Журнал высшей нервной деятельности им. И.П. Павлова, 2020, T. 70, № 4, стр. 543-576
ПОТЕНЦИАЛЬНОЕ РЕШЕНИЕ “ТРУДНОЙ ПРОБЛЕМЫ” СОЗНАНИЯ
Игумен Феофан (В. И. Крюков) *
Данилов монастырь
Москва, Россия
* E-mail: kryukov@msdm.ru
Поступила в редакцию 15.02.2020
После доработки 02.03.2020
Принята к публикации 03.03.2020
Аннотация
Эта статья является продолжением и развитием нашей статьи, посвященной модели памяти и внимания [Игумен Феофан, 2004]. После краткого напоминания результатов этой статьи описаны шесть взаимосвязанных проблем сознания, которые не имеют пока удовлетворительного решения. Показано, что четыре из них могут быть решены с помощью теории внимания О.С. Виноградовой, доминанты А.А. Ухтомского и небольшой модификации ранее опубликованной модели “Нейролокатор”. Приведено большое количество новых данных, согласных с этой моделью или ее подтверждающих. В рамках новой модели так называемая “трудная проблема” не имеет решения, однако наша модель помогает понять, почему эта проблема трудная, а также какие дополнительные свойства памяти необходимо учесть для объяснения субъективного опыта, и почему учение А.А. Ухтомского о доминанте на лицо другого дает решение так называемой “более трудной проблемы” сознания. Статья посвящена памяти О.С. Виноградовой, результаты и идеи которой оказались решающими для настоящей статьи.
ВВЕДЕНИЕ
Центральным событием в изучении сознания явилась формулировка проблемы сознания, предложенная молодым философом Чалмерсом в 1994 г. которая говорит о существовании так называемой “трудной проблемы” сознания и которая звучит так: каким образом и почему мозг способен породить субъективный или феноменальный опыт, почему физическая переработка информации дает начало богатой внутренней жизни? С тех пор и до наших дней не прекращаются попытки либо отвергнуть проблему как иллюзорную, либо объяснить ее как естественное следствие физиологического механизма работы мозга, либо констатировать невозможность ее решения из-за так называемого провала в объяснении, связанного с принципиальными ограничениями познания11.
Сторонники последнего направления, известного в аналитической философии под названием нового мистерианства, утверждают, что природа сознания в принципе не познаваема из-за когнитивных ограничений, возникших в процессе эволюции [Воронов, 2010]. Напротив, в работе [Chalmers, 1995] утверждается, что сознание может быть понято как натуральное явление, и здесь предложен проект нередукционистской теории сознания, основанный на трех принципах: структурной согласованности, организационной инвариантности и двухаспектного представления информации. Однако этот проект не позволяет осознать фундаментальное ограничение на то, почему до сих пор нет решения “трудной проблемы”. В настоящей статье предложена другая стратегия: начать не с теории сознания, а с фундаментальных принципов работы мозга, с помощью которых можно попытаться понять основные когнитивные факты. И лишь потом вернуться к проблеме сознания на основе упомянутой структурной согласованности работы мозга и сознания. В этом случае, как будет показано ниже, есть надежда понять фундаментальное ограничение на решение “трудной проблемы”, т.е. понять, может или не может сознание быть физическим.
ТРИ ГЛАВНЫХ ПРИНЦИПА ФУНКЦИОНИРОВАНИЯ МОЗГА
Принцип доминанты
Принцип доминанты А.А. Ухтомского в кратком изложении состоит в том, что в каждый момент времени в нервной системе существует лишь одна активная доминирующая констелляция (созвездие, очаг) совозбужденных локальных нейронных групп или центров, характеризующаяся единым ритмом и единым действием (поведением) и тормозящая прочие центры. Одни и те же отдельные центры или группы нейронов могут входить в состав различных доминирующих констелляций, причем вхождение в данную констелляцию или выключение из нее определяется способностью этих групп или центров усваивать единый темп и ритм активности. Следы прежних доминант длительно сохраняются в высших отделах нервной системы и при полном или частичном восстановлении первоначальных условий могут вспыхнуть вновь полностью или частично. В высших этажах и в коре полушарий принцип доминанты является физиологической основой акта внимания и предметного мышления.
Кроме перечисленных интегральных свойств доминанты, доминанта характеризуется следующими пятью локальными свойствами:
– повышенная возбудимость: для того, чтобы группа стимулов могла войти в доминанту, порог возбудимости доминанты должен быть ниже, чем сила приходящего возбуждения;
– стойкость возбуждения: для того, чтобы возбуждение могло произвести заметный поведенческий эффект, оно не должно чрезмерно быстро изменяться во времени;
– способность суммировать возбуждение: способность накапливать возбуждение не только от специфической, но и от неспецифической стимуляции;
– инерция, т.е. способность сохранять состояние возбуждения, если первоначальный стимул уже миновал;
– “сопряженное” торможение, т.е. способность исключать из доминанты те центры, чья активность функционально несовместима с активностью доминантной констелляции.
Ухтомский писал: “Доминанта есть не теория и даже не гипотеза, но преподносимый из опыта принцип очень широкого применения, эмпирический закон, вроде закона тяготения, который может быть сам по себе и не интересен, но который достаточно назойлив, чтобы было возможно с ним не считаться. Я считаю ее за “принцип” работы центров не потому, что она кажется мне как-нибудь очень рациональной, но потому что она представляется очень постоянною чертою деятельности центров. В действительности доминанта может становиться и совсем не рациональною чертою работы центров, а только очень устойчивою чертою их работы. Во всяком случае, доминанта – один из скрытых факторов нашей нервной деятельности, притом не невинный, как может показаться сначала” [Ухтомский, 1966].
Принцип фазовых переходов
Идея возникновения торможения из возбуждения в противоположность торможению за счет структурно-фиксированной тормозной субстанции пронизывает все аспекты учения Введенского–Ухтомского. Это учение до сих пор сталкивается с трудностями и непониманием вследствие того, что довольно сложно, малопонятно и нередко парадоксально лежащее в его основе физическое явление фазовых переходов. Инерционность как центральное свойство доминанты, а с ним и все остальные свойства доминанты аналогичны коллективным эффектам в ферромагнетиках, в которых, как известно, существует так называемое критическое замедление кинетики вблизи точки фазового перехода.
Но как доказать, что фазовые переходы в мозге действительно происходят?
В работах [Крюков и др., 1986] и [Kryukov et al., 1990] предложена сравнительно несложная, но достаточно близкая к физиологии модель локальной сети из неформальных нейронов, названная базовой нейронной моделью (БНМ) и строго доказано существование в ней фазового перехода при некоторых нежестких формальных ограничениях. Она учитывает импульсную природу нейронной активности, экспоненциальное затухание мембранного потенциала, рефрактерность и межнейронные связи. Очевидно, что без учета этих факторов говорить о существовании фазовых переходов в нервной системе практически бесполезно. Наоборот, доказав существование фазовых переходов в БНМ, мы получили возможность сопоставить следствия, вытекающие из этого факта, с реальными экспериментами и в конце концов пришли к заключению, что фазовые переходы уже не только в модели, но и в реальном мозге действительно происходят. Изложим кратко последовательность шагов на этом пути.
БНМ математически представляет собой систему взаимодействующих марковских процессов, полученную из большого числа N первоначально независимых марковских процессов, объединенных в систему с помощью локальных связей типа физического парного потенциала. Из теории систем такого типа следует, что существование фазового перехода, когда N стремится к бесконечности, влечет при некоторых дополнительных условиях существование метастабильных состояний22 для конечных N. Численные эксперименты на компьютере подтвердили это положение. При этом был обнаружен новый эффект “сохранения пятна”, состоящий в том, что начальная конфигурация активности нейронов в форме компактного пятна определенного размера или быстро сжимается, или, наоборот, расплывается, в зависимости от силы связи. Однако в случае некоторой критической силы связи “пятно” имеет время жизни, которое на два порядка превышает характерное время релаксации мембранного потенциала. Тем самым существование фазового перехода в БНМ открывает путь для разработки адекватной динамической теории кратковременной нейронной памяти как критического явления, существующего вблизи точки фазового перехода.
Перечислим главные результаты исследования БНМ:
– математически доказано существование “дальнего порядка” как появление значительных корреляций активности весьма удаленных друг от друга нейронов;
– на имитационной модели получено подтверждение предсказанного эффекта метастабильности;
– предложен и исследован нейронный осциллятор, основанный на эффекте метастабильности, с весьма необычными свойствами (например, необычно большой период и его необычно высокая стабильность или малая вариабельность);
– указанные результаты применены для интерпретации трудных для понимания данных из различных мозговых структур: гиппокампа, септума, мозжечка и новой коры;
– сделан вывод о том, что функциональной основой работы мозга могут быть метастабильные и неустойчивые очаги возбуждения, как нейронный субстрат доминанты А.А. Ухтомского.
Существование фазовых переходов в БНМ впервые позволило:
– понять механизм инерционности доминанты как критического замедления кинетики микроочагов;
– устранить основные дефекты реверберационной гипотезы кратковременной памяти, такие как малый период рециркуляции и низкая его стабильность;
– представить непротиворечивую интерпретацию описанных выше пяти локальных свойств доминантного очага;
– объяснить несколько парадоксов, связанных с динамикой электрической активности мозга при выработке и угашении условных рефлексов;
– предложить в качестве нейронного субстрата долговременной памяти микроочаги возбуждения в колонках новой коры;
– решить основную проблему мозжечка о функциональном назначении двух его основных входов, и на этой основе предложить новую модель организации движения как системы фазовой автоподстройки частоты; при этом единство восприятия и действия, требуемое принципом доминанты, реализуется взаимодействием двух идентичных систем фазочастотного регулирования.
Существование долгоживущих микроочагов возбуждения позволяет выдвинуть следующее новое предсказание об иерархической форме связи долговременной и кратковременной памяти. Если кратковременную память отождествить с активностью осцилляторов, не обязательно синхронных, чаще асинхронных, а долговременную память – со стационарным локальным возбуждением микроочагов в корковых слоях, то переход из одной формы в другую должен быть тождественным с хорошо изученным феноменом перехода локального стационарного возбуждения в нейронную активность, распространяющуюся за пределы микроочага. При этом доминанта А.А. Ухтомского как констелляция совозбужденных на одной частоте множества микроколонок – это способ “оживить” неактивные долговременные следы, легко и быстро отобрать из них лишь те, которые были “записаны” когда-то в аналогичной ситуации (т.е. при той же частоте и фазе колебаний), скомбинировать различные модальности в единый интегральный образ и снова перевести результат в неактивную форму стационарного локального возбуждения, хорошо защищенную от внешних и внутренних помех. Это предсказание относится как к сенсорной, так и к моторной системе.
Принцип радара
Мозг во многих отношениях подобен радарной системе, причем как на поведенческом, так и на нейронном уровне. “Всякий раз, как будут повторены прежние условия, необходимые для возникновения доминанты, будут даны и доминантные реакции” [Ухтомский, 1966]. Всякий раз, как имеется симптомокомплекс доминанты, имеется предопределенный ею вектор поведения. Подобную зависимость П.В. Симонов назвал “принципом радара”, понимая под ним “избирательную готовность мозга к ответу на определенный стимул при его появлении в среде, активный поиск этого стимула” [Симонов, 1981]. Похожие идеи в прошлом неоднократно выдвигались и на нейронном уровне. Так, в теории нейронной памяти Джон свою радарную аналогию организует вокруг центрального узла радарной системы – компаратора, осуществляющего сравнение времен прихода сигнала запроса и сигнала отклика из долговременной памяти [John, 1967]. Здесь Джон, опираясь на доминанту А.А. Ухтомского, высказал одну из важных идей теории нейронной памяти о том, что возможен почти мгновенный поиск конкретного памятного следа без всякого сканирования по всей памяти. Им будет наиболее вероятный пространственно-временной паттерн, лучше всего соответствующий заданным условиям внешней и внутренней среды животного, и будет именно тот, который наиболее легко отзывается на сигнал запроса при заданном симптомокомплексе.
Поясним, что здесь под памятью, в соответствии с учением о доминанте, понимается фиксация топологии коактивности нейронов в форме синхронизации по частоте и по фазе различных корковых и гиппокампальных областей на частоте тета-ритма. Считывание и распознавание памятных следов соответствуют возрождению прежде бывшей конфигурации коактивных нейронов при прежних или похожих условиях.
В работе [Vinogradova, 2001] подробно исследовано применение гипотезы компаратора к гиппокампу и новой коре, и эти результаты легли в основу нашей модели. В соответствии с компараторной гипотезой О.С. Виноградовой, мозг в нашей интерпретации, подобно радару, посылает из стволовых структур запросный сигнал во все корковые структуры мозга и после некоторой задержки получает на входе гиппокампа ответный сигнал на свой запрос, наподобие радарного импульса, отраженного от цели. Ответный сигнал с помощью упомянутого компаратора сравнивается с эндогенно генерируемым опорным сигналом септального тета-ритма и при совпадении их по частоте вырабатывается сигнал ошибки, уменьшающий возможное рассогласование по фазе между опорным и ответным сигналом с помощью небольшого изменения частоты тета-ритма.
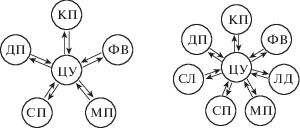
В работе [Игумен Феофан, 2004] приведена блок-схема радарной следящей системы, основные узлы которой соответствуют различным структурам лимбической системы, соединенных между собой так, что они образуют два замкнутых контура. Это – так называемый “велосипед Виноградовой”. В этой же работе приведены допущения радарной модели “Нейролокатор” и основные интегро-дифференциальные уравнения модели, позволяющие объяснить основные свойства внимания и один из самых трудных вопросов о связи внимания и сознания. Упрощенная доминантная структура “Нейролокатора” изображена на рис. 1. Рассмотрим ее структуру и функции более подробно.
Рис. 1.
Сравнение архитектур “Нейролокатор” и “Нейролокатор С”. Обозначения: ДП – долговременная память, КП – кратковременная память, ФВ – фокус внимания, ЦУ – “центральный управитель”, СП – сенсорная память, МП – моторная память, СЛ – свое лицо, ЛД – лицо другого.
Fig. 1. Comparison of the architectures of “Neurolocator” and “Neurolocator C”. Denotation: ДП – long term memory, КП – short term memory, ФВ – focus of attention, ЦУ – “central executor”, СП – sensory memory, МП – motor memory, СЛ – face of himself, ЛД – face of others.
1. Функциональной единицей является не одиночный нейрон, а элементарный осциллятор, т.е. сравнительно небольшая сеть из “физиологических” нейронов, описанная выше как БНМ, в которую добавлен тормозный интернейрон, чтобы в ней могли возникнуть колебания за счет возвратного торможения.
2. Информация “записывается” в системе элементарных осцилляторов, лабильность которых модифицируется в процессе обучения в соответствии с феноменом усвоения ритма от центрального осциллятора. (Фактически – это кодирование графами изолабильности; ребра этого графа соединяют узлы-осцилляторы с собственными частотами, исходно различающимися, скажем, на 10–15%, но способными к синхронизации на общей частоте с усвоением ритма этой частоты.)
3. Центральный осциллятор выполняет функции “центрального управителя”, “дирижера”, способного изменять свою собственную частоту и вовлекать в различные констелляции периферические осцилляторы долговременной и кратковременной памяти, осцилляторы фокуса внимания, а также осцилляторы сенсорной и моторной памяти.
4. Появление внимания соответствует возникновению синхронных и до некоторой степени синфазных колебаний центрального и одного или нескольких параллельно работающих периферических осцилляторов. Переключение внимания с одной группы осцилляторов на другую осуществляется путем изменения частоты центрального осциллятора, работающего в системе фазовой автоподстройки [Линдсей, 1978; Gardner, 1979].
5. Две различные нейронные системы сенсорной и моторной памяти имеют сходные характеристики кодирования и запоминания новой информации и могут работать параллельно (иногда на одной и той же частоте тета-ритма) и могут до некоторой степени заменять одна другую [Крюков и др., 1986].
В этом мы видим причину удивительных данных: об успешности бессознательной двигательной активности, о возможности обучения без сознания, о фактах “слепого зрения” и других загадочных явлениях до сих пор, не имеющих удовлетворительного объяснения. По этой же причине декларативная память, реализуемая с помощью гиппокампальной системы, с точки зрения теории автоматического управления принципиально не будет отличаться от процедурной (бессознательной, автоматической) памяти, если учесть, что последняя, подобно первой, реализуется системой фазо-частотной синхронизации на основе оливо-мозжечковой системы, функционально аналогичной септогиппокампальной системе [Крюков и др., 1986].
Другие, не менее важные данные, вытекающие из принципа доминанты, принципа фазовых переходов и принципа радара, приведены в других наших работах. Так, модель “Нейролокатор” объясняет когнитивную специализацию одиночных нейронов, в том числе “нейронов бабушки” [Игумен Феофан, 2005], все основные теории и эффекты участия гиппокампа в формировании долговременной памяти [Игумен Феофан, 2007; Kryukov, 2008] и ее реконсолидации [Kryukov, 2011а], в формировании и угашении условных рефлексов [Kryukov, 2011б], в том числе наиболее важных для понимания сознания – следовых условных рефлексов [Kryukov, 2012].
ПРОБЛЕМЫ ВНИМАНИЯ И ИХ РЕШЕНИЕ
Поскольку известно, что сознание тесно связано с вниманием и что неясность этой связи представляет главную трудность в научном объяснении феноменального сознания, будет целесообразно напомнить основные проблемы внимания и наше модельное их решение, полученное в работе [Игумен Феофан, 2004]. Попутно приводим и объясняем некоторые новые данные, не имеющие пока модельного объяснения.
I. Проблема селективности стимулов. Каков механизм селективности стимулов: почему некоторые одновременно предъявленные стимулы привлекают внимание и таким образом получают доступ к высшей сенсорной обработке, в то время как другие не получают?
Проблема селективности связана с повышенной возбудимостью доминанты к определенным внешним стимулам, а также с выяснением конкретного механизма отбора этих стимулов. Экспериментально доказано, что внимание воздействует на нейроны в первичных зонах, так что сигналы управления вниманием в большинстве исследований идут сверху вниз, от высших этажей к низшим. Но как эти сигналы приходят на раннюю стадию обработки, чтобы селектировать некоторые синаптические входы из множества других входов, остается неясным. “Нейролокатор”, синхронизируя микроколонки, выбирает группы корковых осцилляторов, кодирующих отдельные свойства объекта или сами объекты, на основе способности этих осцилляторов усваивать единый ритм с помощью фазочастотной синхронизации на частоте тета-ритма. Сам процесс выбора напоминает конкуренцию нескольких групп периферических осцилляторов за захват управления центральным осциллятором. Наша модель способна объяснить выбор и автоматическое сопровождение одновременно до 4–7 независимо движущихся объектов. Те стимулы или объекты, которые не способны вызвать достаточный arousal, или септальный тета-ритм или даже просто reset, будь они совершенно новыми и незнакомыми, не вызовут внимания и не получат доступа к высшей сенсорной обработке. Примером может служить драматический эффект слепоты невнимания (inattentional blindness, см. [Hutchinson, 2019]), важный в теории сознания, но до сих пор не имеющий удовлетворительного модельного объяснения. Наше объяснение его простое: когда тета-сигнал в модели “Нейролокатор” уже запущен стимулом, то в течение некоторого времени никакие другие сигналы не смогут перезапустить (reset) уже текущий ритмический процесс и поэтому не получат доступа к гиппокампальной обработке [Vinogradova, 2001].
II. Проблема долговременной памяти. Каков механизм надежного долгосрочного хранения следов памяти? Как долговременная память связана с кратковременной, и какова при этом роль внимания?
Проблема долговременной памяти – это в терминах доминанты проблема долгоживущих состояний возбуждения, т.е. проблема сохранения следов прошлых доминант. До недавнего времени единственным кандидатом на механизм долговременной памяти была долговременная потенциация (LTP33) в гиппокампе, которая вызывает у многих исследователей глубокие сомнения, хотя бы по причине того, что LTP затухает в течение нескольких часов или дней, в то время как долговременная память работает в течение всей жизни организма. Высокая надежность долговременной памяти объясняется тем, что функциональным элементом нашей модели внимания является не одиночный нейрон, а небольшой ансамбль, локальная группа нейронов, наподобие энграммы44 [Poo et al., 2016] или микроколонки, который способен произвести кооперативный эффект локального стационарного возбуждения (эффект “сохранения пятна”). Под действием внешней стимуляции и неспецифической активации от ретикулярной формации эта локальная активность переходит в колебательную, распространяющуюся по сети активность. В наших работах обосновывается положение, согласно которому переход от синхронизации к локализации является кортикальным механизмом фазового перехода из кратковременной памяти в долговременную и обратно. При этом оказывается, что долговременная память – это не свойство синаптических контактов, но свойство целой нервной ткани кортикальных осцилляторов, способных практически неограниченно долго поддерживать неизменной свою оптимальную лабильность, установленную предшествующей доминантой. Это согласуется с последними данными по выяснению природы и локализации долговременных следов памяти в ретросплениальной коре, но не в гиппокампе [Торопова и др., 2018]. Внимание необходимо как при записи в долговременную память, так и при считывании из долговременной памяти, так как интеграция пространственно рассеянных следов и свойств объектов необходима в обоих случаях.
III. Проблема мультисенсорной интеграции. Поскольку параллельная обработка стимулов происходит путем выделения свойств этих стимулов на ранних стадиях обработки, то возникает вопрос, как и где происходит реконструкция интегрального образа.
Это считается центральной загадкой исследования внимания и сознания: байндинг-проблема. Раскрытие процесса, управляющего мультимодальной интеграцией, продолжает представлять серьезную проблему. Она решается в нашей модели внимания путем фазочастотной синхронизации большого числа удаленных друг от друга корковых осцилляторов, с помощью внимания и “центрального управителя”. Эта интеграция свойств и реконструкция объектов происходят периодически (на частоте тета-ритма), как на поздних (внимание), так и на ранних (предвнимание) стадиях обработки информации, причем перцептивный байндинг визуальной информации опосредуется нейронными колебаниями, привязанными по фазе к волевому действию [Nakayama, Motoyoshi, 2019], что невозможно объяснить одной иерархической архитектурой [Сергин, Сергин, 2019; Анохин, 2013] без синхронизации от “центрального управителя”.
IV. Проблема инерции. Существует большой разрыв между длительностью эффектов внимания, таких, например, как привыкание, и длительностью нейронных событий, скажем, таких, как средний межспайковый интервал. Этот разрыв составляет иногда несколько порядков. Каков физиологический механизм изменения масштаба времени при переходе от нейронов к поведению? Какова нейронная основа сохранения длительного внимания к некоторым стимулам даже и в тех случаях, когда стимул предъявляется на сравнительно короткое время?
Проблема инерции решается в нашей модели автоматическим возникновением в ее компонентах критического режима во время активного восприятия внешних стимулов. Отдельные подсистемы осцилляторов и вся система внимания в целом работают вблизи точки неустойчивого равновесия, являющейся ближайшим аналогом критической точки физического явления фазовых переходов и метастабильности. Последнее сопровождается так называемым критическим замедлением динамики, которое и заполняет временной зазор между психологическим и нейрологическим масштабами времени. Сравнительно долгоживущие кортико-гиппокампальные реверберации обеспечивают состояния, необходимые для решения многих жизненных проблем “на линии” с помощью рабочей памяти.
С проблемой инерции тесно связано существование так называемых “клеток времени” в гиппокампе [Eichenbaum, 2014]. “Клетки времени” до сих пор не имеют объяснения в терминах модельного механизма. В модели “Нейролокатор” “клетки времени” – это нейроны, входящие в линию задержки, составленную из последовательного соединения осцилляторов, и поэтому их активация происходит в короткие интервалы времени внутри последовательности событий, но с очень точным интервалом времени относительно начала очередного цикла последовательности. Более подробно этот механизм описан в статье [Kryukov, 2012] двумя годами раньше открытия “клеток времени”.
Недавно “клетки времени” (порядка нескольких секунд между событиями) были открыты в гиппокампе у человека [Thavabalasingam et al., 2019]. Тем самым не только была подтверждена наша модель следовых рефлексов [Kryukov, 2011а], но и возможность ее обобщения на случай генерации речевой последовательности с большими паузами между словами, т.е. на случай человеческого сознания.
V. Проблема торможения и подавления помех. Какого рода обработке подвергаются стимулы, которым не оказывается внимания: тормозятся ли они активно или просто не допускаются к дальнейшей обработке? Каков механизм торможения мешающих стимулов?
Торможение мешающих объектов осуществляется не только как локальный процесс десинхронизации, но и как глобальный процесс сдвига нулевого уровня фоновой активности (путем снижения arousal) всех представлений, не попавших в фокус внимания. Этот двойной механизм обеспечивает защиту внимания выбранного объекта даже в том случае, когда мешающий объект и цель наложены друг на друга.
VI. Проблема “центрального управителя”. Существует ли отдельная от памяти нейронная модально-неспецифическая структура, координирующая функционирование всех звеньев процесса внимания и памяти, или же внимание есть следствие самоорганизации структур новой коры?
В ЦНС существует отдельная от памяти нейронная модально-неспецифическая структура, выполняющая функции “центрального управителя”. Эти функции очень обширны (см. рис. 4 статьи [Игумен Феофан, 2004]). Сюда относятся: селекция одиночных объектов, селекция группы движущихся объектов, выделение “новизны”, байндинг, пространственное частотно-фазовое кодирование, считывание, консолидация, реконсолидация, генерация последовательностей и различные виды памяти (декларативная, эпизодическая, семантическая, процедурная рабочая, кратковременная, долговременная, одномоментная). “Нейролокатор” единообразно решает проблему моделирования этих функций всех одновременно и совместно. Ключевая идея при этом, кроме доминанты, – применение теории фазовой автоподстройки частоты. “Сердцем” всей этой модели является септо-гиппокампальная структура, подробно изученная в работах О.С. Виноградовой с сотрудниками.
Примером применения могут служить объяснения многочисленных когнитивно-психологических данных Кована [Cowan, 1988, 1995], предложившего концепцию “центрального управителя” и подробно описанную нами в терминах модели “Нейролокатор” [Игумен Феофан, 2004]. До сих пор нейронный механизм “центрального управителя” остается довольно спорным [Buehler, 2018]. Новым примером применения этого принципа может быть наша интерпретация данных по глобальной интеграции и сегрегации информации в мозге и по глобальной “широковещательной”, осцилляторной функциональной связи всех структур мозга; многие из этих данных играют критическую роль в объяснении сознания.
“ТРУДНАЯ ПРОБЛЕМА” СОЗНАНИЯ И СВЯЗАННЫЕ С НЕЙ ПРОБЛЕМЫ
Что такое сознание? В недавней статье [Koch, 2018] в журнале “Nature” пишет: “Сознание – это все то, что вы испытываете. Это мелодия, засевшая в вашей голове, сладость шоколадного мусса, пульсирующая зубная боль, сильная любовь к вашему ребенку и горькое знание, что, в конце концов, все переживания прекратятся”. Это не определение сознания, которого вообще не существует, но это краткая форма современной переориентации научной теории сознания от нейронных коррелят первоначальной проблемы сознания к самой “трудной проблеме” сознания – проблеме феноменального опыта или субъективных переживаний или феноменального сознания. При этом возникает целый ряд взаимосвязанных проблем, без решения которых трудно понять, в чем основная трудность проблемы сознания.
I. Проблема нейронных коррелят сознания (НКС). Она состоит в том, чтобы найти минимальный набор нейронных механизмов, совместно достаточный для любого специфического сознательного опыта [Crick, Koch, 1998]. НКС нейтральны по отношению к физикалистской и дуалистской позиций в теории сознания [Owen, 2019] и не объясняют феноменальный опыт сознания [Thomas, 2019]. Дискуссионные вопросы НКС касаются существования НКС [Noe, Thompson, 2004], локализации НКС в мозге [Mashour, 2018; Sandberg et al., 2016], роли таламуса [Koch et al., 2016], роли осцилляторной активности [Engel, Fries, 2016] и связи НКС со вниманием [Noah, Mangun, 2019]. Одни ученые считают, что основная проблема, связанная с НКС, определить, где они локализованы, – в префронтальной коре [Lau, Rosenthal, 2011] или в зрительной коре [Koch et al., 2016; Koch, 2018], другие считают, что более важная проблема – какова природа и причина возникновения НКС [Sandberg et al., 2016], третьи (и их много) полагают, что главное в проблеме, связанной с НКС, понять, как феноменальное сознание связано с сознанием-доступ. Последнее определяется как сознание, которое участвует в контроле действий, речи, мышления и классифицируется как “легкая” проблема.
II. Проблема соотношения внимания и сознания. Она состоит в том, чтобы решить, какая из трех существующих позиций наилучшим образом соответствует имеющимся данным:
1. Сознание = Внимание [Posner et al., 1994].
2. Сознание комплементарно вниманию (двойная диссоциация) [van Boxtel et al., 2010; Koch, Tsuchiya, 2007; Tsuchiya, van Boxtel, 2013].
3. Внимание необходимо, но не достаточно для сознания [Cohen et al., 2012; Bor, Seth, 2012; Norman et al., 2012; Pitts et al., 2018; Jacobs et al., 2015; Myers, 2017; Noah, Mangun, 2019].
III. “Трудная проблема” сознания (ТПС) [Chalmers, 1995]. Это проблема объяснить, каким образом и почему мозг способен породить субъективный опыт, почему физическая переработка информации дает начало богатой внутренней жизни. При ее обсуждении затрагиваются вопросы философии сознания, эмпирические исследования в нейронауках, психологии и квантовой физике, вопросы о границах применения научной методологии, о связи науки и религии. Подходы к ТПС различны: от отрицания ее существования, как иллюзии, признания ее, но невозможности ее решения, до различных вариантов ее решения, “объясняющих” сознание. Однако даже после успешного объяснения всех когнитивных и поведенческих функций сознания остается вопрос, почему протекание всех функций сопровождается субъективным опытом.
IV. “Легкие проблемы” сознания (ЛПС) [Chalmers, 1995]. ЛПС – это объяснение того, как человеческий мозг осуществляет различные когнитивные функции с помощью специальных нейронных или компьютерных механизмов с использованием стандартной научной методологии. ЛПС включают следующие феномены: фокусировка внимания, контроль поведения, интеграция информации, способность достигать своего определенного внутреннего состояния и сообщать о нем другим, способность дискриминировать, категоризировать и реагировать на внешние стимулы. Детальный механизм ЛПС не обязательно легко предложить и промоделировать: легкие проблемы не такие уж легкие [Hodgson, 1996]. Неизвестно, существует ли единый нейронный механизм, способный объяснить наиболее важные ЛПС, с помощью которых решалась бы следующая вспомогательная, но очень важная мета-проблема сознания.
V. Мета-проблема сознания [Chalmers, 2018]. Это проблема объяснения, почему мы думаем, что сознание представляет трудную проблему, почему трудно понять, как сознание может быть физическим. Она относится к классу “легких проблем”. Она нейтральна по отношению к делению на дуализм и материализм. Она тесно связана с “трудной проблемой”, например, ТПС – иллюзия [Dennett, 2018], ТПС – карикатура внимания [Graziano, 2019] или ТПС исчезает [Solms, 2018]. Она должна подсказывать удовлетворительное решение ТПС. В настоящее время еще нет объяснения, как мета-проблема и проблема сознания тесно связаны между собой.
VI. “Более трудная проблема” сознания. Если “трудная проблема” относится к ситуации, где не требуется концепция сознания другого лица, то “более трудная проблема” тесно связана с этой проблемой. Философ Блок, сформулировавший эту “более трудную проблему”, признается, что не знает, как “трудная проблема” связана с “более трудной проблемой” сознания: включает ли в себя одна другую, или они – только разные аспекты единой проблемы [Block, 2002]. Однако из учения А.А. Ухтомского о доминанте на другое лицо следует, что выработка доминанты на другое лицо тоже оказывается очень трудной проблемой не только теоретически, но и практически, так как “создается большим, чисто физическим насилием над собою, готовностью ломать себя без жалости…, удерживается лишь с большим трудом, самодисциплиной, осторожным охранением совести”. Более того, доминанта на лицо другого, по-видимому, связывает “трудную проблему” и “очень трудную проблему”, как две стороны или две стадии единой проблемы сознания. Поэтому доминанта А.А. Ухтомского должна существенно помочь в решении “трудной” проблемы сознания.
НАИБОЛЕЕ ВАЖНЫЕ ЭКСПЕРИМЕНТАЛЬНЫЕ ДАННЫЕ ПО СОЗНАНИЮ И НЕРЕШЕННЫЕ ВОПРОСЫ ИХ МЕХАНИЗМА
Поскольку проблема сознания в настоящее время стала самой насущной и, возможно, главной проблемой науки, то выделить здесь наиболее важные данные чрезвычайно трудно. Поэтому при отборе данных мы руководствовались в основном вопросами, связанными с нейронным механизмом внимания, памяти и сознания, а также с вопросами, возникшими у исследователей, которые мы приводим (В1–В8) сразу после соответствующих им данных.
Интеграция и сегрегация
Интеграция как способность мозга объединять информацию и “широковещательно” передавать ее в различные области мозга, необходима для сознания [Barttfeld et al., 2015; Deco et al., 2015; Godwin et al., 2015; Bola, Sabel, 2015]; особенно в случае новой информации [Deco et al., 2015], но интеграция может быть и без сознания [Deco et al., 2015; Mudrik et al., 2011]. Сегрегация, как способность мозга к разделению информации между различными модулями, снижается с сознанием [Tagliazucchi et al., 2013], с обучением [Bassett et al., 2015], с увеличением трудности решаемой задачи [Vatansever et al., 2015], с возрастом [Chan et al., 2014; Brier et al., 2014; Geerligs et al., 2015; Esposito et al., 2017; Spreng et al., 2016], с эмоциями и мотивацией [Kinnison et al., 2012] и в эпизодической памяти [Westphal et al., 2017]. Осознание визуальной цели связано с ухудшением модульности из-за увеличения связи соединения между модулями [Godwin et al., 2015]. Увеличение сегрегации происходит до и после выполнения задания [Hearne et al., 2017], в простых двигательных задачах [Shine et al., 2016; Cohen, D’Esposito, 2016], в задачах автоматизации [Mohr et al., 2016] и осуществляется преимущественно за счет уменьшения затрат на метаболизм [Bullmore, Sporns, 2012; Zalesky et al., 2014] и снижения активности нейромодуляторных систем [Shine et al., 2016]. В сознательном состоянии сегрегация и интеграция сосуществуют [Tagliazucchi et al., 2016; Parlatini et al., 2017; Casali et al., 2013] и конкурируют: в частично-сознательном состоянии преобладает сегрегация, а в сознательном – интеграция [Amico et al., 2017; Hudson et al., 2014, Parlatini et al., 2017; Barttfeld et al., 2015]. Сети с высокой модулярностью предпочтительны для простых задач, в то время как сети с низкой модулярностью – для более сложных задач [Yue et al., 2017].
В1. Механизм, с помощью которого кортикальные модули взаимодействуют между собой и происходит их интеграция, остается неясным [Mattar et al., 2015; Deco et al., 2015; Shine et al., 2018; Tang et al., 2017]. “Особенно важно, что дальнейшее исследование принципов сегрегации информации в мозге может дать фундаментальное прозрение самой природы сознания” [Deco et al., 2015]. “Какая нейронная архитектура поддерживает интеграцию внутри и между модулями? Какая информация передается от одного субмодуля к другому в течение функциональной интеграции? По какому принципу субмодули разделяют информацию между собой?” [Park, Friston, 2013].
Глобальный Сигнал
Общий для мозга Глобальный Сигнал (ГС) представляет собой шумоподобные квазипериодические флуктуации, тесно связанные с порождающей их нейронной активностью [Scholvinck et al., 2010]. ГС имеет следующие свойства:
а) амплитуда ГС линейно связана со средней функциональной связью между модулями в сети [Bennett et al., 2015; 2016];
б) конфигурации ГС состоят из широкомасштабных квазипериодических пространственно-временных конфигураций, которые коррелируют с кортикальной активностью, особенно в низкочастотной полосе [Billings, Keilholz, 2018];
в) каждая квазипериодическая конфигурация появляется на специфической фазе профильтрованного ГС [Gutierrez-Barraganet et al., 2018].
В2. “Нейрофизиологическое происхождение Глобального Сигнала остается неясным. Какова роль и нейрофизиологическое происхождение Глобального Сигнала в глобальной МРТ-когерентности и когнитивной деятельности?” [Cabral et al., 2017]. “Распространяется ли нейронная информация в форме дискретных пакетов по аналогии с телекоммуникационными сетями, и если да, то адресуются ли эти пакеты к специфическим целям? Или нейронная коммуникация похожа на широковещательность, т.е. не имеет дискретности?.. Механизм, с помощью которого сигналы распространяются так, что позволяют гибкие и адаптивные вычисления, остается неясным” [Avena-Koenigsberger et al., 2017]. “Моментальное увеличение Глобального Сигнала сопровождается уменьшением сигнала в субкортикальных областях, поддерживающих arousal и бодрствование. Корреляционный анализ не позволяет найти причинную связь между кортикальной и субкортикальной активностью” [Liu et al., 2018].
Функциональные связи
Кроме структурно-анатомических связей существуют функциональные связи (ФС) [Biswal et al., 2010; Park, Friston, 2013; Cabral et al., 2017], от них зависимые [Foster et al., 2016], в том числе даже при отсутствии прямых анатомических связей [Adachi et al., 2012]. Они (ФС) модулируются сознанием [Wu et al., 2015], контекстом задачи [Park, Friston, 2013], эмоциональным и когнитивным состоянием [Biswal et al., 2010]. ФС – не стационарные, а динамические [Zalesky et al., 2014; Liang et al., 2015; Brovelli et al., 2017], изменяющиеся, как функции частоты [Thompson, Fransson, 2015]. Структура ФС различных областей не фиксирована, но образует мягкие ансамбли [Wiltshire et al., 2017; Schultz et al., 2016; Bola, Sabel, 2015], которые динамически изменяются между сегрегацией и интеграцией [Dixon et al., 2017] и в зависимости от текущих когнитивных требований достигают оптимального баланса между ними [Cohen, D’Esposito, 2016; Spielberg et al., 2015]. Степень реконфигурации изменяется в зависимости от уровня сознания [Stitt et al., 2017; Wu et al., 2015], сложности задачи [Shine et al., 2016], внимания [Alnæs et al., 2015], исполнительных функций [Brovelli et al., 2017], arousal [Shine et al., 2018] и творческих способностей [Schultz, Cole, 2016]. ФС бывают не только попарные, но и множественные (так называемые “фокусы взаимодействия” или “динамические сердцевины”), зависящие от общего процесса динамической интеграции [Davison et al., 2015; Chen et al., 2017; Gonzalez-Castillo et al., 2015; Lord et al., 2016; Fuertinger et al., 2015]. Они впервые были описаны А.М. Иваницким [2010] с помощью корреляций сознания и вызванных потенциалов. Они могут иметь глобальную дирекциональность [Weaver et al., 2016; Mitra et al., 2016; Zhigalov et al., 2017]. ФС не однородные, а иерархические, как во времени – от мсек до месяца [Hari, Parkkonen, 2015; Preti et al., 2017], так и в пространстве – от сенсорных до ассоциативных областей [Chaudhuri et al., 2015; Mattar et al., 2016]. На вершине иерархии находятся две, связанные с сознанием, антикоррелированные сети: “дежурная” (DMN)55 и “озадаченная” (TPN)66 [Fox et al., 2005].
В3. “Каково происхождение, механизм и функция функциональных связей? Модулируются ли функциональные связи центральным управителем, таким как префронтальная кора, или они самоорганизуются?” [Hutchison et al., 2013] “Человеческий мозг представляет загадочный и вызывающий парадокс: несмотря на фиксированную анатомию, характеризующую ее связанность, ее функциональный репертуар обширен и позволяет совершать действия, перцепцию и мышление. Нейронные взаимодействия представляют динамику над структурными связями, которые лежат в основании мышления и поведения. Такое расхождение между структурой и функцией является, по-видимому, наиболее интригующим свойством мозга и стимулирует интенсивное исследование мозга в будущем” [Park, Friston, 2013].
Критический режим
Исключительно важное значение для понимания внимания и сознания имеет критический режим и связанные с ним эффекты перехода от хаоса к порядку, а именно: большой динамический диапазон и быстрая адаптация [Shew, Plenz, 2013], максимальная чувствительность к стимуляции (т.е. расходимость восприимчивости), максимально разнообразный репертуар памяти [Moretti, Minoz, 2013], оптимальная обработка информации [Deco et al., 2013; Cocchi et al., 2017; Bettinger, 2017], критическое замедление динамики нейронных сетей (длительные автокорреляции) и связанные с ними метастабильные состояния и метастабильная динамика [Cocchi et al., 2017]. Смещение от критической точки приводит к уменьшению сознания [Priesemann et al., 2013; Scott et al., 2014; Tagliazucchi et al., 2016].
В4. “Неизвестно, какие параметры в мозге следует изменить, чтобы получить критический режим” [Hesse, Gross, 2014; Droste et al., 2013]. “Механизмы, приводящие к критическому поведению, и как они взаимодействуют с такими нейронными процессами, как синаптическая пластичность, не вполне ясны: неясно, сохранится ли критический режим после синаптической модификации, связанной с обучением” [Hernandez-Urbina, Herrmann, 2017].
Метастабильность
Метастабильность – есть кратковременное сохранение критического режима [Крюков и др., 1986]. Она способна обеспечить сенсорную кратковременную память без синаптической модификации [Johnson et al., 2013]. В состоянии покоя мозг человека работает при максимальной метастабильности, т.е. в состоянии максимально быстрого переключения [Deco et al., 2017]. ЭЭГ и ЭКГ показывают, что неокортекс обрабатывает информацию покадрово (как в кино) на основе метастабильности кортикальных модулей, а метастабильные амплитудно-модулированные паттерны этих активных модулей играют роль фреймов или кадров, причем физический фазовой переход обеспечивает окончание каждого кадра и переход к следующему кадру [Kozma, Freeman, 2016; Kaplan et al., 2005; Freeman, 2004 a,b]. Эти фреймы осциллируют на частотах в бета- и гамма-диапазонах в течение доли секунды и создают пространственный паттерн фазовой и амплитудной модуляции несущей частоты в этих частотных диапазонах [Freeman, Holms, 2005].
В5. “Потенциальная роль метастабильности изучается в сенсорном кодировании памяти и принятия решений, однако очень малоизвестно о сетевом механизме, ответственном за его генезис” [Mazzucato et al., 2015]. “Какой механизм разделяет время на дискретные интервалы, закрывает микросостояния и оценивает значение различных нейронных событий, которые появляются в течение персептивных (кино) кадров?” [John, 2001].
Нейронный код сознания
Различные состояния сознания (сознание-доступ, галлюцинации, потеря сознания) характеризуются различным уровнем синхронизации и силы фазово-амплитудной связи (ФАС77) [Nakatani et al., 2014]. ФАС вовлечен в интеграцию и сегрегацию отдельных модулей [Tang et al., 2017, Malekmohammadi et al., 2015], в формирование последовательностей эпизодической памяти [Heusser et al., 2016], в считывание памяти [Kaplan et al., 2014; Shirvalkar et al., 2010; Colgin, 2015], в селекцию внимания [Schroeder, Lakatos, 2009]. ФАС увеличен при успешном обучении [Lega et al., 2016; Colgin, 2015; van Wingerden et al., 2014; Kendrick et al., 2011; Friese et al., 2013] и выработке различных условных рефлексов [Shearkhani et al., 2013; Tort et al., 2009]. ФАС уменьшен при повторном предъявлении одного стимула [Tsunada et al., 2011], фазовом reset [Malerba, Kopell, 2013] и направленном пространственном внимании [Esghaei et al., 2015]. Контрастные характеристики ФАС в сознательном и бессознательном состояниях приведены в табл. 1.
Таблица 1.
Сопоставление данных в двух состояниях сознания Table 1. Comparison of data in two states of consciousness
| СОЗНАТЕЛЬНОЕ СОСТОЯНИЕ | БЕССОЗНАТЕЛЬНОЕ СОСТОЯНИЕ |
|---|---|
| Наличие фазоамплитудной связи (ФАС) [Nakatani et al., 2014; Doesburg et al., 2009; De Ridder et al., 2011; Swann et al., 2016]; тета-ритм является наиболее важной частью нейронного механизма [Doesburg et al., 2009]; сознательно воспринятый стимул имеет тенденцию быть связанным с тета- или дельта-ритмом [Nakatani et al., 2014; Sitt et al., 2014] | ФАС снижается [Swann et al., 2016], нарушается глобальное взаимодействие из-за резкого падения фазовой синхронизации [Pigorini et al., 2015; D’Andola et al., 2017], причем в ФАС максимальная амплитуда высокой частоты сдвигается по фазе с максимума на минимум амплитуды низкой частоты [Purdon et al., 2013] |
| Критический режим динамики [Tagliazucchi et al., 2016; Werner, 2013; Solovey et al.,
2015]; корреляция сознания с большой задержкой, длительной нейронной активностью,
зависимостью от исходного состояния, устойчивой транзиентной нейронной динамикой [Baria
et al., 2017] Комплексный и длительный ответ на транскортикальный сигнал [Casali et al., 2013] |
Уменьшение дальнодействующих корреляций во фронто-таламических структурах [Tagliazucchi
et al., 2016]; глобальное снижение временной изменчивости локальной и дальнодействующей
синхронизации сигналов мозга [Huang et al., 2016] Исчезающий, кратковременный, убывающий транс-кортикальный сигнал [Casali et al., 2013] |
| Положительные и отрицательные корреляции между зонами мозга; богатая функциональная динамика часто отличается от анатомической структуры [Barttfeld et al., 2015] | Отсутствие отрицательных корреляций между зонами мозга [Barttfeld et al., 2015] |
| Роль дефолтной сети (DMN) [Poerio et al., 2017; Hannawi et al., 2015; Amico et al.,
2017; Fernandez-Espejo et al., 2012; Noirhomme et al., 2010; Havlik, 2017] (DMN – default mode network) |
Снижение активности дефолтной сети [Hannawi et al., 2015; Fingelkurts et al., 2012; 2016]; снижение функциональных связей дефолтной сети с кортикальными областями [Fernandez-Espejo et al., 2012; Amico et al., 2017] |
| Наибольшее число возможных конфигураций [Guevara Erra et al., 2016], представляющих содержание сознания [Freeman, 2007] | Уменьшенный функциональный репертуар [Hutchison et al., 2014; Hudetz et al., 2015; Mashour, Hudetz, 2018] |
| Осознание визуальной цели связано с уменьшением модулярности из-за увеличения связи между модулями [Godwin et al., 2015] | Нарушение функциональных связей, ограниченный репертуар и стабилизация динамики коры [Mashour, Hudetz, 2018] |
| Феномен перцептивной интеграции, близкой к субъективному опыту, отделен от сознания-доступа [Fahrenfort et al., 2017; Block, 2005] | Субъективный опыт отсутствует в состоянии комы, при общей анестезии и в определенном состоянии сна [Storm et al., 2017] |
В6. “Весьма возможно, что локализация коррелят сознания менее важна, чем конкретные вычисления, выполняемые соответствующими связанными сетями. Каковы эти вычисления? Какие сети вовлечены? И почему?” [Sandberg et al., 2016].
Неинвазивное “стирание” памяти
Monfils и соавт. [2009] обнаружили, что, если спустя 10–60 мин после реактивации условного рефлекса страха (retrieval) применить угашение (extinction), то спустя месяц, согласно четырем видам тестирования, страх не возвращается (сокращенно эффект ret+ext). Такая процедура надолго уменьшает память страха без использования фармакологических препаратов и дает значительный терапевтический эффект уменьшения рецидивов в наркозависимых клинических популяциях. Эффект ret+ext был многократно повторен на крысах, мышах и людях (см. 12 ссылок в статье [Gershman et al., 2017]. Однако большой проблемой является то, что до настоящего времени механизм, мозговые структуры и условия для появления этого эффекта не ясны. Основным механизмом ret+ext эффекта повсеместно считается срыв реконсолидации на границе угашения. Однако этому противоречит эффект ext+ret, в котором реактивация идет не в начале, а в конце процедуры [Baker et al., 2013], причем в случае применения терапии страха (exposure therapy) дает почти эквивалентное “стирание” памяти страха [Telch et al., 2017]. В последнее время получено прямое доказательство, что ret+ext эффект не зависит от реконсолидации [Cahill et al., 2019].
В7. “Почему многие исследования во всем мире не смогли получить достаточно долгоживущий эффект ret+ext” [Pineyro et al., 2013], а “некоторые исследования получили противоположный эффект?” [Chan et al., 2010; Millan et al., 2013]. “Почему угашение после реактивации эффективно и надолго подавляет память, провоцирующую страх, в то время как одно угашение не способно это сделать?” [Radulovic, Tronson, 2011]. “Неясно, почему этот эффект не наблюдается у крыс, которым дается нормальное обучение угашением?” [Delamater, Westbrook, 2014]. “Эффективность этой парадигмы бросает вызов нашему пониманию взаимоотношений между обучением и памятью” [Gershman et al., 2016].
Связь внимания и сознания
Для сознания внимание необходимо, но недостаточно [Cohen et al., 2012; Bor, Seth, 2012; Norman et al., 2013; Jacob et al., 2015; Myers 2017; Pitts et al., 2018]. В частности, кроме минимального внимания [Pinto et al., 2017], для сознательного восприятия и сообщения о нем требуется у испытуемого превысить порог восприятия [van Vugt et al., 2018], который повышен у пациентов с поврежденной префронтальной корой [Del Cul, 2009] и с шизофренией [Berkovitch et al., 2017]. Сознание модулирует неокортикальные колонки через возбуждающие пирамидные нейроны [Olcese et al., 2016], а внимание – через тормозные интернейроны [Buschman, Kastner, 2015].
Феноменальное сознание научно-методологически пока неотделимо от связанного со вниманием сознания-доступ [Phillips, 2018; Chalmers, 1997], в том числе из-за высокого уровня перцептивных шумов [Gross, Flombaum, 2017]; оно потенциально может быть диссоциировано от сознания-доступ в перцептивной интеграции [Fahrenfort et al., 2017; Block, 2005], причем феноменальное сознание активируется прежде сознания-доступ [Pantani et al., 2018]. Феноменальное сознание модулируется вниманием и связано не с иконической памятью, как утверждалось ранее [Block, 2005], а с промежуточной (между иконической и рабочей) памятью [Simione et al., 2019].
В8. “Феноменальное сознание переполняет ли внимание?” [Myers, 2017]. Перцептивное сознание переполняет ли когнитивный доступ? [Gross, Flombaum, 2017].
МОДЕЛЬ ВНИМАНИЯ, ПАМЯТИ И СОЗНАНИЯ “НЕЙРОЛОКАТОР С”
Эта модель по существу мало чем отличается от более ранней модели внимания и памяти [Игумен Феофан, 2004], но небольшая модификация прежней модели позволяет объяснить значительно большее число новых данных, в том числе данных по сознанию. Большое количество допущений модели (10) не означает ее слабости, но введено для лучшего уяснения роли ее отдельных компонентов и новых экспериментальных подтверждений.
Допущения модели “Нейролокатор С”
1. Медиальный септум является глобальным пейсмекером или центральным осциллятором.
2. Поле СА3 гиппокампа является компаратором и детектором новизны.
3. Лимбический круг, включающий в себя поле СА1, выполняет функцию линии задержки и связи гиппокампа с неокортексом.
4. Тета-ритм играет критическую роль в сознательном и бессознательном восприятии, внимании и памяти (“фильтр внимания”).
5. Функциональной единицей модели является унифицированный субмодуль, напоминающий кортикальную микроколонку и кортикальный “бочонок”.
6. Звездчатая архитектура, состоящая из одного центрального осциллятора и многих кортикальных осцилляторов, работает на частоте тета-ритма, который автоматически регулируется фазо-частотной следящей системой и реализует принцип доминанты Ухтомского.
7. Обучение в модели происходит следующим образом: когда число синхронно работающих корковых осцилляторов достигает критической величины, их собственные частоты сближаются и модифицируются – это так называемое “изолабильное кодирование”, которое значительно усиливается под воздействием нейромодуляции, приходящей от мотивационных структур.
8. Все субмодули и вся система в целом работают на основе принципа физических фазовых переходов как на локальном уровне микроколонок, так и на глобальном системном уровне тета-синхронизации.
9. Социальное сознание, также как внимание, функционально обеспечивается звездчатой архитектурой, но с дополнительными кортикальными осцилляторами, используя один и тот же механизм, как для доминанты на лицо другого, так и для самосознания – доминанты на свое лицо (см. рис. 1).
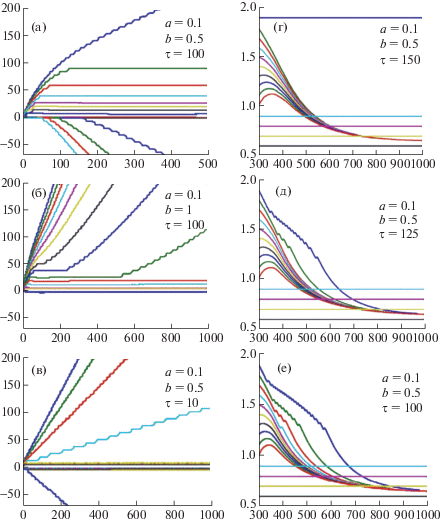
Рис. 2.
Динамика интеграции (а–г) и сегрегации (д–е) в модели “Нейролокатор С”. По вертикальной оси – разность фаз (а–в) и собственная частота осцилляторов (г–е), а по горизонтальной оси – время (см. пояснения в тексте).
Fig. 2. Dynamics of integration (а–г) and segregation (д–е) in the model “Neurolocator C”. The vertical axis is the phase difference (а–в) and the natural frequency of the oscillators (г–е), and the horizontal axis is time (see explanations in the text).
10. Благодаря расходимости восприимчивости при фазовых переходах сознание в звездчатой структуре может быть обеспечено в принципе не только осцилляторами в нео-кортексе, но и синхронными колебаниями в других структурах мозга, сердца и всего тела и даже во внешнем пространстве – по типу эхолокатора у летучих мышей и “космического сознания” у тибетских монахов.
Первоначальная радарная аналогия работы мозга была предложена в книге John [1967] на основе принципа доминанты Ухтомского. Допущения 1–4 адаптированы из работы Vinogradova [2001], допущения 5–8 – из работы [Kryukov et al., 1990], а допущение 9 и 10 – новые, слегка напоминающие социальную гипотезу [Graziano, Kastner, 2011].
Новые подтверждения модели “Нейролокатор С”
В дополнение более ранних подтверждений [Игумен Феофан, 2004] приводим новые данные, подтверждающие каждое из допущений нашей модели (нумерация соответствует номеру допущения модели).
1. Медиальный септум работает как глобальный тета-пейсмекер [Sanchez-Alavez et al., 2014; Amaral, Witter, 2004; Hangya et al., 2009], и генерация тета-активности существенно зависит от его двухсторонних связей с гиппокампом [Kang et al., 2017; Long et al., 2015; Tsanov, 2015].
2. Гиппокамп, интегрируя пространственно-временные субмодули [Schedlbauer et al., 2014], выполняет функцию компаратора [Hunsaker et al., 2007; Lee et al., 2005; Hasselmo, 2005; Forcato et al., 2016; Bannerman et al., 2014; Taylor et al., 2013], что обеспечивает ему центральную роль в обработке информации [Misic et al., 2014] и в механизме сознания [Baars, 2013; Hannula, Green, 2012].
3. Анатомические исследования показали связи гиппокампа с таламусом [Sweeney-Reed et al., 2016], а обратимая инактивация таламического базального ядра сильно подавляет Глобальный Сигнал, идущий из гиппокампа в неокортекс [Turchi et al., 2018]. Таким образом, таламокортикальная система совместно с лимбической системой может работать как радарный передатчик запросного сигнала из гиппокампа в кортикально-таламическое рабочее пространство [Baars et al., 2013], где формируется ответный сигнал, отражающий отбор и интеграцию информации [Bell, Shine, 2016].
4. Запросный или зондирующий сигнал передается в неокортекс на тета-частоте, а отраженный или ответный – на гамма-частоте [Headley, Paré, 2017; Malekmohammadi et al., 2015; Jiang et al., 2015; Spaak et al., 2012; Tang et al., 2017; Weaver et al., 2016].
5. Экспериментально доказано, что кортикальные колонки являются структурными единицами кортикальных функций [Chen et al., 2017].
6. Глобальный Сигнал – это выходной сигнал центрального осциллятора, благодаря которому возникают все свойства функциональных связей. Таким образом, звездчатая архитектура подтверждена многими экспериментами, связанными с Глобальным Сигналом.
7. Долговременная память хранится не в гиппокампе [Lehmann, McNamara, 2011; Торопова и др., 2018], а в неокортексе [Girardeau et al., 2009; Hunsaker, Kesner, 2013; Shema et al., 2015] и кодируется не синаптическими весами [Titley et al., 2017; Attardo et al., 2015], а популяциями нейронов [Montijn et al., 2016] или энграммами [Ryan et al., 2015; Poo et al., 2016], составленными из кортикальных колонок [Chen et al., 2017]. Интегративное “склеивание” элементов памяти и внимания обнаружено в активности с самоцитированием [Sui, Humphreys, 2015].
8. В мозге существуют критический режим и фазовые переходы, обеспечивающие высокую чувствительность к внешним изменениям, огромный допустимый динамический диапазон входных сигналов, большую скорость адаптивных процессов [Shew, Plenz, 2013; Tagliazucchi et al., 2016]. При переходе от сознательного к бессознательному состоянию наблюдается инерционная тенденция (сопротивление) с гистерезисом [Friedman et al., 2010]; при восстановлении сознания синхронизация структур возобновляется с задержкой и внезапно [Kim et al., 2017], причем когерентность в дельта-/тета-диапазоне положительно предсказывает переход от вегетативного состояния к слабому сознанию [Schorr et al., 2016].
9. В вентромедиальной префронтальной коре локализовано нейронное представительство своего лица [Lou et al., 2017; Jenkins et al., 2008; Kim, Johnson, 2015; Heleven, Van Overwalle, 2019a] и отдельно представительство лица других, причем последнее не зависит от нашего знания о них [Heleven, Van Overwalle, 2019b]. Многие эксперименты показывают, что сознание – продукт социального механизма в мозге [Frith, Metzinger, 2016; Combs, Kripner, 2008; Frith, Frith, 2007], и этот механизм тот же самый, который формирует наше самосознание [Graziano, Kasner, 2011]. В частности, самосознание и ссылка на себя (self-reference) действуют как “интегральная склейка” памятных следов в единое целое, что в зависимости от контекста приводит к усилению или разрушению выполняемой задачи [Sui, Humphreys, 2015]. Социоэмоциональное рассуждение подавляет аналитическую форму рассуждения, и, наоборот, последнее подавляет первое [Rochford et al., 2017]. Личный опыт и переживание того, что уже сделанное можно было бы сделать иначе, порождают опыт сожаления. Сожаление – есть сильная негативная эмоция, ведущая к самонаказанию [Frith, Metzinger, 2016]. Аморальные решения связаны с высоким уровнем первичной психопатии [Reniers et al., 2012] и одержимости [Dalpe et al., 2019].
10. У пациента с рассеченным мозгом на левое и правое полушарие наблюдается раздельное восприятие, но нераздельное сознание [Pinto et al., 2017; Pockett, 2013; Sergent, 1987]. Нерефлекторная форма сознания наблюдается у гидроэнцефалических детей и декортикальных людей и крыс, которая обеспечивается субкортикальными структурами [Denton et al., 2009; Merker, 2007; Northoff, 2007]. Воспоминание предсмертного состояния (NDE – near death experience) связано с такими конфигурациями памяти в диапазоне дельта-, тета- и альфа-частот, которые отличаются от памяти воображаемых событий [Palmieri et al., 2014].
Ответы на вопросы по механизму внимания и сознания
Имея в виду, что многие эффекты памяти, внимания и бессознательных состояний были объяснены ранее, мы теперь вместо подробного объяснения приведенных данных в терминах механизма модели “Нейролокатор С” ограничимся лишь ответами (О1–О8) на вопросы (В1–В8), приведенные в предыдущем разделе, из которых, надеемся, будет ясно, как объяснить все приведенные там данные.
О1. Звездчатая архитектура дает ответы на все заданные вопросы по сегрегации и интеграции субмодулей. В качестве математической основы интеграции и сегрегации в модели используются уравнения фазочастотной синхронизации от одного центрального осциллятора, хорошо известные в технике связи под названием фазовой автоподстройки (ФАП) [Линдсей, 1978; Gardner, 1979]. Их нейронная реализация в модели отличается от стандартной ФАП системы следующими свойствами:
1) синхронизация осуществляется одновременно между многими кортикальными осцилляторами от общего центрального осциллятора (см. рис. 1);
2) центральный осциллятор способен ударно возбуждаться от внезапной внешней стимуляции (reset), что обеспечивает быструю фазовую синхронизацию периферических осцилляторов без предварительного, иногда очень длительного, захвата по частоте;
3) синхронизация в широком частотном диапазоне периферических осцилляторов (не обязательно в гамма-диапазоне) обеспечивается особенным нейронным эффектом фазово-амплитудной связи (ФАС), благодаря которому сравнение фаз периферических осцилляторов и центрального осциллятора всегда осуществляется на частоте центрального осциллятора, независимо от собственных частот периферических осцилляторов.
В соответствии с допущением 7, механизм интеграции – единый, но двухстадийный: сначала идет интеграция многих осцилляторов первичных зон мозга с помощью ФАП без модификации собственных частот (рис. 2 (а), 2 (б), 2 (в)), и затем последующая за ней стадия модификации собственных частот для запоминания информации о стимуляции (рис. 2 (г)). Первая стадия корреляционно связана с феноменальным сознанием и с ЭЭГ-компонентой N100/170, а вторая – с сознанием-доступ к памяти и с компонентой Р300. Механизм сегрегации двоякий – пассивный и активный: первый за счет отдыха от выполнения задачи и/или переключение на другую задачу, а второй требует волевой внутренней активности и связан с возможностью отделения феноменального сознания от сознания-доступ (рис. 2 (д), 2 (е)). Подробнее об этом в конце этого раздела.
О2. Глобальный Сигнал – это запросный сигнал “Нейролокатора С”, идущий широковещательно от септального тета-генератора ко всем структурам мозга и вызывающий на своей определенной фазе “ответные” сигналы на гамма-частоте. Последние приходят в гиппокамп, где происходит их сравнение по фазе с колебаниями септального осциллятора, и фазовая ошибка (разность фаз) изменяет глобальную частоту тета-ритма, в соответствии с принципом работы ФАП. До прихода стимула высокая дефолтная активность в поле СА3 гиппокампа тормозит септальный осциллятор и держит всю систему в состоянии готовности. С приходом стимула эта активность резко снижается, и септум ударно растормаживается [Vinogradova, 2001], генерируя тета-ритм Глобального Сигнала. Одновременно через ретикулярную формацию подается сигнал arousal в неокортекс, где устанавливается критически режим в локальных микроколонках, и все процессы идут так, как описано в модели внимания и памяти [Игумен Феофан, 2004]. Линейный корреляционный анализ потому не позволяет найти причинную связь между кортикальной и субкортикальной активностью, что их общий источник, находящийся в септо-гиппокампальной системе, вызывает в неокортексе непредсказуемые нелинейные эффекты, связанные с фазовыми переходами в критическом режиме.
О3. Функциональные связи возникают из-за общего источника – Глобального Сигнала, приходящего почти одновременно во все области мозга. Но так как Глобальный Сигнал формируется из нелинейного взаимодействия входов многоканальной ФАП, то он практически не зависит от более слабых анатомических структурных связей между этими областями. Хотя это расхождение между структурой и функцией при некоторых условиях ослабляется, например, при минимальном сознании [Demertzi et al., 2019] или в критическом состоянии [Lee et al., 2019], но динамика над структурными связями остается доминирующей функциональной основой внимания, памяти, мышления и сознания.
О4. Критический режим в мозге устанавливается автоматически: локально – изменением arousal от ретикулярной формации, а глобально – от ритмической активности септо-гиппокампальной системы. Это – так называемая самоорганизующаяся критичность, которая не имеет нужды во внешней подстройке контрольного параметра [Cocchi et al., 2017]. Наши имитационные эксперименты показали [Крюков и др., 1986; Kryukov et al., 1990], что тета-периодический сигнал, приходящий в неокортекс, модулирует порог срабатывания в кортикальном модуле, и в нем на определенной фазе периодически автоматически устанавливается критический режим с максимальной амплитудой гамма-ритма. Этим объясняется эффект фазоамплитудной связи (ФАС), широко распространенный в нервной системе. В тех же экспериментах было показано, что критический режим после небольшой синаптической модификации сохраняется, и это оставляет локальный осциллятор чувствительным к малым изменениям параметров. На этом основании был предложен новый механизм обучения – “изолабильное кодирование”, в котором постулируется, что при синхронизации модулей их собственные частоты имеют тенденцию сближаться, сохраняя память о том, какая конфигурация модулей была при обучении. При этом синаптическая модификация порядка доли процента не только не разрушает критический режим, но оказывается причиной изменения собственных частот модулей, т.е. нового типа памяти и обучения. Строгое доказательство существования фазовых переходов в нейронной сети кортикального модуля приведено в работе [Крюков и др., 1986; Kryukov et al., 1990].
Другая важная роль критического режима в том, что благодаря расходимости чувствительности, возможно спонтанное возникновение саморегуляции когнитивной и двигательной активности [Libet, 1985], которую можно интерпретировать как “эмерджентное” поведение или как свободную волю. Однако роль свободной воли не в том, чтобы инициировать специфический волевой акт, но скорее в том, чтобы выбрать и контролировать волевое действие в форме разрешения либо предотвращения моторного действия, намерение совершить которое возникает бессознательно [Libet, 1985].
О5. Сетевой механизм метастабильности тот же самый, что и при запоминании конфигурации и обучении, но динамика во времени более тесно связана с фазовыми переходами: последние возникают только в определенном диапазоне фаз низкой частоты и обрываются вне этого критического диапазона. Таким образом, время разделяется на дискретные интервалы, в которые как бы открываются и закрываются перцептивные кадры. Математический анализ метастабильных состояний в нейронной сети (но без внешнего обрывания критического режима) приведен в указанных выше наших работах, а также в работе [Kirillov et al., 1986].
О6. Из предыдущего очевидно, что локализация осцилляторов, участвующих в процессах сознания, не имеет того решающего значения, которое приписывается ей в работах по нейронным коррелятам сознания, что и отражено в допущении 10 нашей модели. В зависимости от поставленной задачи, локальные нейронные сети могут быть как в первичных зонах, например, при зрительном восприятии, так и в центральных зонах, как при социальном сознании. Однако “нейронный код сознания” остается одним и тем же – это фазоамплитудная связь тета- и гамма-колебаний и связанные с ними критические процессы в локальных и глобальных сетях мозга человека и животных.
О7. Проблема механизма ret+ext эффекта по существу идентична проблеме следовых условных рефлексов, так как обе проблемы критически зависят от нейронного субстрата большой задержки (минуты и часы) между условным и безусловным стимулом в первом случае, и между реактивацией и угашением во втором. Поэтому модель “Нейролокатор”, которая решает первую проблему [Kryukov, 2012], решает и вторую [Hegumen Theophan, 2014], и таким образом отвечает на все поставленные вопросы следующим образом. Граничные условия, т.е. значение параметров, когда эффект ret+ext отсутствует, соответствуют граничным условиям следового рефлекса, которые зависят не от одного, а одновременно от многих параметров и от их различных сочетаний, что и объясняет наличие конфликтных результатов в обоих случаях. Одно угашение, т.е. повторение условного стимула без реактивации памяти страха, не позволяет существенно изменить (уменьшить) собственную частоту осцилляторов памяти страха, и страх быстро возвращается, в то время как предварительная реактивация памяти страха и последующая задержка угашения активируют лимбическую линию задержки, что значительно удлиняет время синхронной работы осцилляторов страха, а также значительно меняет собственную частоту осцилляторов страха, затрудняя и даже делая невозможным возвращение страха. Самое замечательное свойство предложенного выше механизма стирания памяти страха в том, что эффект ret+ext почти эквивалентен эффекту ext+ret из-за того, что в модели следового рефлекса центральную роль играет коммутативная математическая операция свертки сигналов условного и безусловного стимулов. Эффект ret+ext помогает понять, в чем отличие сознания человека от сознания животных в отношении “стирания” нежелательной памяти.
О8. В недавнем выпуске журнала “Philosophical Transactions Royal Society B” в большинстве статей теоретически показано, что отделение феноменального сознания от сознания-доступ проблематично и что феноменальное сознание не переполняет сознание-доступ. Основная причина этого – вероятностная природа перцептивных представлений [Gross, Flombaum, 2017; Block, 2018]. Ниже показано, что в модели “Нейролокатор С”, основанной на вероятностной природе фазовых переходов, имеется, тем не менее, принципиальная возможность отделить сознание-доступ от феноменального сознания. С этой целью система дифференциальных уравнений, описывающая внимание и память [Игумен Феофан, 2004], значительно упрощается и численно анализируется следующим образом. Во-первых, операторная форма уравнений (1) из этой работы заменяется эквивалентной системой второго порядка, а шумовой член временно (для ясности эффектов) исключается; во-вторых, общая функция дискриминатора заменяется простой синусоидой и, в-третьих, в правую часть всех уравнений добавляется член, учитывающий динамику собственных частот периферических осцилляторов при синхронизации и обучении. В результате получаем следующую систему уравнений второго порядка:
(1)
$\begin{gathered} \tau \frac{{{{d}^{2}}{{\varphi }_{i}}}}{{{{{(dt)}}^{2}}}} + \frac{{d{{\varphi }_{i}}}}{{dt}} = {{\omega }_{i}} - {{\omega }_{0}} - a\sum\limits_{i = 1}^n {\sin {{\varphi }_{i}}} - b\sin {{\varphi }_{i}}, \\ i = 1, \ldots ,n, \\ \end{gathered} $Результаты компьютерных вычислений при n = 15, c = 0.01, ω0 = 1, ωi = 2 – 0.1i, i = 0, …14 при различных значениях параметров a, b, τ, представленные на рис. 2, показали следующее.
Динамика синхронизации многих осцилляторов, т.е. выход их разностей фаз на горизонтальные линии рис. 2 (а) и (б) устанавливается не сразу, а с задержкой, зависящей от собственных частот осцилляторов, удовлетворяющих условию захвата |ωi – ω0| < ak, (k < n) [Gardner, 1979]. Остальные осцилляторы десинхронизованы. При уменьшении τ задержки синхронизации сильно уменьшаются (рис. 2 (в)), объясняя факт ранней мультимодальной интеграции. После установившейся синхронизации некоторого критического числа осцилляторов включается механизм обучения (рис. 2 (г)), при котором все вошедшие в синхронизм осцилляторы меняют во времени свои собственные частоты, сближая их между собой.
Динамика собственных частот осцилляторов при обучении показывает, что при уменьшении τ (рис. 2 (д) и 2 (е)) интегрированные осцилляторы имеют тенденцию к сегрегации. Другими словами, при определенных условиях “склеивание” осцилляторов, показанное на рис. 2 (г), может постепенно ослабиться настолько, что их траектории будут приближаться к горизонтальным линиям (есть отдельные эксперименты) и “склеенные” осцилляторы будут синхронизованы так же, как и “несклеенные”. В содержательных терминах это означает, что память, связанная с сознанием-доступ, прекращает свое “разрушительное” действие и освобождает “склеенные” осцилляторы для участия в представительстве феноменального сознания. Этот процесс постепенный, и на некотором этапе феноменальное сознание по терминологии Block [2005] “переполняет” сознание-доступ, хотя, по сути, здесь идет не переполнение, а переход осцилляторов из одной популяции в другую. Правда, для этого дополнительно необходимо значительно уменьшить уровень перцептивных шумов, иначе нельзя будет получить богатую информацию феноменального сознания, даже если прежде “склеенные” осцилляторы будут “отклеены”. Это очевидно из того, что функция распределения разности фаз стандартной ФАП имеет большую дисперсию при уменьшении отношения сигнал/шум [Gardner, 1979], что было численно подтверждено после добавления шума в правую часть уравнения (1).
СРАВНЕНИЕ С ДРУГИМИ МОДЕЛЯМИ СОЗНАНИЯ
Обзор многочисленных теорий и моделей сознания выходит за рамки настоящей статьи. Здесь мы ограничимся лишь кратким обзором некоторых моделей с определенными чертами, общими с нашей моделью “Нейролокатор С”. Все вместе они предсказывают единый механизм сознания, объясняющий большое число имеющихся данных, за исключением феноменального сознания.
Гиперсетевая модель сознания [Анохин, 2013]
Это теоретическая иерархическая модель сознания, нижний уровень которой составляет обычная нейронная сеть или коннектом, а верхний уровень, называемый когнитом, генерирует сознание как функциональное свойство иерархической системы. Более конкретно, отдельные узлы коннектома, даже если они пространственно разнесены, объединяются в комплексы, называемые коги, которые способны выполнять различные когнитивные функции. Коги в свою очередь являются узлами когнитома, образуя гиперсеть, функциональные и топологические свойства которой предположительно могут быть сопоставлены с некоторыми свойствами сознания. Эта модель имеет общие черты с нашей моделью: 1) она иерархична; 2) память в ней на основе системно-адаптивного свойства сети, не обязательно на синапсических связях; 3) не объясняет субъективного опыта; 4) предсказывает верхний предел познаваемости мира. Основное отличие в том, что эта модель не требует, кроме нейронов, какого-либо дополнительного “субстрата” сознания, но и не объясняет феноменального сознания. Другое важное отличие в том, что она коннекционистская, и поэтому не объясняет осцилляторные нейронные корреляты сознания и носит характер проекта будущих исследований сознания. Одним из таких возможных направлений исследования, предложенным А.М. Иваницким [2010], может быть уточнение гиперсетевой модели сознания с учетом ранее полученного авторами [Anokhin et al., 2002] эффекта реконсолидации памяти.
Модель унификации сознания и самосознания [Berkovich-Ohana, Gliksohn, 2014]
Модель описывает “двухъярусное” сознание в трех координатах: величины сознания-доступ, силы эмоций и течения времени. Модель опирается на большое количество экспериментов в различных состояниях сознания, в том числе, патологических и медитативных, но не касается вопросов о роли каких-либо нейронных коррелят сознания. Эта модель может служить прототипом для описания антагонизма между сознанием своего лица и сознанием другого лица, так как свойства ее центрального ядра сильно отличаются от свойств ее периферической части в отношении самосознания.
Квантово-кинематографическая модель сознания [Freeman, Vitiello, 2016]
Внешний мир (запахи) отображается в обонятельной системе как амплитудно-модифицированные пакеты паттернов на гамма-частоте, повторяющиеся во времени на более низкой частоте бета-диапазона, аналогично ФАС и кадрам в кино. Физические фазовые переходы в этой системе происходят лишь в определенные моменты времени, связанные с фазой низкой частоты, вполне аналогично фазовым переходам в “Нейролокаторе С”, с той лишь разницей, что вместо тета-диапазона частот здесь используется бета-диапазон, а память образуется в отдельном пакете, в то время, как у нас она – результат интеграции многих изолабильных микроколонок. Еще одно отличие от нашей модели в том, что модель обоняния не имеет центрального управителя для глобальной синхронизации системы, но требует квантово-механической теории, как для объяснения кинематографических кадров, так и для фазовых переходов. В остальном сходство с “Нейролокатором С” несомненное, отражающее универсальные структурные и динамические принципы структур мозга разной модальности.
Механизм сознания как информационный синтез [А.М. Иваницкий, 2010]
Это попытка понять феноменальное сознание на основе пространственно-временной корреляции процессов в мозге с возвращением возбуждения в проекционные зоны мозга после его обработки в центральных отделах. Как и в модели “Нейролокатор С”, основная идея взаимодействия входного возбуждения с процессами памяти и мотивации подсказана радарной методикой обнаружения слабых сигналов на фоне сильных шумов. При этом гиппокамп и неокортекс обеспечивают синтез сигналов и сохранение в памяти последовательности событий. Непрерывное сознание возникает из последовательностей образов длительностью 100–150 миллисекунд, что согласуется с кинематографической моделью [Freeman, Vitiello, 2016]. Еще одна важная характеристика, общая с моделью “Нейролокатор С”, состоит в том, что понимание, как субъективное чувство, возникает вследствие активации поля своего сознания в вентромедиальной префронтальной коре после прихода сигналов удовлетворения и награды от мотивационного центра. Это поднимает вопрос о необходимости морального воспитания в отношении к другим людям.
“Анатомическая” модель сознания [Faw, Faw, 2016]
Это одна из немногих моделей сознания, в которой гиппокамп играет критически важную роль: он, подобно его роли в модели “Нейролокатор С”, принимает активацию от многих распределенных структур мозга, организует и связывает ее в новую эпизодическую память и затем “широковещательно” посылает ее обратно в неокортекс. Таким образом, говорят авторы, гиппокамп играет роль генератора или симулятора субъективного опыта, хотя событие переживания этого опыта происходит вне гиппокампа. Модель объясняет трудный случай остаточного сознания знаменитого пациента Н.М. с удаленным гиппокампом, случай внимания без сознания в практике автоводителей и некоторые другие интересные случаи. Но, как замечает [Behrendt et al., 2017], из модели неясно, как распределенные неокортикальные области могут объединяться в связанные конфигурации, которые превращаются в единое феноменальное сознание и почему сознательный опыт возникает из неокортикальной активности, если известно, что сознание наблюдается даже в случае декортикации. Кроме того, модель, по-видимому, не различает феноменальное сознание и сознание-доступ.
Осцилляторная модель внимания [Kazanovich, 2019]
Хотя эта модель не касается проблем сознания, однако математический и вычислительный аппарат имеет много общего с моделью “Нейролокатор C”, так как последняя по существу является модификацией модели внимания. Описанные выше вычислительные эксперименты (рис. 2) были проведены совместно с Я.Б. Казановичем и основаны на небольшой модификации его уравнений, и отличаются от них введением фильтра нижних частот. Еще одно отличие в том, что при обучении в “Нейролокаторе С” изменяются собственные частоты периферических осцилляторов лишь после достижения определенной степени синхронизации группы осцилляторов (“изолабильное кодирование”)в то время, как в осцилляторной модели Казановича собственная частота каждого осциллятора адаптируется к своей текущей частоте. Но самое главное – это не различие, а сходство наших моделей в том, что в обоих случаях применена архитектура с одним центральным осциллятором и множеством независимых периферических осцилляторов – это следствие того, что мы учились у одного учителя – О.С. Виноградовой, предложившей медиальный септум в качестве глобального пейсмекера мозга [Vinogradova, 2001].
Модель социального сознания [Graziano, Kastner, 2011]
Это модель сознательного поведения других людей на основе перспективной реконструкции их внимания к внешнему миру. Она является вариантом решения мета-проблемы сознания, т.е. она не решает “трудную проблему”, но объясняет, почему эта проблема трудная. Точнее, она подсказывает, чего не достает модели внимания, чтобы стать моделью сознания, а именно требует особого внимания на лицо другого и на свое лицо. Эта модель, согласно авторским замечаниям, имеет три недостатка:
1) не может объяснить феноменальное сознание,
2) не решает проблему интеграции разных модальностей,
3) требует доработки для дополнительного согласования с существующей имитационной моделью, основанной на эмпатии.
Хотя модель “Нейролокатор С” также не может объяснить феноменальное сознание, зато проблема интеграции в ней решена [Игумен Феофан, 2004], а связь ее с моделью эмпатии может быть обоснована с помощью доминанты на лицо другого.
Квантово-макроскопическая модель сознания [Keppler, 2012, 2016, 2018]
До недавнего времени считалось, что декогерентность в зашумленном, теплом и влажном мозге препятствует применению квантовой механики для объяснения сознания. Однако Кепплер, опираясь на новую теорию стохастической электродинамики, предложил модель сознания, в которой весь мозг или его отдельные области имеют поведение когерентной макроскопической квантовой системы без разделения на микрокосм и макрокосм: все физические свойства материи теперь понимаются как возникающие из взаимодействия с нулевым фоновым полем (ZPF zero point field). В новой модели постулируется, что мозг посылает информацию в ZPF на частоте тета-ритма и обратно получает богатый информацией отклик в виду того, что метастабильные электромагнитные аттракторы ZPF фазо-частотно синхронизированы на этой же частоте тета-ритма между собой и мозгом, осуществляя, таким образом, запись и считывание феноменального сознательного опыта многих людей. Автор признает, что модель не решает “трудную проблему” сознания [Shani, Keppler, 2018] и проблему фазовых переходов на всех уровнях модели [Keppler, 2016], но зато подробно объясняет связь квантовой физики с восточной метафизикой [Keppler, 2012].
ОБСУЖДЕНИЕ И ПРЕДСКАЗАНИЯ
Теперь перейдем к особенности нашего подхода при решении каждой из проблем сознания.
I. Проблема НКС. Мы согласны с утверждением [Sandberg et al., 2016] в том, что главное в проблеме НКС не локализация сознания, а выяснение природы и причин возникновения НКС. “Нейролокатор С” объясняет, что причина корреляций и антикорреляций активности различных структур мозга, и значит всех НКС – это селективная и интегративная активность септо-гиппокампальной системы, которая и должна быть включена в НКС как основная причина корреляций в проблеме НКС. При этом какая из структур мозга оказывается минимально необходимой, зависит от контент-содержания сознания и выбранной методики: если сознание тестируется с помощью внешнего зрения, то НКС в зрительной коре, а если через “внутреннее” или зрение высокого уровня, то в префронтальной коре, а если нерефлекторная форма гидроэнцефалов и декортикальных, то вне неокортекса. Этим закрывается, казалось бы, непримиримый конфликт между сторонниками двух основных теорий НКС [Koch et al., 2016; Koch, 2018; Lau, Rosenthal, 2011].
II. Проблема соотношения внимания и сознания. В модели “Нейролокатор С” эта проблема решается принятием допущения о том, что внимание и сознание имеют в принципе один и тот же нейронный механизм, подробно описанный в нашей работе [Игумен Феофан, 2004]. Внимание необходимо, но не достаточно для сознания, так как требуется дополнительный механизм социальной активности, частным, но наиболее часто встречающимся случаем которого является эгоцентрический механизм самосознания и очень редко – доминанта на лицо другого.
III. “Трудная проблема” сознания. Мы считаем, что эта проблема не имеет решения в рамках научной методологии, но без научного изучения она навсегда останется одной из непостижимых тайн нового мистерианства [Воронов, 2010]. В чем же основная трудность “трудной проблемы”? Этот вопрос остается до настоящего времени невыясненным, если не считать, что есть много ученых и философов, которых Chalmers [Chalmers, 2018] называет “иллюзионистами”, т.е. считающих “трудную проблему” иллюзией. Мы считаем, что выяснение этого вопроса невозможно без решения “легких проблем” сознания, которые должны подсказать “узкое место” в научном объяснении сознания. Действительно, таким “узким местом” оказалось снижение сегрегации при эмоциональном обучении и повышенной интеграции информации. “Трудная проблема” сознания не имеет решения в рамках предложенной модели “Нейролокатор С”, но модель объясняет, что трудность проблемы принципиально связана с механизмом внимания, обучения и памяти (“изолабильное кодирование”), сдвигающим баланс между интеграцией и сегрегацией в сторону уменьшения разрешающей способности когнитивной системы.
IV. “Легкие проблемы” сознания. Это такие проблемы, решение которых возможно в рамках научной методологии. На деле “легкие проблемы” для современной нейробиологии не являются легкими в обычном смысле по следующим причинам. Во-первых, почти повсеместно считается, что мозг – это как бы оркестр без дирижера [Singer, 2005; Palm, 2016; Сергин, Сергин, 2019], в то время как данные по сознанию невозможно объяснить без “центрального управителя”, т.е. без септо-гиппокампальной системы. Во-вторых, считается, что нейронная кратковременная или рабочая память локализована в гиппокампе [Bannerman et al., 2014], и поэтому гиппокамп не должен играть существенной роли в феноменальном сознании, которое по информационному содержанию должно быть значительно богаче, чем рабочая память. Наконец, принцип доминанты А.А. Ухтомского, как главный принцип работы мозга, не признается фундаментальным для понимания внимания, памяти и сознания (исключение – работа [John, 1967]. Поэтому пока не будет принят единый доминантный механизм, объясняющий единообразно все “легкие проблемы” сознания, не будет понятно, почему “трудная проблема” не решается в рамках научной методологии. По существу, эту же мысль высказал Chalmers [Chalmers, 2018] в форме мета-проблемы сознания, к решению которой он призвал всех ученых, независимо от их априорной философской позиции в изучении сознания. “Легкие” проблемы сознания решаются единым нейронным механизмом модели “Нейролокатор С” с особой ролью фазовых переходов и фазово-амплитудной связи. Без решения “легких проблем” невозможно понять внимание и память, а значит, все остальные проблемы сознания.
V. Мета-проблема. В отличие от многих кандидатов на решение мета-проблемы, описанных в обзоре Chalmers [2018], модель “Нейролокатор С” выделяется тем, что она подсказывает естественный механизм возникновения “узкого места”, из-за которого, как мы считаем, нельзя понять механизм богатого по информационному содержанию субъективного опыта, нельзя понять феноменальное сознание. Это – способность мозга к интеграции и сегрегации информации, которая тесно связана с механизмом обучения, как это сформулировано в допущении 7 нашей модели. Особенно важно, что сегрегация снижается с сознанием, обучением, усилением внимания, увеличением трудности задачи, потому что при этом уменьшается “разрешающая способность” сознания по причине интегрального “склеивания” функциональных модулей восприятия [Sui, Humphrey, 2015]. Это и есть наше решение мета-проблемы сознания. Это решение нейтрально по отношению к делению на дуализм и материализм. Оно объясняет, почему трудно понять, как сознание может быть физическим. Как же это решение может подсказать удовлетворительное решение “трудной проблемы”? Во-первых, необходимо учесть, что естественных механизмов мозга (сон, отдых, культура и т.д.) недостаточно, чтобы значительно увеличить сегрегацию кортикальных модулей и уменьшить остаточную интеграцию модулей. Во-вторых, необходимо учесть решающую роль социального сознания, как это представлено в новых данных, подтверждающих допущение 9 нашей модели, а в обычной жизни закреплено в культурной традиции народа в виде необходимости борьбы со своими страстями. Лучше всего это можно понять на примере решения “более трудной” проблемы сознания.
VI. “Более трудная” проблема сознания. После того, как выяснено, в чем трудность “трудной проблемы”, естественно возникает вопрос, как в принципе можно преодолеть эту трудность в теории и в своей жизни. Ответ содержится в нравственной компоненте принципа доминанты – в доминанте на лицо другого. То, что А.А. Ухтомский описывает как подсознательные процессы, в психологии [Dalpe et al., 2019] называется страстями, которые подразделяются на гармонические и обсцессивные. Обсцессивные страсти разрушительны для сознания человека и приводят к одержимости. Но хотя в обычной жизни обсцессивные страсти внешне бывают малозаметны, они радикально меняют феноменальное сознание по причине сужения сознания с преобладанием памяти о “страстных” предметах (сознание-доступа) и, во-вторых, по причине высокого уровня перцептивных шумов, связанных, например, с чрезвычайно высоким уровнем скорости метаболизма при эгоцентричном сознании [Lou et al., 2017]. Борьба с обсцессивными страстями до некоторой степени возможна в рамках многих религиозных и психологических практик, например, флуктуации в сознании заметно уменьшаются после длительной практики в медитации [Carter et al., 2005]. Но доминанту на лицо другого понять очень трудно, так как страстная память не стирается без большого труда и без постоянного большого “чисто физического насилия над собой”. Выработка доминанты на лицо другого решает “более трудную” проблему сознания, опираясь на двухтысячелетний свято-отеческий опыт, которым владел академик А.А. Ухтомский. Итак, “более трудная” проблема, решенная в теории и в личной жизни А.А. Ухтомского, учит нас, что сознание другого лица объективно непознаваемо, но субъективно-опытно открыто всякому человеку, имеющему доминанту на другое лицо. Отсюда вытекает следующее следствие-предсказание А.А. Ухтомского: “[Н]аука не может пойти плодотворно, пока внутренняя горница человека не вычищена,… истина открывается сердцу не всякому, но лишь очистившемуся от страстей… тому, кто потрудился и пролил пот при работе над сердцем своим … Истина открывается деятельному духу, насколько он очищает свое сердце, а затем ум, т.е. воле, сердцу и уму вместе”. Еще одно предсказание относительно “трудной проблемы” сознания в том, что ее потенциальное решение в принципе экспериментально проверяемо. Дело в том, что “изолабильное кодирование” информации в мозге, особенно с пристрастной мотивацией и страстями, фундаментально ограничивает познавательные способности человека и животных. И наоборот, очищение от страстей, как процесс сегрегации функциональных единиц ЦНС, расширяет познавательные способности. Последнее предсказание, судя по большому количеству работ по сегрегации, в принципе проверяется, особенно в условиях уединения, разумного воздержания от пищи и сна, размышления о смерти и др.
ВЫВОДЫ
Учение о доминанте на лицо другого А.А. Ухтомского, теория компараторной функции гиппокампа О.С. Виноградовой и основанная на них математическая модель “Нейролокатор С” позволяют сделать следующие основные выводы:
1. Без решения “легких проблем” в форме единого механизма, объясняющего основные данные по сознанию, невозможно в рамках научной методологии понять, почему существует “трудная проблема” сознания.
2. Существует фундаментальное ограничение на научное изучение феноменального сознания в виду “склеивания” элементарных единиц памяти и самосознания [Sui, Humphreys, 2015], в результате которого интеграция информации преобладает над сегрегацией.
3. А.А. Ухтомский открыл уникальный способ преодоления указанного выше фундаментального ограничения через выработку доминанты на лицо другого человека.
4. Наша математическая модель сознания “Нейролокатор С” путем решения “легких” проблем и, главным образом, мета-проблемы сознания, дает потенциальное решение “трудной проблемы” сознания, указывая на принципиальную возможность отделения феноменального сознания от сознания-доступ.
5. Эта модель через объяснение эффекта ret+ext, т.е. через неинвазивное стирание страстной памяти, предлагает нейробиологическую основу для специфического обучения, с помощью которого потенциальное решение “трудной проблемы” может стать актуальным.
Выражаю благодарность игумену Иннокентию, Ольге Жедяевой за помощь и Якову Казановичу за помощь и критические замечания. Работа выполнена без финансовой поддержки от грантов, фондов или научных программ.
Список литературы
Анохин К.В. Коды вавилонской библиотеки мозга. В мире науки. 2013. 5: 83–89.
Воронов Н.А. Что такое новое мистерианство. Вестник МГУ. Сер. 7. Философия. 2010. 5: 18–33.
Иваницкий А.М. Наука о мозге на пути к решению проблемы сознания. Вестник РАН. 2010. 80 (5): 447–455.
Игумен Феофан. Модель внимания и памяти, основанная на принципе доминанты и компараторной функции гиппокампа. М.: Журн. высш. нервн. деят. им. И.П. Павлова. 2004. 54 (1): 10–29.
Игумен Феофан. Принципы сенсорной интеграции: иерархичность и синхронизация. М.: Журн. высш. нервн. деят. им. И.П. Павлова. 2005. 55 (2): 163–169.
Игумен Феофан. Роль гиппокампа в долговременной памяти: системно-динамический подход. 2007. 57 (3): 1–278.
Крюков В.И., Борисюк Г.Н., Борисюк Р.М., Кириллов А.Б., Коваленко Е.И. Метастабильные и неустойчивые состояния в мозге. Ред. А.М. Молчанов. Пущино: ОНТИ НЦБИ АН СССР. 1986. 114 с.
Линдсей В. Системы синхронизации в связи и управлении. М.: “Советское радио”, 1978. 600 с.
Сергин В.Я., Сергин А.В. Иерархическая модель восприятия без комбинаторного взрыва. Журн. высш. нервн. деят. им. И.П. Павлова. 2019. 69 (5): 629–654.
Симонов П.В. Эмоциональный мозг. М.: Наука, 1981. 215 с.
Торопова К.А., Трошев Д.В., Ивашкина О.И., Анохин К.В. Активация экспрессии c-fos в ретросплениальной коре, но не гиппокампе, сопровождает формирование ассоциации между обстановкой и безусловным стимулом и ее последующее извлечение у мышей. 2018. 68 (6): 756–770.
Ухтомский A.A. Доминанта. М.-Л.: Наука, 1966. 273 с.
Adachi Y., Osada T., Sporns O., Watanabe T., Matsui T., Miyamoto K., Miyashita Y. Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb. Cortex. 2012. 22 (7): 1586–1592.
Alnæs D., Kaufmann T., Richard G., Duff E.P., Sneve M.H., Endestad T., Nordvik J.E., Andreassen O.A., Smith S.M., Westlye L.T. Attentional load modulates large-scale functional brain connectivity beyond the core attention networks. Neuroimage. 2015. 109: 260–272.
Amaral D.G., Witter M.P. Hippocampal formation. In: G. Paxinos, Editor, The Rat NervousSystem (2nd edition ed.) Academic Press, San Diego, 2004. pp. 635–704.
Amico E., Marinazzo D., Di Perri C., Heine L., Annen J., Martial C., Dzemidzic M., Kirsch M., Bonhomme V., Laureys S., Goñi J. Mapping the functional connectome traits of levels of consciousness. Neuroimage. 2017. 148: 201–211.
Anokhin K.V., Tiunova A.A., Rose S.P. Reminder effects – reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive-avoidance task in young chicks. Eur. J. Neurosci. 2002. 15 (11): 1759–1765.
Attardo A., Fitzgerald J.E., Schnitzer M.J. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature. 2015. 523 (7562): 592–596.
Avena-Koenigsberger A., Misic B., Sporns O. Communication dynamics in complex brain networks. Nat. Rev. Neurosci. 2017. 19 (1): 17–33.
Baars B.J. A scientific approach to silent consciousness. Front Psychol. 2013. 4: 678.
Baars B.J., Franklin S., Ramsoy T.Z. Global workspace dynamics: cortical “binding and propagation” enables conscious contents. Front. Psychol. 2013. 4: 200.
Baker K.D., McNally G.P., Richardson R. Memory retrieval before or after extinction reduces recovery of fear in adolescent rats. Learn. Mem. 2013. 20 (9): 467–473.
Bannerman D.M., Sprengel R., Sanderson D.J., McHugh S.B., Rawlins J.N., Monyer H., Seeburg P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014. 15 (3): 181–192.
Baria A.T., Maniscalco B., He B.J. Initial-state-dependent, robust, transient neural dynamics encode conscious visual perception. PLoS Comput. Biol. 2017. 13 (11): e1005806.
Barttfeld P., Uhrig L., Sitt J.D., Sigman M., Jarraya B., Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proc. Natl. Acad. Sci. U. S. A. 2015. 112 (3): 887–892.
Bassett D.S., Yang M., Wymbs N.F., Grafton S.T. Learning-induced autonomy of sensorimotor systems. 2015. Nat. Neurosci. 2015. (5): 744–751.
Behrendt R.P. Hippocampus as a wormhole: gateway to consciousness. Wiley Interdiscip. Rev. Cogn. Sci. 2017. 8 (5): e1446.
Bell P.T., Shine J.M. Subcortical contributions to large-scale network communication. Neurosci. Biobehav. Rev. 2016. 71: 313–322.
Bennett M.R., Farnell L., Gibson W.G., Lagopoulos J. Cortical network models of firing rates in the resting and active states predict BOLD responses. PLoS One. 2015. 10 (12): e0144796.
Bennett M.R., Farnell L., Gibson W., Lagopoulos J. On the origins of the 'global signal' determined by functional magnetic resonance imaging in the resting state. J. Neural. Eng. 2016. 13 (1): 016012.
Berkovich-Ohana A., Glicksohn J. The consciousness state space (CSS) – a unifying model for consciousness and self. Front. Psychol. 2014. 5: 341.
Berkovitch L., Dehaene S., Gaillard R. Disruption of conscious access in schizophrenia. Trends Cogn. Sci. 2017. 21 (11): 878–892.
Bettinger J.S. Comparative approximations of criticality in a neural and quantum regime. Prog. Biophys. Mol. Biol. 2017. 131: 445–462.
Billings J., Keilholz S. The not-so-global blood oxygen level-dependent signal. Brain Connect. 2018. 8 (3): 121–128.
Biswal B.B., Mennes M., Zuo X.N. Gohel S., Kelly C., Smith S.M., Beckmann C.F., Adelstein J.S., Buckner R.L., Colcombe S., Dogonowski A.M., Ernst M., Fair D., Hampson M., Hoptman M.J., Hyde J.S., Kiviniemi V.J., Kötter R., Li S.J., Lin C.P., Lowe M.J., Mackay C., Madden D.J., Madsen K.H., Margulies D.S., Mayberg H.S., McMahon K., Monk C.S., Mostofsky S.H., Nagel B.J., Pekar J.J., Peltier S.J., Petersen S.E., Riedl V., Rombouts S.A., Rypma B., Schlaggar B.L., Schmidt S., Seidler R.D., Siegle G.J., Sorg C., Teng G.J., Veijola J., Villringer A., Walter M., Wang L., Weng X.C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y.F., Zhang H.Y., Castellanos F.X., Milham M.P. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U. S. A. 2010. 107 (10): 4734–4739.
Block N. The harder problem of consciousness. 2002. Journal of Philosophy. 99 (8): 391–425.
Block N. Two neural correlates of consciousness. Trends Cogn. Sci. 2005. 9 (2): 46–52.
Block N. If perception is probabilistic, why does it not seem probabilistic? Philos. Trans. R Soc. Lond. B Biol. Sci. 2018. 373 (1755).
Bola M., Sabel B.A. Dynamic reorganization of brain functional networks during cognition. Neuroimage. 2015. 114: 398–413.
Bor D., Seth A.K. Consciousness and the prefrontal parietal network: insights from attention, working memory, and chunking. Front Psychol. 2012. 3: 63.
Brier M.R., Thomas J.B., Fagan A.M., Hassenstab J., Holtzman D.M., Benzinger T.L., Morris J.C., Ances B.M. Functional connectivity and graph theory in preclinical Alzheimer’s disease. Neurobiol. Aging. 2014. 35 (4): 757–768.
Brovelli A., Badier J.M., Bonini F., Bartolomei F., Coulon O., Auzias G. Dynamic Reconfiguration of Visuomotor-Related Functional Connectivity Networks. J. Neurosci. 2017. 37 (4): 839–853.
Buehler D. The central executive system. Synthese. 2018. 195 (5): 1969–1991.
Bullmore E., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012. 13 (5): 336–349.
Buschman T.J., Kastner S. From Behavior to Neural Dynamics: An integrated theory of attention. Neuron. 2015. 88 (1): 127–144.
Cabral J., Kringelbach M.L., Deco G. Functional connectivity dynamically evolves on multiple time-scales over a static structural connectome: Models and mechanisms. Neuroimage. 2017. 160: 84–96.
Cahill E.N., Wood M.A., Everitt B.J., Milton A.L. The role of prediction error and memory destabilization in extinction of cued-fear within the reconsolidation window. Neuropsychopharmacology. 2019. 44 (10): 1762–1768.
Carter O.L., Presti D.E., Callistemon C., Ungerer Y., Liu G.B., Pettigrew J.D. Meditation alters perceptual rivalry in Tibetan Buddhist monks. 2005. Current Biology. 15 (11): R412-3.
Casali A.G., Gosseries O., Rosanova M., Boly M., Sarasso S., Casali K.R., Casarotto S., Bruno M.A., Laureys S., Tononi G., Massimini M. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 2013. 5 (198): 198ra105.
Chalmers D.J. Facing up to the problem of consciousness. Journal of Consciousness Studies. 1995. 2 (3): 200–219.
Chalmers D.J. Availability: The Cognitive Basis of Experience? Behavioral and Brain Sciences. 1997. 20 (1): 148–149.
Chalmers D.J. The Meta-problem of consciousness. Journal of Consciousness Studies. 2018. 25 (9–10): 6–61.
Chan M.Y., Park D.C., Savalia N.K., Petersen S.E., Wig G.S. Decreased segregation of brain systems across the healthy adult lifespan. Proc. Natl. Acad. Sci. U. S. A. 2014. 111 (46): E4997–E5006.
Chan W.Y., Leung H.T., Westbrook R.F., McNally G.P. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn. Mem. 2010. 17 (10): 512–521.
Chaudhuri R., Knoblauch K., Gariel M.A., Kennedy H., Wang X.J. A large-scale circuit mechanism for hierarchical dynamical processing in the primate cortex. Neuron. 2015. 88 (2): 419–431.
Chen J.E., Glover G.H., Greicius M.D., Chang C. Dissociated patterns of anti-correlations with dorsal and ventral default-mode networks at rest. Hum. Brain Mapp. 2017. 38 (5): 2454–2465.
Cocchi L., Gollo L.L., Zalesky A., Breakspear M. Criticality in the brain: a synthesis of neurobiology, models and cognition. Prog. Neurobiol. 2017. 158: 132–152.
Cohen J.R., D’Esposito M. The segregation and integration of distinct brain networks and their relationship to cognition. J. Neurosci. 2016. 36 (48): 12083–12094.
Cohen M.A., Cavanagh P., Chun M.M., Nakayama K. The attentional requirements of consciousness. Trends Cogn. Sci. 2012. 16 (8): 411–417.
Colgin L.L. Theta-gamma coupling in the entorhinal-hippocampal system. Curr. Opin. Neurobiol. 2015. 31: 45–50.
Combs A., Kripner S. Collective consciousness and the social brain. Journal of Consciousness Studies. 2008. 15 (10–11): 264–276.
Cowan N. Attention and memory: an integrated framework. Oxford Psychol. Ser. № 26. N.Y.: Oxford Univ. Press, 1995 (Paperback edition: 1997). xv. 321 p.
Cowan N. Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychol. Bull. 1988. 104 (2): 163–191.
Crick F., Koch C. Consciousness and Neuroscience. Cereb. Cortex. 1998. 8: 97–107.
Dalpe J., Demers M., Verner-Filion J., Vallerand R.J. From personality to passion: The role of the Big Five factors. Personality and Individual Differences. 2019. 138: 280–285.
D’Andola M., Rebollo B., Casali A.G., Weinert J.F., Pigorini A., Villa R., Massimini M., Sanchez-Vives M.V. Bistability, causality, and complexity in cortical networks: an in vitro perturbational study. Cereb. Cortex. 2017. 28 (7): 2233–2242.
Davison E.N., Schlesinger K.J., Bassett D.S., Lynall M.E., Miller M.B., Grafton S.T., Carlson J.M. Brain network adaptability across task states. PLoS Comput. Biol. 2015. 11 (1): e1004029.
Deco G., Jirsa V.K., McIntosh A.R. Resting brains never rest: computational insights into potential cognitive architectures. Trends Neurosci. 2013. 36 (5): 268–274.
Deco G., Kringelbach M.L., Jirsa V.K., Ritter P. The dynamics of resting fluctuations in the brain: metastability and its dynamical cortical core. Sci. Rep. 2017. 7 (1): 3095.
Deco G., Tononi G., Boly M., Kringelbach M.L. Rethinking segregation and integration: contributions of whole-brain modeling. Nat. Rev. Neurosci. 2015. 16 (7): 430–439.
Del Cul A., Dehaene S., Reyes P., Bravo E., Slachevsky A. Causal role of prefrontal cortex in the threshold for access to consciousness. Brain. 2009. 132 (Pt 9): 2531–2540.
Delamater A.R., Westbrook R.F. Psychological and neural mechanisms of experimental extinction: a selective review. Neurobiol. Learn. Mem. 2014. 108: 38–51.
Demertzi A., Tagliazucchi E., Dehaene S., Deco G., Barttfeld P., Raimondo F., Martial C., Fernández-Espejo D., Rohaut B., Voss H.U., Schiff N.D., Owen A.M., Laureys S., Naccache L., Sitt J.D. Human consciousness is supported by dynamic complex patterns of brain signal coordination. Sci. Adv. 2019. 5 (2): eaat7603.
Dennett D.C. Facing up to the hard question of consciousness. Philos. Trans. R Soc. Lond. B Biol. Sci. 2018. 373 (1755).
Denton D.A., McKinley M.J., Farrell M., Egan G.F. The role of primordial emotions in the evolutionary origin of consciousness. Conscious Cogn. 2009. 18 (2): 500–514.
De Ridder D., van der Loo E., Vanneste S., Gais S., Plazier M., Kovacs S., Sunaert S., Menovsky T., van de Heyning P. Theta-gamma dysrhythmia and auditory phantom perception. J. Neurosurg. 2011. 114 (4): 912–921.
Dixon M.L., Andrews-Hanna J.R., Spreng R.N., Irving Z.C., Mills C., Girn M., Christoff K. Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage. 2017. 147: 632–649.
Doesburg S.M., Green J.J., McDonald J.J., Ward L.M. Rhythms of consciousness: binocular rivalry reveals large-scale oscillatory network dynamics mediating visual perception. PLoS One. 2009. 4 (7): e6142.
Droste F., Do A.L., Gross T. Analytical investigation of self-organized criticality in neural networks. J. R. Soc. Interface. 2013. 10: 20120558.
Eichenbaum H. Time cells in the hippocampus: a new dimension for mapping memories. Nat. Rev. Neurosci. 2014. 15 (11): 732–744.
Engel A.K., Fries P. Neuronal oscillations, coherence, and consciousness. In The Neurology of Conciousness. Cognitive Neuroscience and Neuropathology. 2016. pp. 49–60.
Esghaei M., Daliri M.R., Treue S. Attention decreases phase-amplitude coupling, enhancing stimulus discriminability in cortical area MT. Front Neural Circuits. 2015. 9: 82.
Esposito R., Cieri F., Chiacchiaretta P., Cera N., Lauriola M., Di Giannantonio M., Tartaro A., Ferretti A. Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav. 2017. 12 (1): 127–141.
Fahrenfort J.J., van Leeuwen J., Olivers C.N., Hogendoorn H. Perceptual integration without conscious access. Proc. Natl. Acad. Sci. U. S. A. 2017. 114 (14): 3744–3749.
Faw M., Faw B. Neurotypical subjective experience is caused by a hippocampal simulation. Wiley Interdiscip. Rev. Cogn. Sci. 2016. 8 (5): e1412.
Fernandez-Espejo D., Soddu A., Cruse D., Palacios E.M., Junque C., Vanhaudenhuyse A., Rivas E., Newcombe V., Menon D.K., Pickard J.D., Laureys S., Owen A.M. A role for the default mode network in the bases of disorders of consciousness. Ann. Neurol. 2012. 72 (3): 335–343.
Fingelkurts A.A., Fingelkurts A., Bagnato S., Boccagni C., Galardi G. DMN operational synchrony relates to self-consciousness: evidence from patients in vegetative and minimally conscious states. Open. Neuroimag. J. 2012. 6: 55–68.
Fingelkurts A.A., Fingelkurts A., Bagnato S., Boccagni C., Galardi G. The chief role of frontal operational module of the brain default mode network in the potential recovery of consciousness from the vegetative state: a preliminary comparison of three case reports. Open. Neuroimag. J. 2016. 10: 41–51.
Forcato C., Bavassi L., De Pino G., Fernández R.S., Villarreal M.F., Pedreira M.E. Differential left hippocampal activation during retrieval with different types of reminders: an fmri study of the reconsolidation process. PLoS One. 2016. 11 (3): e0151381.
Foster B.L., He B.J., Honey C.J., Jerbi K., Maier A., Saalmann Y.B. Spontaneous neural dynamics and multi-scale network organization. Front. Syst. Neurosci. 2016. 10: 17.
Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 2005. 102 (27): 9673–9678.
Freeman W.J., Holmes M.D. Metastability, instability, and state transition in neocortex. Neural. Netw. 2005. 18 (5–6): 497–504.
Freeman W.J., Vitiello G. Matter and mind are entangled in two streams of images guiding behavior and informing the subject through awareness. Imprint. Academic. 2016. 14 (1): 7–24.
Freeman W.J. Origin, structure, and role of background EEG activity. Part 1. Analytic amplitude. Clin. Neurophysiol. 2004a. 115 (9): 2077–2088.
Freeman W.J. Origin, structure, and role of background EEG activity. Part 2. Analytic amplitude. Clin. Neurophysiol. 2004b. 115 (9): 2089–2107.
Freeman W.J. Indirect biological measures of consciousness from field studies of brains as dynamical systems. Neural Netw. 2007. 20 (9): 1021–1031.
Friedman E.B., Sun Y., Moore J.T., Hung H.T., Meng Q.C., Perera P., Joiner W.J., Thomas S.A., Eckenhoff R.G., Sehgal A., Kelz M.B. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One. 2010. 5 (7): e11903.
Friese U., Köster M., Hassler U., Martens U., Trujillo-Barreto N., Gruber T. Successful memory encoding is associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations in human scalp-recorded EEG. Neuroimage. 2013. 66: 642–647.
Frith C.D., Frith U. Social cognition in humans. Curr. Biol. 2007. 17 (16): R724–R732.
Frith C.D., Metzinger T. What’s the use of consciousness? How the stab of conscience made us really conscious, in Engel, A.K., Friston, K.J., Kragic. D. (eds.) The Pragmatic Turn: Toward Action-Oriented Views in Cognitive Science, Strungmann Forum Reports, 2016. vol. 18, Lupp, J. (series ed.), Cambridge, MA: MIT Press.
Fuertinger S., Horwitz B., Simonyan K. The functional connectome of speech control. PLoS Biol. 2015. 13 (7): e1002209.
Gardner F.M. Phaselock Techniques. John Wiley and Sons. New York, 2nd edition. 1979. 426 p.
Geerligs L., Renken R.J., Saliasi E., Maurits N.M., Lorist M.M. A brain-wide study of age-related changes in functional connectivity. Cereb. Cortex. 2015. 25 (7): 1987–1999.
Gershman S.J., Monfils M.H., Norman K.A., Niv Y. The computational nature of memory reconsolidation. eLife. 2016. bioRxiv
Gershman S.J., Monfils M.H., Norman K.A., Niv Y. The computational nature of memory modification. eLife. 2017. 6: e23763.
Girardeau G., Benchenane K., Wiener S.I., Buzsáki G., Zugaro M.B. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 2009. 12 (10): 1222–1223.
Godwin D., Barry R.L., Marois R. Breakdown of the brain’s functional network modularity with awareness. Proc. Natl. Acad. Sci. U. S. A. 2015. 112 (12): 3799–3804.
Gonzalez-Castillo J., Hoy C.W., Handwerker D.A., Robinson M.E., Buchanan L.C., Saad Z.S., Bandettini P.A. Tracking ongoing cognition in individuals using brief, whole-brain functional connectivity patterns. Proc Natl. Acad. Sci. USA. 2015. 112 (28): 8762–8767.
Graziano M.S., Kastner S. Human consciousness and its relationship to social neuroscience: A novel hypothesis. Cogn. Neurosci. 2011. 2 (2): 98–113.
Graziano M.S. Attributing awareness to others: The attention schema theory and its relationship to behavioural prediction. Journal of Consciousness Studies. 2019. 26 (3–4): 17–37.
Gross S., Flombaum J. Does perceptual consciousness overflow cognitive access? The challenge from probabilistic, hierarchical processes. Mind and Language. 2017. 32 (3): 358–391.
Guevara Erra R., Mateos D.M., Wennberg R., Perez Velazquez J.L. Statistical mechanics of consciousness: Maximization of information content of network is associated with conscious awareness. Phys. Rev. E. 2016. 94 (5-1): 052402.
Gutierrez-Barragan D., Basson M.A., Panzeri S., Gozzi A. Oscillatory brain states govern spontaneous fMRI network dynamics. 2018. bioRxiv preprint first posted online Aug. 20, 2018; https://doi.org/10.1101/393389
Hangya B., Borhegyi Z., Szilágyi N., Freund T.F., Varga V. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. J. Neurosci. 2009. 29 (25): 8094–8102.
Hannawi Y., Lindquist M.A., Caffo B.S., Sair H.I., Stevens R.D. Resting brain activity in disorders of consciousness: a systematic review and meta-analysis. Neurology. 2015. 84 (12): 1272–1280.
Hannula D.E., Greene A.J. The hippocampus reevaluated in unconscious learning and memory: at a tipping point? Front. Hum. Neurosci. 2012. 6: 80.
Hari R., Parkkonen L. The brain timewise: how timing shapes and supports brain function. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015. 370 (1668).
Hasselmo M.E. What is the function of hippocampal theta rhythm? Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005. 15 (7): 936–949.
Havlík M. From Anomalies to essential scientific revolution? Intrinsic brain activity in the light of kuhn’s philosophy of science. Front. Syst. Neurosci. 2017. 11: 17.
Headley D.B., Paré D. Common oscillatory mechanisms across multiple memory systems. NPJ Sci. Learn. 2017. 2.
Hearne L.J., Cocchi L., Zalesky A., Mattingley J.B. Reconfiguration of brain network architectures between resting-state and complexity-dependent cognitive reasoning. J. Neurosci. 2017. 37 (35): 8399–8411.
Hegumen Theophan (V.I. Kryukov). The problem of non-invasive memory erasure and its solution. Annual Pavlovian Society Meeting. Sept 1820. 2014. Hilton Seattle. Seattle WA https://campus.albion.edu/pavlovian/files/2014/09/2014programAbs.pdf
Heleven E., Van Overwalle F. The neural representation of the self in relation to close others using fMRI repetition suppression. Soc. Neurosci. 2019a. 13: 1–12.
Heleven E., Van Overwalle F. Neural representations of others in the medial prefrontal cortex do not depend on our knowledge about them. Soc. Neurosci. 2019b. 14 (3): 286–299.
Hernandez-Urbina V., Herrmann J.M. Self-organized criticality via retro-synaptic signals. Front. Phys. 2017. 4 (54): 1–12.
Hesse J., Gross T. Self-organized criticality as a fundamental property of neural systems. Front. Syst. Neurosci. 2014. 8 (166): 1–14.
Heusser A.C., Poeppel D., Ezzyat Y., Davachi L. Episodic sequence memory is supported by a theta-gamma phase code. Nat. Neurosci. 2016. 19 (10): 1374–1380.
Hodgson D. Nonlocality, local indeterminism and consciousness. Ratio. 1996. 9 (1): 1–22.
Huang Z., Zhang J., Wu J., Qin P., Wu X., Wang Z., Dai R., Li Y., Liang W., Mao Y., Yang Z., Zhang J., Wolff A., Northoff G. Decoupled temporal variability and signal synchronization of spontaneous brain activity in loss of consciousness: An fMRI study in anesthesia. Neuroimage. 2016. 124 (Pt A): 693–703.
Hudetz A.G., Liu X., Pillay S. Dynamic repertoire of intrinsic brain states is reduced in propofol-induced unconsciousness. Brain Connect. 2015. 5 (1): 10–22.
Hudson A.E., Calderon D.P., Pfaff D.W., Proekt A. Recovery of consciousness is mediated by a network of discrete metastable activity states. Proc. Natl. Acad. Sci. U. S. A. 2014. 111 (25): 9283–9288.
Hunsaker M.R., Kesner R.P. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci. Biobehav. Rev. 2013 37 (1): 36–58.
Hunsaker M.R., Mooy G.G., Swift J.S., Kesner R.P. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatialand nonspatial (visual object) information processing. Behav. Neurosci. 2007. 121 (4): 742–750.
Hutchison R.M., Hutchison M., Manning K.Y., Menon R.S., Everling S. Isoflurane induces dose-dependent alterations in the cortical connectivity profiles and dynamic properties of the brain’s functional architecture. Hum Brain Mapp. 2014. 35 (12): 5754–5775.
Hutchison R.M., Womelsdorf T., Allen E.A., Bandettini P.A., Calhoun V.D., Corbetta M., Della Penna S., Duyn J.H., Glover G.H., Gonzalez-Castillo J., Handwerker D.A., Keilholz S., Kiviniemi V., Leopold D.A., de Pasquale F., Sporns O., Walter M., Chang C. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage. 2013. 80: 360–378.
Hutchinson B.T. Toward a theory of consciousness: A review of the neural correlates of inattentional blindness. 2019. Neurosci. Biobehav. Rev. 104: 87–99.
Jacob J., Jacobs C., Silvanto J. Attention, working memory, and phenomenal experience of WM content: memory levels determined by different types of top-down modulation. Front. Psychol. 2015. 6: 1603.
Jenkins A.C., Macrae C.N., Mitchell J.P. Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proc. Natl. Acad. Sci. U. S. A. 2008. 105 (11): 4507–4512.
Jiang H., Bahramisharif A., van Gerven M.A., Jensen O. Measuring directionality between neuronal oscillations of different frequencies. Neuroimage. 2015. 118: 359–367.
John E.R. Mechanisms of memory. New York: Academic press, 1967.
John E.R. A Field Theory of Consciousness. Conscious Cogn. 2001. 10 (2): 184–213.
Johnson S., Marro J., Torres J.J. Robust short-term memory without synaptic learning. PLoS One. 2013. 8 (1): e50276.
Kang D., Ding M., Topchiy I., Kocsis B. Reciprocal interactions between medial septum and hippocampus in theta generation: granger causality decomposition of mixed spike-field recordings. Front. Neuroanat. 2017. 11: 120.
Kaplan A.Y., Fingelkurts A.A., Fingelkurts A.A., Borisov S.V., Darkhovsky B.S. Nonstationary nature of the brain activity as revealed by EEG/MEG: Methodological, practical and conceptual challenges. Signal Processing. 2005. 85 (11): 2190–2212.
Kaplan R., Horner A.J., Bandettini P.A., Doeller C.F., Burgess N. Human hippocampal processing of environmental novelty during spatial navigation. Hippocampus. 2014. 24 (7): 740–750.
Kazanovich Y. Modelling brain cognitive functions by oscillatory neural networks. Optical Memory and Neural Networks. 2019. 28 (3): 175–184.
Kendrick K.M., Zhan Y., Fischer H., Nicol A.U., Zhang X., Feng J. Learning alters theta amplitude, theta-gamma coupling and neuronal synchronization in inferotemporal cortex. BMC Neurosci. 2011. 12: 55.
Keppler J. A conceptual framework for consciousness based on a deep understanding of matter. Open Journal of Philosophy. 2012. 6 (4): 689–703.
Keppler J. On the universal mechanism underlying conscious systems and the foundations for a theory of consciousness. Open Journal of Philosophy. 2016. 6: 346–367.
Keppler J. The role of the brain in conscious processes: a new way of looking at the neural correlates of consciousness. Front. Psychol. 2018. 9: 1346.
Kim K., Johnson M.K. Activity in ventromedial prefrontal cortex during self-related processing: positive subjective value or personal significance? Soc. Cogn. Affect. Neurosci. 2015. 10 (4): 494–500.
Kim M., Kim S., Mashour G.A., Lee U. Relationship of topology, multiscale phase synchronization, and state transitions in human brain networks. Front. Comput. Neurosci. 2017. 11: 55.
Kinnison J., Padmala S., Choi J.M., Pessoa L. Network analysis reveals increased integration during emotional and motivational processing. J. Neurosci. 2012. 32 (24): 8361–8372.
Kirillov A.B., Borisyuk G.N., Borisyuk R.M., Kovalenko Ye.I., Kryukov V.I. Short-term memory as a metastable state. III. Diffusion approximation. Cybernetics and Systems: Int. J. 1986. 17: 2–3, 169–182.
Koch C., Massimini M., Boly M., Tononi G. Neural correlates of consciousness: progress and problems. Nat. Rev. Neurosci. 2016. 17 (5): 307–321.
Koch C., Tsuchiya N. Attention and consciousness: two distinct brain processes. Trends Cogn. Sci. 2007. 11 (1): 16–22.
Koch C. What Is Consciousness? Nature. 2018. 557 (7704): S8–S12.
Kozma R., Freeman W.J. Interpretation of experimental results as cortical phase transitions. In: Cognitive phase transitions in the cerebral cortex – enhancing the neuron doctrine by modeling neural fields. Studies in Systems, Decision and Control. Springer. New York. 2016. 262 p.
Kryukov V.I., Borisyuk G.N., Borisyuk R.M., Kirillov A.B., Kovalenko Ye.I. “Metastable and unstable states in the brain,”in: Stochastic Systems: Ergodicity, Memory, Morphogenesis, R.L. Dobrushin, V.I. Kryukov, an A.L. Toom (eds.), Manchester University Press, Manchester, UK, 1990. 226–357.
Kryukov V.I. The role of the hippocampus in long-term memory: is it memory store or comparator? J. Integr. Neurosci. 2008. 7 (1): 117–184.
Kryukov V.I. (Hegumen Theophan). Towards a unified model of Pavlovian conditioning: A solution to the reconsolidation problem. In: Mastarakis N., Mladenov V., Bojkovic Z., Topalis F., Psarris K., Barbulescu A., Karimi H.R., Tsekouras G.J., Salem A-B.M., Vladareanu L., Nikolic., Simian D., Hausnerova B., Berber S, Bardis N, Zaharim A., Subramaniam C. (eds.) Recent researches in geography, geology, energy, evironment and biomedicine: 193–202, Proceedings of the 5th International conference on energy and Development – Environment – Biomedicine (EDEB ‘11), WSEAS Press, Greece. 2011a.
Kryukov V.I. (Hegumen Theophan). Towards a unified model of Pavlovian conditioning: Solution to the extinction problem. In Braissant O. et al. (eds.) Recent researches in modern medicine: 330–340, 2nd International conference on MEDICAL PHYSIOLOGY (PHYSIOLOGY ‘11), WSEAS Press, Cambridge, UK. 2011в.
Kryukov V.I. Towards a unified model of Pavlovian conditioning: short review of trace conditioning models. Cogn. Neurodyn. 2012. 6 (5): 377–398.
Lau H., Rosenthal D. Empirical support for higher-order theories of conscious awareness. Trends Cogn. Sci. 2011. 15 (8): 365–373.
Lee I., Hunsaker M.R., Kesner R.P. The role of hippocampal subregions in detecting spatial novelty. Behav. Neurosci. 2005. 119 (1): 145–153.
Lee H., Golkowski D., Jordan D., Berger S., Ilg R., Lee J., Mashour G.A., Lee U.; ReCCognition. Study Group. Relationship of critical dynamics, functional connectivity, and states of consciousness in large-scale human brain networks. Neuroimage. 2019. 188: 228–238.
Lega B., Burke J., Jacobs J., Kahana M.J. Slow-theta-to-gamma phase–amplitude coupling in human hippocampus supports the formation of new episodic memories. Cereb. Cortex. 2016. 26 (1): 268–278.
Lehmann H., McNamara K.C. Repeatedly reactivated memories become more resistant to hippocampal damage. Learn. Mem. 2011. 18 (3): 132–135.
Liang Z., Liu X., Zhang N. Dynamic resting state functional connectivity in awake and anesthetized rodents. Neuroimage. 2015. 104: 89–99.
Libet B. Unconscious cerebral initiative and the role of conscious will in voluntary action. Behav. and Brain Sci. 1985. 8: 529–566.
Liu X., de Zwart J.A., Schölvinck M.L., Chang C., Ye F.Q., Leopold D.A., Duyn J.H. Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat. Commun. 2018. 9 (1): 395.
Long L.L., Bunce J.G., Chrobak J. Theta variation and spatiotemporal scaling along the septotemporal axis of the hippocampus. Front. Syst. Neurosci. 2015. 9: 37.
Lord L.D., Expert P., Fernandes H.M., Petri G., Van Hartevelt T.J., Vaccarino F., Deco G., Turkheimer F., Kringelbach M.L. Insights into brain architectures from the homological scaffolds of functional connectivity networks. Front. Syst. Neurosci. 2016. 10: 85.
Lou H.C., Changeux J.P., Rosenstand A. Towards a cognitive neuroscience of self-awareness. Neurosci. Biobehav. Rev. 2017. 83:765–773.
Malekmohammadi M., Elias W.J., Pouratian N. Human thalamus regulates cortical activity via spatially specific and structurally constrained phase-amplitude coupling. Cereb. Cortex. 2015. 25 (6): 1618–1628.
Malerba P., Kopell N. Phase resetting reduces theta-gamma rhythmic interaction to a one-dimensional map. J. Math. Biol. 2013. 66 (7): 1361–1386.
Mashour G.A. The controversial correlates of consciousness. Science. 2018. 360 (6388): 493–494.
Mashour G.A., Hudetz A.G. Neural correlates of unconsciousness in large-scale brain networks. Trends Neurosci. 2018. 41 (3): 150–160.
Mattar M.G., Cole M.W., Thompson-Schill S.L., Bassett D.S. A functional cartography of cognitive systems. PLoS Comput. Biol. 2015. 11 (12): e1004533.
Mattar M.G., Kahn D.A., Thompson-Schill S.L., Aguirre G.K. Varying timescales of stimulus integration unite neural adaptation and prototype formation. Curr. Biol. 2016. 26 (13): 1669–1676.
Mazzucato L., Fontanini A., La Camera G. Dynamics of multistable states during ongoing and evoked cortical activity. J. Neurosci. 2015. 35 (21): 8214–8231.
Merker B. Consciousness without a cerebral cortex: a challenge for neuroscience and medicine. Behav. Brain Sci. 2007. 30 (1): 63–81.
Millan E.Z., Milligan-Saville J., McNally G.P. Memory retrieval, extinction, and reinstatement of alcohol seeking. Neurobiol. Learn. Mem. 2013. 101: 26–32.
Misic B., Goñi J., Betzel R.F., Sporns O., McIntosh A.R. A network convergence zone in the hippocampus. PLoS Comput. Biol. 2014. 10 (12): e1003982.
Mitra A., Snyder A.Z., Hacker C.D., Pahwa M., Tagliazucchi E., Laufs H., Leuthardt E.C., Raichle M.E. Human cortical-hippocampal dialogue in wake and slow-wave sleep. Proc. Natl. Acad. Sci. U.S.A. 2016. 113 (44): E6868–E6876.
Mohr H., Wolfensteller U., Betzel R.F., Mišić B., Sporns O., Richiardi J., Ruge H. Integration and segregation of large-scale brain networks during short-term task automatization. Nat. Commun. 2016. 7: 13217.
Monfils M.H., Cowansage K.K., Klann E., LeDoux J.E. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009. 324 (5929): 951–955.
Montijn J.S., Meijer G.T., Lansink C.S., Pennartz C.M. Population-level neural codes are robust to single-neuron variability from a multidimensional coding perspective. Cell Rep. 2016. 16 (9): 2486–2498.
Moretti P., Minoz M.A. Griffiths phases and the stretching of criticality in brain networks. Nat. Commun. 2013. 4: 2521.
Mudrik L., Breska A., Lamy D., Deouell L.Y. Integration without awareness: expanding the limits of unconscious processing. Psychol. Sci. 2011. 22 (6): 764–770.
Myers J. Does phenomenal consciousness overflow attention? An argument from feature-integration. Florida Philosophical Review. 2017. 17 (1): 28–44.
Nakatani C., Raffone A., van Leeuwen C. Efficiency of conscious access improves with coupling of slow and fast neural oscillations. J. Cogn. Neurosci. 2014. 26 (5): 1168–1179.
Nakayama R., Motoyoshi I. Attention periodically binds visual features as single events depending on neural oscillationsphase-locked to action. J. Neursci. 2019. 39 (21): 4153–4161.
Noah S., Mangun G.R. Recent evidence that attention is necessary, but not sufficient, for conscious perception. Ann. N.Y. Acad. Sci. 2019. Special Issue: The Year in Cognitive Neuroscience: 1–12.
Noe A., Thompson E. Are there neural correlates of consciousness? Journal of Consciousness Studies. 2004. 11 (1): 3–28.
Noirhomme Q., Soddu A., Lehembre R., Vanhaudenhuyse A., Boveroux P., Boly M., Laureys S. Brain connectivity in pathological and pharmacological coma. Front. Syst. Neurosci. 2010. 4: 160.
Norman K.A., Joel R. Quamme J.R., Ehren L. Newman E.L. Multivariate methods for tracking cognitive states. In Neuroimaging of Human Memory: Linking Cognitive Processes to Neural Systems. 2 ed. 2012. 488 p.
Norman L.J., Heywood C.A., Kentridge R.W. Object-based attention without awareness. Psychol. Sci. 2013. 24 (6): 836–843.
Northoff G. Psychopathology and pathophysiology of the self in depression – neuropsychiatric hypothesis. J. Affect. Disord. 2007. 104 (1–3): 1–14.
Olcese U., Bos J.J., Vinck M., Lankelma J.V., van Mourik-Donga L.B., Schlumm F., Pennartz C.M. Spike-based functional connectivity in cerebral cortex and hippocampus: loss of global connectivity is coupled to preservation of local connectivity during non-rem sleep. J. Neurosci. 2016. 36 (29): 7676–7692.
Owen A.M. The search for consciousness. Neuron. 2019. 102 (3): 526–528.
Palm G. Neural information processing in cognition: we start to understand the orchestra, but where is the conductor? Front. Comput. Neurosci. 2016. 10: 3.
Palmieri A., Calvo V., Kleinbub J.R., Meconi F., Marangoni M., Barilaro P., Broggio A., Sambin M., Sessa P. “Reality” of near-death-experience memories: evidence from a psychodynamic and electrophysiological integrated study. Front. Hum. Neurosci. 2014. 8: 429.
Pantani M., Tagini A., Raffone A. Phenomenal consciousness, access consciousness and self across waking and dreaming: bridging phenomenology and neuroscience. Phenomenology and Cognitive Sciences. 2018. 17 (1): 175–197.
Park H.J., Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013. 342 (6158): 1238411.
Parlatini V., Radua J., Dell’Acqua F., Leslie A., Simmons A., Murphy D.G., Catani M., Thiebaut de Schotten M. Functional segregation and integration within fronto-parietal networks. Neuroimage. 2017. 146: 367–375.
Phillips I. The methodological puzzle of phenomenal consciousness. Philos. Trans. R Soc. Lond. B Biol. Sci. 2018. 373 (1755).
Pigorini A., Sarasso S., Proserpio P., Szymanski C., Arnulfo G., Casarotto S., Fecchio M., Rosanova M., Mariotti M., Lo Russo G., Palva J.M., Nobili L. Massimini M. Bistability breaks-off deterministic responses to intracortical stimulation during non-REM sleep. Neuroimage. 2015. 112: 105–113.
Pineyro M.E., Ferrer Monti R.I., Alfei J.M., Bueno A.M., Urcelay G.P. Memory destabilization is critical for the success of the reactivation-extinction procedure. Learn. Mem. 2013. 21 (1): 46–54.
Pinto Y., Neville D.A., Otten M., Corballis P.M., Lamme V.AF., de Haan E.H.F., Foschi N., Fabri M. Split brain: divided perception but undivided consciousness. Brain. 2017. 140 (5): 1231–1237.
Pitts M.A., Lutsyshyna L.A., Hillyard S.A. The relationship between attention and consciousness: an expanded taxonomy and implicationsfor “no-report” paradigms. Philos. Trans. R Soc. Lond. B Biol. Sci. 2018. 373 (1755): 20170348.
Pockett S. Field theories of consciousness. Scholarpedia, 2013. 8 (12): 4951. http://www.scholarpedia.org/article/Field_theories_of_consciousness
Poerio G.L., Sormaz M., Wang H.T., Margulies D., Jefferies E., Smallwood J. The role of the default mode network in component processes underlying the wandering mind. Soc. Cogn. Affect. Neurosci. 2017. 12 (7): 1047–1062.
Poo M.M., Pignatelli M., Ryan T.J., Tonegawa S., Bonhoeffer T., Martin K.C., Rudenko A., Tsai L.H., Tsien RW., Fishell G., Mullins C., Gonçalves J.T., Shtrahman M., Johnston S.T., Gage F.H., Dan Y., Long J., Buzsáki G., Stevens C. What is memory? The present state of the engram. BMC Biol. 2016. 14: 40.
Posner M.I. Attention: the mechanisms of consciousness. Proc. Natl. Acad. Sci. U. S. A. 1994. 91 (16): 7398–7403.
Preti M.G., Bolton T.A., Van De Ville D. The dynamic functional connectome: State-of-the-art and perspectives. Neuroimage. 2017. 160: 41–54.
Priesemann V., Valderrama M., Wibral M., Le Van Quyen M. Neuronal avalanches differ from wakefulness to deep sleep – evidence from intracranial depth recordings in humans. PLoS Comput. Biol. 2013. 9 (3): e1002985.
Purdon P.L., Pierce E.T., Mukamel E.A., Prerau M.J., Walsh J.L., Wong K.F., Salazar-Gomez A.F., Harrell P.G., Sampson A.L., Cimenser A., Ching S., Kopell N.J., Tavares-Stoeckel C., Habeeb K., Merhar R., Brown E.N. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc. Natl. Acad. Sci. U. S. A. 2013. 110 (12): E1142–E1151.
Radulovic J., Tronson N.C. Receptors in (e)motion. Nat. Neurosci. 2011. 14 (10): 1222–1224.
Reniers R.L., Corcoran R., Völlm B.A., Mashru A., Howard R., Liddle P.F. Moral decision-making, ToM, empathy and the default mode network. Biol. Psychol. 2012. 90 (3): 202–210.
Rochford K.C., Jack A.I., Boyatzis R.E., French S.E. Ethical leadership as a balance between opposing neural networks. J. Bus. Ethics. 2017. 144 (4):755–770.
Ryan T.J., Roy D.S., Pignatelli M., Arons A., Tonegawa S. Memory. Engram cells retain memory under retrograde amnesia. Science. 2015. 348 (6238): 1007–1013.
Sanchez-Alavez M., Robledo P., Wills D.N., Havstad J., Ehlers C.L. Cholinergic modulation of event-related oscillations (ERO). Brain Res. 2014. 1559: 11–25.
Sandberg K., Frässle S., Pitts M. Future directions for identifying the neural correlates of consciousness. Nat. Rev. Neurosci. 2016. 17 (10): 666.
Schedlbauer A.M., Copara M.S., Watrous A.J., Ekstrom A.D. Multiple interacting brain areas underlie successful spatiotemporal memory retrieval in humans. Sci. Rep. 2014. 4: 6431.
Scholvinck M.L., Maier A., Ye F.Q., Duyn J.H., Leopold D.A. Neural basis of global resting-state fMRI activity. Proc. Natl. Acad. Sci. U. S. A. 2010. 107 (22): 10238–10243.
Schorr B., Schlee W., Arndt M., Bender A. Coherence in resting-state EEG as a predictor for the recovery from unresponsive wakefulness syndrome. J. Neurol. 2016. 263 (5): 937–953.
Schroeder C.E., Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009. 32 (1): 9–18.
Schultz D.H., Cole M.W. Higher intelligence is associated with less task-related brain network reconfiguration. J. Neurosci. 2016. 36 (33): 8551–8561.
Schultz D.H., Balderston N.L., Baskin-Sommers A.R., Larson C.L., Helmstetter F.J. Psychopaths show enhanced amygdala activation during fear conditioning. Front. Psychol. 2016. 7: 348.
Scott G., Fagerholm E.D., Mutoh H., Leech R., Sharp D.J., Shew W.L., Knöpfel T. Voltage imaging of waking mouse cortex reveals emergence of critical neuronal dynamics. J. Neurosci. 2014. 34 (50): 16611–16620.
Sergent J. A new look at the human split brain. Brain. 1987. 110 (Pt 5): 1375–1392.
Shani I., Keppler J. Beyond combination: How cosmic consciousness grounds ordinary experience. Journal of the American Philosophical Association. 2018. 4 (3): 390–410.
Shearkhani O., Takehara-Nishiuchi K. Coupling of prefrontal gamma amplitude and theta phase is strengthened in trace eyeblinkconditioning. Neurobiol. Learn. Mem. 2013. 100: 117–126.
Shema R., Kulicke R., Cowley G.S., Stein R., Root D.E., Heiman M. Synthetic lethal screening in the mammalian central nervous system identifies Gpx6 as a modulator of Huntington’s disease. Proc. Natl. Acad. Sci. U. S. A. 2015. 112 (1): 268–272.
Shew W.L., Plenz D. The functional benefits of criticality in the cortex. Neuroscientist. 2013. 19 (1): 88–100.
Shine J.M., Aburn M.J., Breakspear M., Poldrack R.A. The modulation of neural gain facilitates a transition between functional segregation and integration in the brain. eLife. 2018. 7: e31130.
Shine J.M., Bissett P.G., Bell P.T., Koyejo O., Balsters J.H., Gorgolewski K.J., Moodie C.A., Poldrack R.A. The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron. 2016. 92 (2): 544–554.
Shirvalkar P.R., Rapp P.R., Shapiro M.L. Bidirectional changes to hippocampal theta-gamma comodulation predict memory for recent spatial episodes. Proc. Natl. Acad. Sci. U. S. A. 2010. 107 (15): 7054–7059.
Simione L., Di Pace E., Chiarella S.G., Raffone A. Visual attention modulates phenomenal consciousness: evidence from a change detectionstudy. Front. Psychol. 2019. 10: 2150.
Singer W. The Brain – An Orchestra without a Conductor. Max Planck Research. 2005. 15–18.
Sitt J.D., King J.R., El Karoui I., Rohaut B., Faugeras F., Gramfort A., Cohen L., Sigman M., Dehaene S., Naccache L. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain. 2014. 137 (Pt 8): 2258–2270.
Solms M. The hard problem of consciousness and the free energy principle. Front. Psychol. 2018. 9: 2714.
Solovey G., Alonso L.M., Yanagawa T., Fujii N., Magnasco M.O., Cecchi G.A, Proekt A. Loss of consciousness is associated with stabilization of cortical activity. J. Neurosci. 2015. 35 (30): 10866–10877.
Spaak E., Bonnefond M., Maier A., Leopold D.A., Jensen O. Layer-specific entrainment of γ-band neural activity by the α rhythm in monkey visual cortex. Curr. Biol. 2012. 22 (24): 2313–2318.
Spielberg J.M., Miller G.A., Heller W., Banich M.T. Flexible brain network reconfiguration supporting inhibitory control. Proc. Natl. Acad. Sci. U.S.A. 2015. 112 (32): 10020–10025.
Spreng R.N., Stevens W.D., Viviano J.D., Schacter D.L. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol. Aging. 2016. 45: 149–160.
Stitt I., Hollensteiner K.J., Galindo-Leon E., Pieper F., Fiedler E., Stieglitz T., Engler G., Nolte G., Engel A.K. Dynamic reconfiguration of cortical functional connectivity across brain states. Sci. Rep. 2017. 7 (1): 8797.
Storm J.F., Boly M., Casali A.G., Massimini M., Olcese U., Pennartz C.M.A., Wilke M. Consciousness regained: disentangling mechanisms, brain systems, and behavioral responses. J. Neurosci. 2017. 37 (45): 10882–10893.
Sui J., Humphreys G.W. The integrative self: how self-reference integrates perception and memory. Trends Cogn. Sci. 2015. 19 (12): 719–728.
Swann N.C., de Hemptinne C., Maher R.B., Stapleton C.A., Meng L., Gelb A.W., Starr P.A. Motor system interactions in the beta band decrease during loss of consciousness. J. Cogn. Neurosci. 2016. 28 (1): 84–95.
Sweeney-Reed C.M., Zaehle T., Voges J., Schmitt F.C., Buentjen L., Kopitzki K., Richardson-Klavehn A., Hinrichs H., Heinze H.J., Knight R.T., Rugg M.D. Clinical, neuropsychological, and pre-stimulus dorsomedial thalamic nucleus electrophysiological data in deep brain stimulation patients. Data Brief. 2016. 8: 557–561.
Tagliazucchi E., von Wegner F., Morzelewski A., Brodbeck V., Borisov S., Jahnke K., Laufs H. Large-scale brain functional modularity is reflected in slow electroencephalographic rhythms across the human non-rapid eye movement sleep cycle. Neuroimage. 2013. 70: 327–339.
Tagliazucchi E., Chialvo D.R., Siniatchkin M., Amico E., Brichant J.F., Bonhomme V., Noirhomme Q., Laufs H., Laureys S. Large-scale signatures of unconsciousness are consistent with a departure from critical dynamics. J. R Soc. Interface. 2016. 13 (114): 20151027.
Tang W., Liu H., Douw L., Kramer M.A., Eden U.T., Hämäläinen M.S., Stufflebeam S.M. Dynamic connectivity modulates local activity in the core regions of the default-mode network. PNAS. 2017. 114 (36): 9713–9718.
Taylor A.M., Bus T., Sprengel R., Seeburg P.H., Rawlins J.N., Bannerman D.M. Hippocampal NMDA receptors are important for behavioural inhibition but not for encoding associative spatial memories. Philos. Trans. R Soc. Lond. B Biol. Sci. 2013. 369 (1633): 20130149.
Telch M.J., York J. Lancaster C.L., Monfils M.H. Use of a brief fear memory reactivation procedure for enhancing exposure therapy. Clinical Psychological Science. 2017. 5 (2): 367–378.
Thavabalasingam S., O’Neil E.B., Tay J., Nestor A., Lee A.C. Evidence for the incorporation of temporal duration information in human hippocampal long-term memory sequence representations. Proc. Natl. Acad. Sci. U. S. A. 2019. 116 (13): 6407–6414.
Thomas J.I. Current status of consciousness research from the neuroscience perspective. Acta Scientific Neurology. 2019. 2 (1): 38–44.
Thompson W.H., Fransson P. The frequency dimension of fMRI dynamic connectivity: Network connectivity, functional hubs and integration in the resting brain. Neuroimage. 2015. 121: 227–242.
Titley H.K., Brunel N., Hansel C. Toward a neurocentric view of learning. Neuron. 2017. 95 (1): 19–32.
Tort A.B., Komorowski R.W., Manns J.R., Kopell N.J., Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc. Natl. Acad. Sci. U. S. A. 2009. 106 (49): 20942–20947.
Tsanov M. Septo-hippocampal signal processing: breaking the code. Prog. Brain Res. 2015. 219: 103–120.
Tsuchiya N., van Boxtel J. Introduction to research topic: attention and consciousness in different senses. Front. Psychol. 2013. 4: 249.
Tsunada J., Baker A.E., Christison-Lagay K.L., Davis S.J., Cohen Y.E. Modulation of cross-frequency coupling by novel and repeated stimuli in the primate ventrolateral prefrontal cortex. Front. Psychol. 2011. 2: 217.
Turchi J., Chang C., Ye F.Q., Russ B.E., Yu D.K., Cortes C.R., Monosov I.E., Duyn J.H., Leopold D.A. The basal forebrain regulates global resting-state fmri fluctuations. Neuron. 2018. 97 (4): 940–952.e4.
van der Meij R., Kahana M., Maris E. Phase-amplitude coupling in human electrocorticography is spatially distributed and phase diverse. J. Neurosci. 2012. 32 (1): 111–123.
van Vugt B., Dagnino B., Vartak D., Safaai H., Panzeri S., Dehaene S., Roelfsema P.R. The threshold for conscious report: Signal loss and response bias in visual and frontal cortex. Science. 2018. 360 (6388): 537–542.
van Wingerden M., van der Meij R., Kalenscher T., Maris E., Pennartz C.M. Phase-amplitude coupling in rat orbitofrontal cortex discriminates between correct and incorrect decisions during associative learning. J. Neurosci. 2014. 34 (2): 493–505.
Vatansever D., Menon D.K., Manktelow A.E., Sahakian B.J., Stamatakis E.A. Default mode dynamics for global functional integration. J. Neurosci. 2015. 35 (46): 15254–15262.
Vinogradova O.S. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001. 11 (5): 578–598.
van Boxtel J.J., Tsuchiya N., Koch C. Opposing effects of attention and consciousness on afterimages. Proc. Natl. Acad. Sci. U. S. A. 2010. 107 (19): 8883–8888.
Weaver K.E., Wander J.D., Ko A.L., Casimo K., Grabowski T.J., Ojemann J.G., Darvas F. Directional patterns of cross frequency phase and amplitude coupling within the resting state mimic patterns of fMRI functional connectivity. Neuroimage. 2016. 128: 238–251.
Werner G. Consciousness viewed in the framework of brain phase space dynamics, criticality, and the renormalization group. Chaos, Solitons and Fractals. 2013. 55: 3–12.
Westphal A.J., Wang S., Rissman J. Episodic Memory Retrieval Benefits from a Less Modular Brain Network Organization. J. Neurosci. 2017. 37 (13): 3523–3531.
Wiltshire T.J., Euler M.J., McKinney T.L., Butner J.E. Changes in dimensionality and fractal scaling suggest soft-assembled dynamics in human EEG. Front. Physiol. 2017. 8: 633.
Wu X., Zou Q., Hu J., Tang W., Mao Y., Gao L., Zhu J., Jin Y., Wu X., Lu L., Zhang Y., Zhang Y., Dai Z., Gao J.H., Weng X., Zhou L., Northoff G., Giacino J.T., He Y., Yang Y. Intrinsic functional connectivity patterns predict consciousness level and recovery outcome in acquired brain injury. J. Neurosci. 2015. 35 (37): 12932–12946.
Yue Q., Martin R.C., Fischer-Baum S., Ramos-Nuñez A.I., Ye F., Deem M.W. Brain modularity mediates the relation between task complexity and performance. J. Cogn. Neurosci. 2017. 29 (9): 1532–1546.
Zalesky A., Fornito A., Cocchi L., Gollo L.L., Breakspear M. Time-resolved resting-state brain networks. Proc. Natl. Acad. Sci. U. S. A. 2014. 111 (28): 10341–10346.
Zhigalov A., Arnulfo G., Nobili L., Palva S., Palva J.M. Modular co-organization of functional connectivity and scale-free dynamics in the human brain. Netw Neurosci. 2017. 1 (2): 143–165.
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


