репортеры внутриклеточного кальция являются самыми распространенными оптическими зондами для детекции активности нейрона. Синтетические низкомолекулярные и генетически кодируемые белковые соединения обеспечивают огромное разнообразие доступных методов регистрации сигналов разной интенсивности на разных уровнях изучения нервной системы, начиная от синаптических бутонов и дендритных шипиков и заканчивая регистрацией активности центральной нервной системы в свободном поведении in vivo. В работе проводится сравнительное описание методик оптической регистрации изменений в концентрации внутриклеточного кальция и сопоставляются оригинальные экспериментальные данные, полученные каждым из описываемых методов. Описаны преимущества и недостатки, а также очерчены возможные области применения каждого из коммерчески доступных и широко используемых видов кальций-чувствительных репортеров.
ВВЕДЕНИЕ
Среди методов визуализации нейрональной активности по праву выделяется регистрация ионов внутриклеточного кальция как наиболее массовый и востребованный метод. Концентрация кальция в нейроне в норме очень низкая и изменяется при генерации потенциала действия на несколько порядков (Gleichmann, Mattson, 2011), что выгодно отличает кальций от других ионов, концентрации которых изменяются не столь значительно. Кроме того, нейроны обладают развитым механизмом буферизации кальция, который эффективно восстанавливает концентрацию кальция после окончания потенциала действия до фоновых значений (Matthews, Dietrich, 2015). Различные вариации регистрации кальция позволяют записывать дискретную спайковую активность многочисленных нейронов in vivo и in situ на уровне нейронной сети, регистрировать надпороговые и подпороговые изменения концентрации кальция на субклеточном уровне в компартментах нейрона, таких как аксон и пресинаптические бутоны (Koester, Johnston, 2005; Roshchin et al., 2018), дендриты и дендритные шипики (Higley, Sabatini, 2012). Регистрируемые кальциевые сигналы не только помогают распознать генерацию потенциалов действия, но и имеют самостоятельное функциональное значение. Так, например, уровень внутриклеточного кальция определяет выброс медиатора из синаптической терминали и амплитуду вызванного постсинаптического потенциала (Koester, Johnston, 2005; Roshchin et al., 2018). Регистрация потенциалов действия представляет собой относительно более простую задачу по сравнению с регистрацией подпороговых сигналов, поскольку при этом активируются не только низкопороговые потенциал-зависимые кальциевые каналы (LVA), но и высокопороговые каналы с высокой проводимостью (LVA, включая L-, P/Q-, N- и R-типы (Catterall, 2000; Grienberger, Konnerth, 2014)), поэтому при достижении определенного порога возникает непропорционально высокий оптический сигнал. Кроме этого, использование оптических зондов позволяет регистрировать и низкоамплитудные флуктуации внутриклеточного кальция, не связанные с изменениями мембранного потенциала (Ross, 2012).
В свете всего сказанного детальная проработка оптических методов регистрации кальция, включая характеристики самих зондов (структура, уровень сродства, стабильность, яркость, выцветание, фототоксичность, линейность и т.п.), способы их доставки и детали оптической системы, необходимой для регистрации, имеют огромное значение для разработки схемы и проведения успешного оптофизиологического эксперимента на соответствующем объекте. В этой работе мы детально рассматриваем наиболее известные методы, позволяющие успешно применить оптическую регистрацию кальция, которые уже многократно доказали свою полезность и важность для выявления принципиально новых деталей физиологии нейронов и их сетей, недоступных другим методам.
НИЗКОМОЛЕКУЛЯРНЫЕ СИНТЕТИЧЕСКИЕ ЗОНДЫ
Низкомолекулярные зонды – производные кальциевых хелаторов
К низкомолекулярным зондам (по сравнению с высокомолекулярными белковыми зондами) кальциевой активности относят синтетические химические соединения-красители, созданные на основе кальциевых хелаторов и флуоресцентных групп, изменяющие интенсивность своей флуоресценции при связывании со свободным внутриклеточным кальцием (табл. 1). Одним из первых зондов, получивших широкое применение, стал синтезированный на основе салицилового альдегида в восьмидесятых годах прошлого века краситель Fura-2 (Grynkiewicz et al., 1985). Зонд Fura-2 не только позволял регистрировать уровень свободного кальция, но также был рациометрическим, т.е. обладал линейностью ответа в достаточном диапазоне, и, при возможности калибровки, позволял получать реалистичные численные значения внутриклеточной концентрации кальция, измеряя отношение флуоресценции (обычно в полосе ~450–550 нм) при возбуждении 340 нм/380 нм (Fowler, Tiger, 1997). Однако из-за требований учета трехмерной структуры образца при калибровке в реальном эксперименте надежные рациометрические измерения с использованием Fura-2 были практически ограничены монослойными культуральными клетками и первичными культурами нейронов, где этот зонд активно используется для быстрого скрининга ответов рецепторов на аппликацию фармакологических агентов (Lin et al., 1999; Blockstein et al., 2012). Зонд Fura-2 имеет множество недостатков, таких как возбуждение УФ-спектром (необходимо использовать кварцевую оптику), недостаточные стабильность в растворе и фотостабильность, а также несовместимость с лазерными источниками возбуждения (Lin et al., 1999). С другой стороны, смещение спектров зондов Fura в фиолетовую область облегчает их комбинированное одновременное применение с красными зондами, такими как стириловые репортеры мембранного потенциала (JPW-1114, JPW-3027/8), из-за минимального перекрытия спектров (Canepari et al., 2008). На смену им пришли предложенные производителем Molecular Probes зонды серии Oregon Green BAPTA (OGB) и Calcium Green, выгодно отличающиеся повышенной стабильностью, яркостью и соотношением сигнал/шум при регистрации относительного изменения во флуоресценции (Sah, Clements, 1999; Zheng et al., 2015). Эти зонды были оптимизированы под максимум возбуждения 488 нм (полоса широкодоступных газовых и твердотельных лазеров) и флуоресценцию от 510 нм и выше. OGB1 на основе хелатора BAPTA до сегодняшнего дня является одним из самых широко используемых зондов для исследований кальциевых сигналов в нейрональных компартментах нейронов млекопитающих (см. обзор (Grienberger, Konnerth, 2014)) и беспозвоночных (Kemenes et al., 2006; Nikitin et al., 2005, Nikitin et al., 2013). Зонд обеспечивает регистрацию с сигналом до десятков процентов по отношению к фоновой флуоресценции, позволяет отводить сигнал от тонких отростков нейронов на препаратах переживающей ЦНС или срезов мозга (рис. 1). Поскольку базовая флуоресценция тонких отростков, заполненных OGB1, очень низкая и часто не отличается от фоновой флуоресценции, в добавление к OGB1 обычно используют дополнительный, более яркий флуоресцентный морфологический трейсер – краситель с эмиссией в другой полосе и обладающий высокой фотостабильностью, такой как Alexa Fluor 594 (Koester, Johnston, 2005), необходимый для того, чтобы высветить контур интересующего отростка и облегчить фокусировку и эффективное считывание кальциевого сигнала. Кроме того, для оптической регистрации одновременно с активацией фоточувствительных соединений (таких как caged-Ca2+) была создана версия OGB2 (аналог Calcium Green-2), имеющая близкие полосы возбуждения и эмиссии, но содержащая две флуоресцентные группы, гасящие друг друга при отсутствии кальция, что обеспечивает низкую фоновую флуоресценцию и, как следствие, отсутствие ложного сигнала и фотовыцветания при фотоактивации (Wang, Augustine, 1995; Roshchin et al., 2018). Другие варианты – OGB5 и 6 – обладают более низким сродством к кальцию, чем OGB1, и они предназначаются для детекции более сильных изменений концентрации кальция (табл. 1). Реже применяемая для регистрации нейронов линейка аналогичных зондов выпускается под маркой Fluo (2–5).
Таблица 1.
Сравнение основных коммерчески доступных кальциевых зондов, используемых для регистрации нейрональной активности. Сравниваются недостатки и преимущества каждого зонда, определяющие область применения в нейрофизиологии. * – Линейка современных зондов с варьирующим сродством к кальцию для детекции сигналов разной интенсивности. Недавно выпущены более новые зонды серии jGCaMP7 (Dana et al., 2019), но они пока широко не используются Table 1. Comparison of the most common and commercially available probes of calcium that are employed for imaging of neuronal activity. Advantages and disadvantages of each probe are compared to determine their best possible usage in neurophysiology. * – a range of probes with varying calcium affinity for detection of different signal amplitudes. Newer probes jGCaMP7 (Dana et al., 2019) are already available, but they are not commonly used yet
| Названия зондов | Способ доставки в нейрон | Преимущества | Недостатки |
|---|---|---|---|
| Fura-2 | Внутриклеточная инъекция и патч-перфузия | Рациометрические измерения, контролируемая концентрация зонда | Возбуждение УФ, низкие сигнал и стабильность, высокое выцветание |
| Oregon Green BAPTA 1 (5, 6), Calcium Green 1 | Внутриклеточная инъекция, патч-перфузия | Сигнал/шум, умеренная фоновая флуоресценция, контролируемая концентрация зонда | Умеренное выцветание, узкая палитра спектров |
| Oregon Green BAPTA 2, Fluo-3(4, 5) | Внутриклеточная инъекция, патч-перфузия | Высокий сигнал/шум, контролируемая концентрация зонда, применение вместе с фотоактивируемыми веществами | Низкая фоновая флуоресценция |
| Calcium Green 1 –AM, Fura-2 AM, Fluo-4 (5) AM | Прямое нанесение раствора на ткани | Сигнал/шум, мультинейронная регистрация, умеренно контролируемая концентрация зонда | Нестабильность эфирных соединений, сложность аппликации, ограниченная область |
| Oregon Green BAPTA-1 dextran, Calcium Green 1 dextran | Внутриклеточная инъекция, пиноцитоз, электропорация | Применение in vivo, ретроградное окрашивание, стабильность | Сложность применения, ограниченная область |
| GCaMP6s (f, m)* зеленый, RGeco (1) красный | Электропорация, вирусная трансдукция, химическая трансфекция, генная пушка | Генетический таргетинг популяций и компартментов нейронов, фотостабильность, высокий сигнал/шум, применение in vivo, палитра спектров | Широкие спектры, нет контроля концентрации, фоновая флуоресценция, взаимодействие с белками |
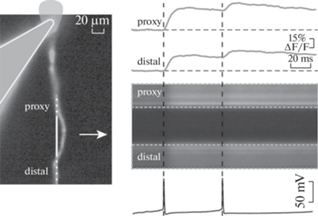
Рис. 1.
Пример оптической регистрации потенциалов действия в компартментах нейрона (здесь – апикальный дендрит) с использованием флуоресцентного зонда OGB1, доставленного внутриклеточно через патч-пипетку, и сканирования линии. Слева показан конфокальный кадр с эпифлуоресцентным изображением нейрона и патч-пипетки (контур слева). Отмечены области линии, соответствующие проксимальной (proxy) и дистальной (distal) частям апикального дендрита. Справа показана временная развертка сканирования линии. Серыми прямоугольниками выделены области усреднения. Сверху от развертки показан усредненный кальциевый ответ от времени, снизу – электрофизиологическая регистрация потенциалов действия. Моменты потенциалов действия отмечены вертикальными черными прерывистыми линиями для синхронизации с оптической регистрацией. Получено авторами.
Fig. 1. Example of optical recording of action potentials in the neuronal compartments (here, an apical dendrite) using the linescan and epifluorescent probe OGB1 delivered through the patch pipette. Left, a confocal frame of the neuron and patch pipette (silhouette on the left side). The linescan regions corresponded to proximal (proxy) and more distal (distal) parts of the apical dendrite are shown. Right, time series of linescan. Gray squares demarcates the areas of signal averaging. Top left, trace show averaged calcium transients against time. Bottom left, electrophysiological recording of action potentials (timing: vertical dotted lines). Recordings obtained by the authors.
Способы доставки низкомолекулярных зондов
Существует два основных способа доставки низкомолекулярных зондов внутрь нейрона: (1) внутриклеточно через регистрирующую патч-пипетку или острый электрод под давлением для мембранно-непроникающих зондов или (2) аппликация специально модифицированной AM-формы (мембранно-проникающая форма, ацетоксиметиловый эфир) зонда на препарат в виде раствора.
Первый способ позволяет очень точно контролировать концентрацию зонда, которую в случае патч-пипетки в режиме целой клетки (whole cell) принимают равной концентрации в пипетке, поскольку клетка перфузируется через открытое отверстие из кончика пипетки. Это позволяет избежать чрезмерной буферизации цитоплазмы зондом (поскольку он обладает кальций-связывающими свойствами) и получать воспроизводимую форму потенциала действия. При аппликации давлением через острый электрод тоже можно контролировать концентрацию зонда, варьируя число коротких импульсов давления (Nikitin et al., 2013).
При аппликации в раствор эфирная форма зонда (AM) легко проникает сквозь мембрану и гидролизуется внутри нейрона эстеразами, переводя зонд в мембранно-непроницаемую форму. Таким способом невозможно окрасить одиночные нейроны, поскольку зонд находится в растворе. Сложность применения состоит в низкой стабильности эфирной формы, а также необходимости использовать свежий полностью безводный диметилсульфоксид для растворения зонда перед окраской. Однако таким способом на культурах и срезах мозга можно регистрировать мультинейронную активность нейронной сети и выделять активность отдельных клеток по их контурам (рис. 2). При значительном усложнении методики возможно даже зарегистрировать единичную активность нескольких нейронов in vivo (Grewe et al., 2010).
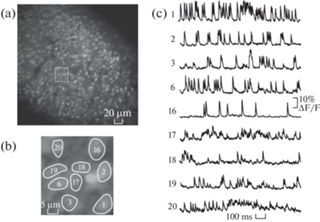
Рис. 2.
Регистрация спонтанной активности нейронов (около 400) переживающего среза энторинальной коры с использованием красителя Oregon Green BAPTA 1-AM и конфокального микроскопа LSM 5 Live. (a) – общий вид кадра, получаемого с использованием объектива 63x и сканирования 256 × 256 (256 строк по 256 пикселей). (b) – репрезентативная область кадра (выделена прямоугольником на (a)) с отмеченными телами нейронов, сигнал от которых усредняется по области. (с) Усредненные сигналы (“трейсы”) от областей тел нейронов, отмеченных на (b), измеренные как относительное изменение флуоресценции от времени. Получено авторами.
Fig. 2. Optical recording of multineuronal activity (~400) in the acute slice of the enthorinal cortex using the probe Oregon Green BAPTA 1-AM and a confocal microscope LSM 5 Live. (a) Frame obtained with a single 256 × 256 scan using a 63x objective lens. (b) Representative frame (shown on (a) with a square) with highlighted neuronal bodies as zones of signal averaging. (c) Calcium traces obtained for these areas of neuronal bodies, shown in (b) were measured as relative change in fluorescence against time. Data obtained by the authors.
Кроме того, существует еще один несколько необычный способ окрашивания мономолекулярными зондами, подразумевающий использование декстран-конъюгированых соединений зонда с повышенной стабильностью, – это совместная инкубация с агентом, стимулирующим пиноцитоз (Friedrich, Kor-sching, 1997) или приложение электрического тока (электропорация (Hovis et al., 2010)) для облегчения проникновения зонда в клетки. Например, таким способом были впервые визуализированы ответы гломерул обонятельных луковиц после ретроградного окрашивания с нанесением декстрановой формы зонда на обонятельный эпителий (Wachowiak, Cohen, 2001). Однако из-за трудоемкости метод применялся очень ограниченным числом лабораторий, и сейчас уже доступны гораздо более удобные альтернативы с использованием генетически-кодируемых зондов (Storace et al., 2015), и, в частности, для ретроградного окрашивания успешно используются векторы на основе вируса бешенства, проникающие через синаптическую щель (Osakada et al., 2012), и векторы на основе аденоассоциированного вируса, проникающие через активные синаптические терминали нейронов (Tervo et al., 2016).
Низкомолекулярные зонды красного спектра
Кроме красителей зеленого спектра (OGB1 и аналоги, возбуждение: ~488 нм, эмиссия: 510LP) были разработаны подобные кальциевые зонды (Fura-red, Calcium Orange, Rhod-2/3), но со сдвигом спектра в красную область, что позволило бы применять их независимо и одновременно с красителями зеленого спектра или, например, совместно с такими широко используемыми белками, как зеленый GFP в условиях наложения флуоресцентного сигнала. Однако были выявлены проблемы с загрузкой и неравномерным распределением этих красителей внутри нейрона, что критически затрудняет их использование для регистрации нейрональной активности (Wilson et al., 2007). Впоследствии Rhod-3-AM (в виде ацетоксиметилового эфира) использовался в отдельных работах для имаджинга суммарного кальциевого сигнала срезов мозга, не требующих дискриминации сигналов отдельных нейронов (Kumada, Komuro, 2004; Wang et al., 2014), но дальнейшего распространения для регистрации нейрональной активности этот зонд не получил и полностью устарел в свете появления генетически-кодируемых красных белков-репортеров.
БЕЛКОВЫЕ ЗОНДЫ
Генетически кодируемые кальциевые репортеры
На основе флуоресцентных белков, получивших самое широкое распространение в биомедицинских исследованиях последних десятилетий, были созданы генетически кодируемые флуоресцентные кальциевые репортеры как альтернатива низкомолекулярным зондам. Самый известный из них был сконструирован путем слияния трех белков: зеленого флуоресцентного белка GFP, кальций-чувствительного белка кальмодулина (CaM, умеренное сродство к кальцию (Mangialavori et al., 2010)) и последовательности легкой цепи миозина (Nakai et al., 2001). Когда GCaMP связывается с кальцием, его флуоресценция дает пик на волне ~510 нм при максимуме возбуждающего спектра ~480 нм (Barnett et al., 2017). После этого структура репортера GCaMP подверглась многочисленным доработкам и оптимизации для увеличения чувствительности и сигнала (Tian et al., 2009; Akerboom et al., 2012), что практически позволило им сравняться по этим характеристикам с низкомолекулярными синтетическими зондами свободного кальция. В современных исследованиях последним наиболее широко используемым зондом стал GCaMP6 (табл. 1), оптимизированный специально для регистрации отдельных потенциалов действия в соме, дендритах и дендритных шипиках нейронов in situ и in vivo (Chen et al., 2013). Всего было произведено три разновидности GCaMP6 – это “s” (наивысшая амплитуда сигнала), “m” (промежуточный) и “f” (наилучшая скорость возврата к базовой флуоресценции. Амплитуда сигнала GCaMP6s на диссоциированной культуре нейронов достигала 30% ΔF/F при одном потенциале действия и 50% ΔF/F при десяти вызванных потенциалах действия (Chen et al., 2013).
Направление белковых зондов в определенные типы клеток и их компартменты (таргетинг)
Для того чтобы получить сигнал от генетически кодируемого репортера в нейроне, необходимо вызвать достаточную его экспрессию в этом нейроне, т.е. с помощью собственных клеточных механизмов вызвать трансляцию белка с инородного гена, доставленного в клетку генно-инженерными способами. Для этого используются плазмиды, содержащие промоторы, обеспечивающие считывание и производство флуоресцентного белка в клетке. Одним из самых простых вариантов является промотор цитомегаловируса (CMV), не обладающий специфичностью к классам зараженных клеток и вызывающий заражение нейронов и глии (van den Pol et al., 1999). Этот промотор подходит для трансфекции культуры электропорацией или химическими методами, а также позволяет трансфицировать клетки переживающих срезов с использованием генной пушки (Matlashov et al., 2015; Roshchin et al., 2018). Поскольку при использовании промотора CMV для трансфекции эмбриона наблюдается подавление экспрессии во взрослом состоянии, для преодоления этого был создан модифицированный промотор pCAG путем добавления энхансера бета-актина (Alexopoulou et al., 2008). Хотя pCAG не обладает клеточной избирательностью, тем не менее с его помощью можно трансфицировать выбранную группу нейронов коры, если проводить электропорацию in utero (или вирусную), инъецируя в соответствующий желудочек на определенный день развития, когда формируются предшественники нейронов, например, пирамидных клеток пятого слоя (E14-E15) (LoTurco et al., 2009).
Возможность более точного и удобного направления белковых зондов в специфическую группу нейронов предоставляет клеточно-специфический таргетинг, использующий промоторы, которые позволяют экспрессировать зонд только при попадании вектора в определенный тип клеток. Например, промотор a-CaMKII, широко применяемый в современных исследованиях, позволяет получить специфичность трансфекции глутаматергических нейронов до 90% на моделях in vivo (Wang et al., 2013; Watakabe et al., 2015). Специфические промоторы были также сконструированы для классов интернейронов SST+ (соматостатин) и PV+ (парвальбумин) (Mehta et al., 2019).
Однако наиболее благоприятные возможности, недостижимые для низкомолекулярных зондов, предоставляет таргетинг белковых зондов в специфические группы нейронов in vivo с использованием группоспецифических промоторов и субклеточный таргетинг в определенные компартменты нейронов, опирающийся на мотивы внутриклеточного транспорта. Слияние GCaMP с последовательностью синаптофизина (цитоплазматического белка, ассоциированного с синаптическими везикулами) позволило регистрировать изменения в пресинаптическом кальции в области синапсов (Dreosti et al., 2009). Соответственно, направление зонда GCaMP в дендриты (Mao et al., 2008) и дендритные шипики (Reese, Kavalali, 2016) позволило оптически регистрировать сигналы от этих структур. Элегантное применение было найдено для таргетинга GCaMP, позволившее значительно улучшить регистрацию сигналов нейронов in vivo. Поскольку в мозге у нейронов много переплетающихся отростков, при использовании in vivo оптики с достаточно низкой числовой апертурой (для увеличения числа нейронов в поле зрения) сигналы разных нейронов и их отростков накладываются и суммируются по области регистрации. Для локализации преодоления смешивания сигналов активных нейронов было предложено использовать локализующийся в ядре H2B-GCaMP (рис. 3), полученный путем слияния с последовательностью ядерного гистона (Cong et al., 2017). Этот зонд слияния позволяет определять спайковую активность нейрона только в месте расположения его ядра и сомы, но не в областях проекций его отростков.
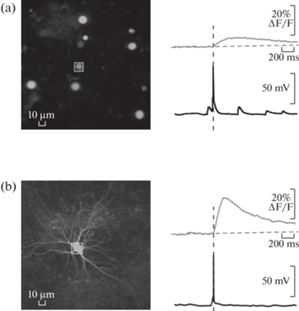
Рис. 3.
Сравнение оптической регистрации потенциалов действия с использованием флуоресцентных зондов GCaMP6 ядерной (a) и цитоплазматической (b) локализации на диссоциированной нейрональной культуре. Слева показаны конфокальные кадры с эпифлуоресцентным изображением ядра (a) и всего нейрона (b), одновременно регистрируемого стеклянным патч-электродом (невидим) с областью усреднения внутри квадратов. Справа показаны регистрации потенциала от времени (снизу) и кальциевого ответа (записи сверху над потенциалами действия, усреднение по области). Моменты потенциала действия отмечены вертикальными черными прерывистыми линиями. Получено авторами.
Fig. 3. Comparison of optical recordings of action potentials obtained with GCaMP6 probes localized either in the nucleus (a) or in the cytoplasm (b) of the cultured neurons. Left, confocal frames with epifluorescent nuclei (a) or the whole neuron (b), recorded simultaneously with a patch pipette (invisible for epifluorescent imaging) with averaging within the square-marked areas. Right, recordings of the membrane potential and averaged calcium transient responses (above each action potential) are plotted against time. Action potential timings are shown by vertical dotted lines. Data obtained by the authors.
Интерференция с кальмодулином и способы ее преодоления
Поскольку наиболее широко используемые белки GCaMP созданы на основе модуляторного белка CaM (кальмодулина), то сам зонд потенциально способен аллостерически модулировать функциональные белки и каналы нейрона (Adams et al., 2014; Chang et al., 2018), и, в частности, есть данные по взаимодействию GCaMP-зондов и кальциевых Cav1-каналов (Yang et al., 2018). Подобная интерференция может не только нарушить взаимодействие канала с нормальным белком CaM, но и повлиять на уровень внутриклеточного кальция и кальциевый сигнал, вызывая аномальную нейрональную активность (Steinmetz et al., 2017). С целью исправления этого недостатка был создан видоизмененный зонд GCaMP-X, конструирование которого было попыткой снизить нежелательные взаимодействия последовательности CaM, добавляя к ней дополнительный мотив apoCaM, затрудняющий связывание с каналами Cav1 (Yang et al., 2018). Тем не менее полного описания интерференции CaM-мотивов зондов GCaMP и внутриклеточных мишеней CaM на сегодняшний день не существует. В качестве альтернативы предлагается вообще заменить кальций-чувствительную часть зонда, используя вместо CaM другие белки-сенсоры, не обладающие столь же многофункциональными модулирующими свойствами. Были предложены несколько вариантов сенсоров на основе тропонина-С (Heim, Griesbeck, 2004), продемонстрировавшие более линейный сигнал по сравнению с оригинальными GCaMP и подходящие, в том числе, для визуализации потенциалов действия нейронов (Barykina et al., 2016).
ЗАКЛЮЧЕНИЕ
На сегодняшний день репортеры уровня внутриклеточного кальция являются самыми распространенными оптическими зондами для детекции активности нейрона. Синтетические низкомолекулярные и белковые генетически кодируемые соединения обеспечивают огромное разнообразие доступных методов для регистрации сигналов разной интенсивности на разных уровнях изучения нервной системы, начиная от синаптических бутонов и дендритных шипиков и заканчивая регистрацией активности комплексных нейронных сетей в свободном поведении in vivo.
Изучение низкомолекулярных синтетических зондов выполнено при поддержке гранта РНФ 20-15-00408. Эксперименты с генетически кодируемыми белками-репортерами выполнены при поддержке гранта РНФ 20-75-00090.
Список литературы
Adams P.J., Ben-Johny M., Dick I.E., Inoue T., Yue D.T. Apocalmodulin itself promotes ion channel opening and Ca(2+) regulation. Cell. 2014. 159 (3): 608–622.
Akerboom J., Chen T.W., Wardill T.J., Tian L., Marvin J.S., Mutlu S., Calderón N.C., Esposti F., Borghuis B.G., Sun X.R., Gordus A., Orger M.B., Portugues R., Engert F., Macklin J.J., Filosa A., Aggarwal A., Kerr R.A., Takagi R., Kracun S., Shigetomi E., Khakh B.S., Baier H., Lagnado L., Wang S.S., Bargmann C.I., Kimmel B.E., Jayaraman V., Svoboda K., Kim D.S., Schreiter E.R., and Looger L.L. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012. 32 (40): 13819–13840.
Alexopoulou A.N., Couchman J.R., Whiteford J.R. The CMV early enhancer/chicken beta actin (CAG) promoter can be used to drive transgene expression during the differentiation of murine embryonic stem cells into vascular progenitors. BMC cell biology. 2008. 9: 2–2.
Barnett L.M., Hughes T.E., Drobizhev M. Deciphering the molecular mechanism responsible for GCaMP6m's Ca2+-dependent change in fluorescence. PLoS One. 2017. 12 (2): e0170934.
Barykina N.V., Subach O.M., Doronin D.A., Sotskov V.P., Roshchina M.A., Kunitsyna T.A., Malyshev A.Y., Smirnov I.V., Azieva A.M., Sokolov I.S., Piatkevich K.D., Burtsev M.S., Varizhuk A.M., Pozmogova G.E., Anokhin K.V., Subach F.V., Enikolopov G.N. A new design for a green calcium indicator with a smaller size and a reduced number of calcium-binding sites. Scientific Reports. 2016. 6 (1): 34447.
Blockstein L., Luk C.C., Mudraboyina A.K., Syed N.I., Yadid-Pecht O. A PVAc-Based Benzophenone-8 Filter as an Alternative to Commercially Available Dichroic Filters for Monitoring Calcium Activity in Live Neurons via Fura-2 AM. IEEE Photonics Journal. 2012. 4 (3): 1004–1012.
Canepari M., Vogt K., Zecevic D. Combining voltage and calcium imaging from neuronal dendrites. Cell Mol Neurobiol. 2008. 28 (8): 1079–1093.
Catterall W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000. 16: 521–555.
Chang A., Abderemane-Ali F., Hura G.L., Rossen N.D., Gate R.E., Minor D.L., Jr. A Calmodulin C-Lobe Ca(2+)-Dependent Switch Governs Kv7 Channel Function. Neuron. 2018. 97 (4): 836–852 e6.
Chen T.-W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A., Schreiter E.R., Kerr R.A., Orger M.B., Jayaraman V., Looger L.L., Svoboda K., Kim D.S. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013. 499 (7458): 295–300.
Cong L., Wang Z., Chai Y., Hang W., Shang C., Yang W., Bai L., Du J., Wang K., Wen Q. Rapid whole brain imaging of neural activity in freely behaving larval zebrafish (Danio rerio). eLife. 2017. 6: e28158.
Dana H., Sun Y., Mohar B., Hulse B.K., Kerlin A.M., Hasseman J.P., Tsegaye G., Tsang A., Wong A., Patel R., Macklin J.J., Chen Y., Konnerth A., Jayaraman V., Looger L.L., Schreiter E.R., Svoboda K., Kim D.S. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat Methods. 2019. 16 (7): 649–657.
Dreosti E., Odermatt B., Dorostkar M.M., Lagnado L. A genetically encoded reporter of synaptic activity in vivo. Nat Methods. 2009. 6 (12): 883–889.
Fowler C.J., Tiger G. Calibration of Fura-2 signals introduces errors into measurement of thrombin-stimulated calcium mobilisation in human platelets. Clin Chim Acta. 1997. 265 (2): 247–261.
Friedrich R.W., Korsching S.I. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997. 18 (5): 737–52.
Gleichmann M., Mattson M.P. Neuronal calcium homeostasis and dysregulation. Antioxidants & redox signaling. 2011. 14 (7): 1261–1273.
Grewe B.F., Langer D., Kasper H., Kampa B.M., Helmchen F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nature Methods. 2010. 7 (5): 399–405.
Grienberger C., Konnerth A. Imaging calcium in neurons. Neuron. 2012. 73 (5): 862–85.
Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985. 260 (6): 3440–3450.
Heim N., Griesbeck O. Genetically encoded indicators of cellular calcium dynamics based on troponin C and green fluorescent protein. J Biol Chem. 2004. 279 (14): 14280–14286.
Higley M.J., Sabatini B.L. Calcium signaling in dendritic spines. Cold Spring Harbor perspectives in biology. 2012. 4 (4): a005686-a005686.
Hovis K.R., Padmanabhan K., Urban N.N. A simple method of in vitro electroporation allows visualization, recording, and calcium imaging of local neuronal circuits. Journal of neuroscience methods. 2010. 191 (1): 1–10.
Kemenes I., Straub V.A., Nikitin E.S., Staras K., O’Shea M., Kemenes G., Benjamin P.R. Role of Delayed Nonsynaptic Neuronal Plasticity in Long-Term Associative Memory. Current Biology. 2006. 16 (13): 1269–1279.
Koester H.J., Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science. 2005. 308 (5723): 863–866.
Kumada T., Komuro H. Completion of neuronal migration regulated by loss of Ca(2+) transients. Proceedings of the National Academy of Sciences of the United States of America. 2004. 101 (22): 8479–8484.
Lin K., Sadée W., Quillan J.M. Rapid measurements of intracellular calcium using a fluorescence plate reader. Biotechniques. 1999. 26 (2): 318–22, 324–6.
LoTurco J., Manent J.-B., Sidiqi F. New and improved tools for in utero electroporation studies of developing cerebral cortex. Cerebral cortex (New York, N.Y.: 1991). 2009. 19 Suppl 1(Suppl 1): i120–i125.
Mangialavori I., Ferreira-Gomes M., Pignataro M.F., Strehler E.E., Rossi J.P. Determination of the dissociation constants for Ca2+ and calmodulin from the plasma membrane Ca2+ pump by a lipid probe that senses membrane domain changes. J Biol Chem. 2010. 285 (1): 123–130.
Mao T., O’Connor D.H., Scheuss V., Nakai J., Svoboda K. Characterization and Subcellular Targeting of GCaMP-Type Genetically-Encoded Calcium Indicators. PLOS ONE. 2008. 3 (3): e1796.
Matlashov M.E., Bogdanova Y.A., Ermakova G.V., Mishina N.M., Ermakova Y.G., Nikitin E.S., Balaban P.M., Okabe S., Lukyanov S., Enikolopov G., Zaraisky A.G., Belousov V.V. Fluorescent ratiometric pH indicator SypHer2: Applications in neuroscience and regenerative biology. Biochimica et Biophysica Acta (BBA) - General Subjects. 2015. 1850 (11): 2318–2328.
Matthews E.A., Dietrich D. Buffer mobility and the regulation of neuronal calcium domains. Frontiers in cellular neuroscience. 2015. 9: 48–48.
Mehta P., Kreeger L., Wylie D.C., Pattadkal J.J., Lusignan T., Davis M.J., Turi G.F., Li W.-K., Whitmire M.P., Chen Y., Kajs B.L., Seidemann E., Priebe N.J., Losonczy A., Zemelman B.V. Functional Access to Neuron Subclasses in Rodent and Primate Forebrain. Cell reports. 2019. 26 (10): 2818–2832.e8.
Nakai J., Ohkura M., Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001. 19 (2): 137–141.
Nikitin Evgeny S., Balaban Pavel M., Kemenes G. Nonsynaptic Plasticity Underlies a Compartmentalized Increase in Synaptic Efficacy after Classical Conditioning. Current Biology. 2013. 23 (7): 614–619.
Nikitin E.S., Zakharov I.S., Samarova E.I., Kemenes G., Balaban P.M. Fine tuning of olfactory orientation behaviour by the interaction of oscillatory and single neuronal activity. European Journal of Neuroscience. 2005. 22 (11): 2833–2844.
Osakada F., Mori T., Cetin A.H., Marshel J.H., Virgen B., Callaway E.M. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron. 2011. 71 (4): 617–631.
Roshchin M.V., Matlashov M.E., Ierusalimsky V.N., Balaban P.M., Belousov V.V., Kemenes G., Staras K., Nikitin E.S. A BK channel-mediated feedback pathway links single-synapse activity with action potential sharpening in repetitive firing. Sci Adv. 2018. 4 (7): eaat1357.
Ross W.N. Understanding calcium waves and sparks in central neurons. Nat Rev Neurosci. 2012. 13 (3): 157–168.
Sah P., Clements J.D. Photolytic manipulation of [Ca2+]i reveals slow kinetics of potassium channels underlying the afterhyperpolarization in hippocampal pyramidal neurons. J Neurosci. 1999. 19 (10): 3657–3664.
Steinmetz N.A., Buetfering C., Lecoq J., Lee C.R., Peters A.J., Jacobs E.A.K., Coen P., Ollerenshaw D.R., Valley M.T., de Vries S.E.J., Garrett M., Zhuang J., Groblewski P.A., Manavi S., Miles J., White C., Lee E., Griffin F., Larkin J.D., Roll K., Cross S., Nguyen T.V., Larsen R., Pendergraft J., Daigle T., Tasic B., Thompson C.L., Waters J., Olsen S., Margolis D.J., Zeng H., Hausser M., Carandini M., and Harris K.D. Aberrant Cortical Activity in Multiple GCaMP6-Expressing Transgenic Mouse Lines. eNeuro. 2017. 4 (5).
Storace D.A., Braubach O.R., Jin L., Cohen L.B., Sung U. Monitoring Brain Activity with Protein Voltage and Calcium Sensors. Scientific Reports. 2015. 5 (1): 10212.
Tervo D.G., Hwang B.Y., Viswanathan S., Gaj T., Lavzin M., Ritola K.D., Lindo S., Michael S., Kuleshova E., Ojala D., Huang C.C., Gerfen C.R., Schiller J., Dudman J.T., Hantman A.W., Looger L.L., Schaffer D.V., Karpova A.Y. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron. 2016. 92 (2):372–382. https://doi.org/10.1016/j.neuron.2016.09.021
Tian L., Hires S.A., Mao T., Huber D., Chiappe M.E., Chalasani S.H., Petreanu L., Akerboom J., McKinney S.A., Schreiter E.R., Bargmann C.I., Jayaraman V., Svoboda K., Looger L.L. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009. 6 (12): 875–881.
van Den Pol A.N., Mocarski E., Saederup N., Vieira J., Meier T.J. Cytomegalovirus cell tropism, replication, and gene transfer in brain. The Journal of Neuroscience: the official journal of the Society for Neuroscience. 1999. 19 (24): 10948–10965.
Wachowiak M., Cohen L.B. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001. 32 (4): 723–735.
Wang S.S., Augustine G.J. Confocal imaging and local photolysis of caged compounds: dual probes of synaptic function. Neuron. 1995. 15 (4): 755–760.
Wang T., Kumada T., Morishima T., Iwata S., Kaneko T., Yanagawa Y., Yoshida S., Fukuda A. Accumulation of GABAergic neurons, causing a focal ambient GABA gradient, and downregulation of KCC2 are induced during microgyrus formation in a mouse model of polymicrogyria. Cerebral cortex (New York, N.Y.: 1991). 2014. 24 (4): 1088–1101.
Watakabe A., Ohtsuka M., Kinoshita M., Takaji M., Isa K., Mizukami H., Ozawa K., Isa T., Yamamori T. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci Res. 2015. 93: 144–157.
Wilson J.M., Dombeck D.A., Díaz-Ríos M., Harris-Warrick R.M., Brownstone R.M. Two-Photon Calcium Imaging of Network Activity in XFP-Expressing Neurons in the Mouse. Journal of Neurophysiology. 2007. 97 (4): 3118–3125.
Yang Y., Liu N., He Y., Liu Y., Ge L., Zou L., Song S., Xiong W., Liu X. Improved calcium sensor GCaMP-X overcomes the calcium channel perturbations induced by the calmodulin in GCaMP. Nature Communications. 2018. 9 (1): 1504.
Zheng K., Bard L., Reynolds J.P., King C., Jensen T.P., Gourine A.V., Rusakov D.A. Time-Resolved Imaging Reveals Heterogeneous Landscapes of Nanomolar Ca(2+) in Neurons and Astroglia. Neuron. 2015. 88 (2): 277–288.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


