Журнал высшей нервной деятельности им. И.П. Павлова, 2023, T. 73, № 5, стр. 622-636
Увеличение пиковой частоты альфа-ритма ээг при предъявлении собственного имени во время глубокой анестезии
Г. В. Портнова 1, А. О. Канцерова 1, Л. Б. Окнина 1, Д. И. Пицхелаури 2, В. В. Подлепич 2, Я. О. Вологдина 2, Е. Л. Машеров 2
1 ФГБУН Институт Высшей Нервной Деятельности и Нейрофизиологии РАН
Москва, Россия
2 ФГАУ “НМИЦ Нейрохирургии им. ак. Н.Н. Бурденко”
Москва, Россия
Поступила в редакцию 31.03.2023
После доработки 31.05.2023
Принята к публикации 03.07.2023
- EDN: TRWMSZ
- DOI: 10.31857/S0044467723050106
Аннотация
Восприятие эмоционально значимых звуков у пациентов с угнетением сознания остается актуальной темой. Данные литературы свидетельствуют о том, что собственное имя, подаваемое в виде звукового сигнала, является одним из стимулов, способных оказывать активирующее действие на субъектов, находящихся в бессознательном состоянии. В данной работе мы исследовали электрофизиологическую реакцию пациентов, находящихся под глубоким наркозом во время проведения нейрохирургической операции, с целью оценить влияние глубины наркоза на восприятие звуков, в том числе и собственного имени, и исследовать области мозга, вовлеченные в их анализ. Мы регистрировали ЭЭГ синхронно от 12 скальповых электродов и на уровне среднего мозга от двух глубинных электродов в состоянии покоя и при предъявлении звуковых стимулов (собственное имя, чужое имя и шум) у одиннадцати пациентов сразу после удаления опухоли задних отделов третьего желудочка или четвертого желудочка. Результаты показали, что пациенты, находящиеся под глубоким наркозом, могли реагировать на свое имя в виде увеличения пиковой частоты альфа-ритма, по сравнению с чужими именами, шумами, а также в сравнении с ЭЭГ в состоянии покоя, как на уровне скальповой ЭЭГ в височных областях, так и на уровне среднего мозга.
ВВЕДЕНИЕ
Одной из актуальных проблем клинической нейрофизиологии является вопрос о способности пациента, находящегося в бессознательном состоянии, воспринимать и реагировать на внешние раздражители, при отсутствии поведенческих признаков подобных реакций. Наличие реакции на те или иные раздражители у пациентов в состоянии общей анестезии во время нейрохирургической операции является важной проблемой, с учетом того, что эффект анестезии может изменяться в процессе хирургического вмешательства и должен постоянно контролироваться анестезиологом (Kannabiran, Bidkar, 2018). Актуальным также является вопрос о диагностической ценности и возможностях скальпового ЭЭГ-мониторинга для оценки уровня сознания пациента и его способности реагировать на раздражители во время операции. Одним из наиболее значимых для человека стимулов является звук собственного имени, который способен выполнять сигнальную и активирующую функцию в различных жизненных ситуациях. Значимость своего имени и его стимулирующее действие было подтверждено сомнологическими и психологическими исследованиями, в которых была продемонстрирована более высокая “активирующая” способность звуков собственного имени по сравнению с другими звуками (Oswald et al., 1960), (Portas et al., 2000). В частности, исследования, проведенные с добровольцами, находящимися в глубокой фазе сна, показали, что при предъявлении собственного имени испытуемые пробуждались значительно чаще, чем при предъявлении имен других людей, (Oswald et al., 1960). Другими исследовательскими группами было показано, что звук имени субъекта, в отличие от звуков других имен и простых тонов, способствовал либо его пробуждению, либо переходу в менее глубокий сон, а также сопровождался реакцией со стороны вегетативной нервной системы, а именно увеличением частоты сердечных сокращений (Voss, Harsh, 1998). Также было показано, что во второй стадии сна звук своего имени способен вызвать дифференцированную когнитивную реакцию, наблюдаемую также на уровне вызванного ответа мозга (Perrin et al., 1999; Bastuji et al., 2002).
Несмотря на то, что биологическая значимость звуков собственного имени не вызывает сомнения, нейрофизиологические механизмы его активирующего влияния остаются недостаточно изученными. В частности, остаются вопросы об участии различных структур мозга в анализе звуков собственного имени. Так, исследования, проведенные на пациентах со значительными нарушениями корковых функций вследствие тяжелой ЧМТ, показали, что, даже при отсутствии какой-либо реакции на аналогичные по физическим характеристикам звуковые стимулы, у данной группы пациентов фиксируются изменения электрической активности мозга на звуки собственного имени (Cheng et al., 2013). Аналогичные данные были получены и другими исследователями, которые зарегистрировали различия в мозговой активности между звуками своего имени и других имен у пациентов в состоянии комы различной этиологии или в персистирующем вегетативном состоянии, и при этом выраженность реакции на звуки собственного имени коррелировала с тяжестью состояния пациента и прогнозом его заболевания (Fischer et al., 2008; Cheng et al., 2013; Vanhaudenhuyse et al., 2008; Holeckova et al., 2008). Существует множество показателей ЭЭГ, свидетельствующих о повышении уровня активности в состоянии активного бодрствования, однако не все они подходят для исследований пациентов с угнетенным состоянием сознания.
Одним из показателей, который может быть рассмотрен как маркер возбуждения или активации в бессознательном состоянии, согласно данным литературы, является пиковая частота (ПЧА) и мощность альфа-ритма (Barry et al., 2020). Уровень активности человека в состоянии активного бодрствования сопровождается значимыми изменениями мощности и частоты альфа-ритма (Shaw, 2003), однако, в отличие от пациентов, находящихся в бессознательном состоянии, данные изменения не всегда однозначны. В частности, появление и исчезновение альфа-ритма во время сна является одним из критериев изменения глубины сна (De Gennaro et al., 2001). В других исследованиях во время различных стадий сна обнаружены значимые изменения альфа-ритма при восприятии звуков собственного имени по сравнению с простыми и сложными тонами (Ruby et al., 2013b), а в фазе быстрого сна было обнаружено увеличение мощности альфа-ритма выше для звуков собственного имени по сравнению с чужими именами (Ruby et al., 2013a). Однако в последнем случае подобная реакция наблюдалась только в стадии парадоксального сна и отсутствовала в состоянии бодрствования, более того, у добровольцев, находящихся в сознании, напротив, наблюдалось уменьшение мощности альфа-ритма (Ruby et al., 2013a). В других исследованиях было продемонстрировано, что увеличение альфа-ритма, с одной стороны, связано с большей нагрузкой на память и внимание (Scheeringa et al., 2009), но при этом также отражает торможение активности зон мозга, не связанных напрямую с выполнением конкретной задачи (Jensen, Mazaheri, 2010; Haegens et al., 2010). Подобные результаты свидетельствуют о том, что изменение альфа-ритма в состоянии активного бодрствования и в состоянии угнетения сознания может иметь различную функцию и происхождение; и если в состоянии бодрствования увеличение мощности альфа-ритма может наблюдаться в областях, не связанных с выполняемой деятельностью, и свидетельствовать о торможении, то в бессознательном состоянии появление альфа-ритма и увеличение его частоты с большей вероятностью сигнализирует о повышении уровня сознания и активации индивидуума (Jensen, Mazaheri, 2010; De Gennaro, 2001).
В настоящее время существуют неоднозначные данные об участии коры и подкорковых структур в генерации альфа-ритма и регуляции его частоты и амплитуды (Feige et al., 2005), (Manshanden et al., 2002), однако при этом различные показатели альфа-ритма, включающие в себя мощность, амплитуду, частоту и индекс, имеют огромное значение для анализа уровня активности человека и особенно состояний сна и бодрствования (Patel et al., 2023; Schwabedal et al., 2016; Li et al., 2022). В частности, данные литературы свидетельствуют о том, что такие мозговые структуры, как мост, средний мозг, гипоталамус, таламус, миндалевидное тело, а также базальная префронтальная кора и островок, могут быть напрямую связаны с генерацией альфа-ритма (Schwabedal et al., 2016; Schreckenberger et al., 2004; Sadato et al., 1998). При этом локализация, частота, амплитуда и другие характеристики альфа-ритма могут различаться в зависимости от структуры мозга, с которой связывают его происхождение. Например, регистрируемый в височной области альфа-ритм, по данным некоторых исследователей, связывается с активностью гиппокампа (Boldyreva, 1997), более того, изменение его частоты часто наблюдается при заболеваниях, сопровождающихся атрофией гиппокампальной извилины (Moretti et al., 2011). Согласно другим данным, альфа-ритм, связанный с активностью ствола мозга, имеет более низкую частоту и более сглаженную топографию по сравнению с альфа-ритмом, происхождение которого ассоциировано со структурами неокортекса и лимбической системы (Omata et al., 2013). Также, исходя из данных литературы, существуют значимые различия между характеристиками альфа-ритма, генерируемого таламусом и средним мозгом (Feige et al., 2005).
Основной целью данной работы было выделение специфических изменений ЭЭГ в ответ на предъявление звука имени пациента в состоянии глубокого медикаментозного сна, что свидетельствовало бы о возможности распознавания собственного имени среди других звуков и шумов. В ходе работы был поставлен вопрос о том, является ли реакция на звук своего имени результатом когнитивной активности пациента и связана ли с распознаванием, или же обусловлена тем, что в процессе онтогенеза звук собственного имени приобретает дополнительную биологическую значимость и является активирующим сигналом, не требующим для распознавания активности речевых центров. Для ответа на данный вопрос были применены два метода регистрации активности ЭЭГ: регистрация электрической активности с поверхности головы за счет скальповых электродов, а также регистрация сигнала среднего мозга при помощи глубинного электрода, устанавливаемого на уровне водопровода мозга. Анализировались частотные и топографические изменения регистрируемых изменений электрической активности мозга.
МEТОДИКА
Испытуемые
В исследование включено 11 пациентов с опухолями задних отделов третьего желудочка или четвертого желудочка, которым было показано хирургическое удаление опухоли передним транскортикальным трансвентрикулярным доступом или срединной субокципитальной краниотомией с последующим доступом в четвертый желудочек (табл. 1). В обоих случаях траектория операционного доступа обеспечивала хорошую видимость водопровода головного мозга на всем его протяжении после удаления опухоли (табл. 1).
Таблица 1.
Характеристики пациентов. SC – срединный подзатылочный доступ; ТА – транскортикально-трансвентрикулярный доступ; СА – водопровод головного мозга Table 1. Characteristics of patients. SC – median subcutaneous approach; TA – transcortical-transventricular approach; CA – cerebral aqueduct
| Пациенты | Пол | Возраст | Хирургический доступ | Контактный электрод | Запись контактов |
|---|---|---|---|---|---|
| P001 | F | 56 | SC | IV ventricle | CA |
| P002 | M | 31 | SC | IV ventricle | CA |
| P003 | M | 22 | TA | III ventricle | CA |
| P004 | M | 54 | SC | IV ventricle | CA |
| P005 | M | 45 | SC | IV ventricle | CA |
| P006 | F | 65 | TA | III ventricle | CA |
| P007 | M | 41 | SC | IV ventricle | CA |
| P008 | M | 39 | SC | IV ventricle | CA |
| P009 | M | 30 | TA | III ventricle | CA |
| P010 | M | 42 | SC | IV ventricle | CA |
| P011 | M | 52 | SC | IV ventricle | CA |
Обязательным условием включения пациентов в исследование было отсутствие вовлечения среднего мозга в опухолевый процесс, что было подтверждено в результате предоперационной магнитно-резонансной томографии (МРТ). В предоперационном периоде всем пациентам было проведено стандартное клиническое обследование в Центре нейрохирургии им. Бурденко. Все пациенты, включенные в исследование, не имели проблем со слухом и явных когнитивных расстройств. Участие в исследовании было добровольным, и все пациенты дали на него свое письменное информированное согласие. Этическое одобрение методов исследования получено от Комитета по этике научных исследований Центра нейрохирургии им. Бурденко (протокол № 1/2016). Все больные оперированы в Центре нейрохирургии им. Бурденко одним из соавторов статьи (ПДИ). Все операции прошли успешно, без каких-либо осложнений.
Общая анестезия
Запись электроэнцефалограммы (ЭЭГ) выполняли в состоянии тотальной внутривенной анестезии с использованием комбинации продленной инфузии пропофола и фентанила. Анестезиологи рассчитывали дозу с учетом клинической оценки пациента, течения операции и параметров интраоперационного нейрофизиологического мониторинга. Для контроля оптимального уровня анестезии регистрировали ЭЭГ с 12 скальповых электродов с одновременной регистрацией потенциалов на уровне среднего мозга (табл. 2).
Таблица 2.
Количественные и качественные данные анализа ЭЭГ. BS – паттерн “вспышка-подавление”, NBS – отсутствие паттерна “вспышка-подавление”. Доза пропофола в мг/кг/час. * – пациент, исключенный из группового анализа Table 2. Quantitative and qualitative EEG analysis data. BS – “burst-suppression” pattern. NBS – no “burst-suppression” pattern. The dose of propofol in mg/kg/hour. * – the patient excluded from the group analysis
| Пациенты | Визуальная оценка ЭЭГ | Пропофол мг/кг/ч во время стимуляции | Доминантная частота |
|---|---|---|---|
| P001 | BS | 8.5 | 3.6 |
| P002 | BS | 5.3 | 2.7 |
| P003 | NBS | 8.4 | 2.3 |
| P004 | BS | 10.7 | 3.3 |
| P005* | NBS | 8.3 | 4.5 |
| P006 | BS | 7 | 3.1 |
| P007 | NBS | 7 | 2.3 |
| P008 | BS | 7.8 | 3.3 |
| P009 | BS | 10.4 | 2.8 |
| P010 | BS | 6.09 | 3.2 |
| P011 | BS | 5.81 | 3.1 |
Нейропсихологическое обследование
Нейропсихологическое обследование планировалось на дооперационном этапе и включает скрининговые методы (МоКА, ФАБ (Freitas et al., 2012)), батарею тестов А.Р. Лурия (Luria, 1980), дихотическое прослушивание, адаптированную для русского языка методику Е.П. Кока. Основное внимание уделялось оценке состояния речевых функций. Состояние речевых функций по батарейным тестам Лурия оценивали по четырехбалльной шкале, разработанной в лаборатории нейропсихологии А.Р. Лурия в Институте нейрохирургии имени Бурденко.
До операции все обследованные пациенты, кроме больного Р005, были полностью ориентированы во времени, пространстве и собственной личности. Выполнение полноценного обследования пациента P005 был затруднительно из-за языкового барьера.
Когнитивные функции пациентов оценивались совокупно по шкалам МоКА, ФАБ, и батареи тестов А.Р. Лурия. Критерием исключения было наличие среднетяжелых и тяжелых когнитивных нарушений: меньше 26 баллов по шкале МоКА, менее 12 баллов по ФАБ и наличие умеренного или выраженного дефицита сенсорных и моторных компонентов речи согласно результатам батареи тестов А.Р. Лурия. По данным МоКА когнитивная сфера у всех пациентов соответствовала нормативным значениям (среднее значение – 28.2). По данным ФАБ, легкие когнитивные нарушения выявлены у двух из десяти пациентов (среднее значение – 13.5); у остальных какие-либо нарушения выявлены не были. Согласно результатам нейропсихологического обследования, у трех пациентов был выявлен легкий дефицит сенсорных компонентов, у двух пациентов – незначительный дефицит моторных компонентов речи.
Стимулы
В ходе нейропсихологического обследования было выбрано наиболее актуальное и частотное произношение имени каждого пациента. Помимо этого, были выделены и исключены значимые для больного имена (близкие родственники, друзья и др.), а подбор контрольного имени (другое имя) осуществлялся с учетом сходства звучания и длительности своего и чужого имени. Все стимулы были получены в ходе аудиозаписи голоса диктора (одного и того же носителя русского языка). Стимуляция осуществлялась при помощи программы “Presentation” (Neurobehavioral Systems, Inc., США) через наушники Neurosoft TDH-39. Все стимулы обрабатывались в программе WaveLab 10 (Steinberg) и выравнивались по средней частоте и громкости. Последний тип стимула – шум – был сгенерирован с помощью интерфейса Matlab на основе звука собственного имени (мы применили функцию розового шума с мощностью шума (дБ) и фильтром высоты тона (Гц), соответствующими звуку собственного имени (см. табл. 3)).
Таблица 3.
Физические характеристики предъявляемых звуков (порог вычисления средней громкости – 50 дБ) Table 3. Physical characteristics of the sounds presented (the threshold for calculating the average volume is 50 dB)
| Длительность, м сек сред. ± ст. откл. | Средняя частота, Гц сред. ± ст. откл. | Громкость/RMS, дБ сред. ± ст. откл. | |
|---|---|---|---|
| Свое имя | 588 ± 64 | 2112 ± 64 | –12.7 ± 0.9 |
| Чужое имя | 578 ± 49 | 2104 ± 68 | –13.1 ± 0.7 |
| Шум | 580 ± 66 | 2120 ± 51 | –12.4 ± 0.6 |
Процедура исследования
После удаления опухоли, на завершающем этапе операции, для регистрации ЭЭГ устанавливали одноразовый цилиндрический электрод с трехкольцевыми контактами из нержавеющей стали AISI 321 (12Х18Н10Т) (рис. 1 (а)). Прибор изготовлен ООО “Нейроэлект”, Россия. Диаметр электрода 2.7 мм. К дистальному концу направляющих отведений прикрепляли трехкольцевые контакты (два контакта ближе к концу электрода – регистрирующие (D1 и D2), а проксимальный – референтный (R)), шириной 3 мм, на расстоянии 6.5 мм друг от друга. Дистальный конец электрода с двумя записывающими контактами плотно прилегает к стенкам водопровода среднего мозга (рис. 1 (б)). Референтный электрод располагался в третьем или четвертом желудочках и был покрыт хирургической марлей для предотвращения прямого контакта с эпендимальным слоем желудочков. Звуки собственного имени испытуемого, чужое имя и шум предъявлялись с произвольным межстимульным интервалом 1500–2500 мс. Каждый стимул повторялся 35 раз в случайном порядке (рис. 1).
Рис. 1.
Схематическое изображение трех осесимметричных кольцевых контактов на направляющем проводе и его расположение в водопроводе мозга: (а) – схематическое изображение цилиндрического глубинного электрода. Диаметр электрода – 2.7 мм, ширина каждого кольцевого электрода – 3 мм, расстояние между кольцевыми контактами – 6.5 мм. (б)–(в) – положение контактов глубинного электрода относительно структур ствола мозга; D1 – ростральный контакт, D2 – каудальный контакт, R – референтный контакт. SC – верхнее двухолмие. IC – нижнее двухолмие. PG – периакведуктальное серое вещество. Fig. 1. Schematic presentation of three axisymmetric ring contacts on directional lead and its location in the aqueduct: (а) – a schematic presentation of the cylindrical depth electrode. The electrode diameter – 2.7 mm, width of every ring electrode – 3 mm, distance between ring contacts – 6.5 mm. (б)–(в) – position of the depth electrode contacts relative to brainstem structures D1 – rostral contact, D2 – caudal contact, R – reference contact. SC – superior colliculus. IC – inferior colliculus. PG – periaqueductal gray.
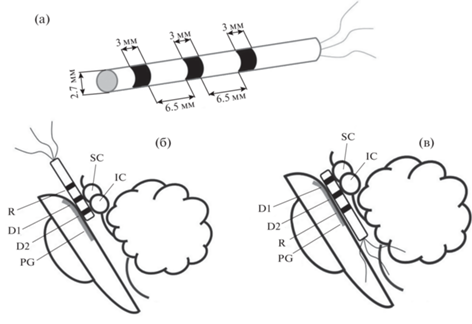
После окончания регистрации глубинный электрод удаляли, рану послойно ушивали. У всех пациентов послеоперационный период протекал без осложнений со стороны среднемозговой симптоматики и признаков нарушения слуха.
Регистрация ЭЭГ
Для регистрации ЭЭГ в просвет водопровода головного мозга свободно устанавливали вышеописанный электрод. Электроды в водопровод располагали так, что первые два электрода выступали под верхним и нижним бугорками четверохолмной пластинки соответственно (рис. 1). Третий электрод использовался в качестве референта.
Регистрацию скальповой ЭЭГ выполняли с помощью системы интраоперационного нейрофизиологического мониторинга “Нейро-ИОМ-32/С” (включает 12 электродов: FP1, FP2, F3, F4, C3, C4, P3, P4, T3, T4, O1, O2) ООО “Нейрософт” с частотой дискретизации 10000 Гц и фильтрацией 0.01–4000 Гц. Заземляющий электрод был общим как для скальповой ЭЭГ, так и для участков среднего мозга, и располагался в проекции плечевого сустава. Референтным электродом для скальпового ЭЭГ служил левый ушной электрод. Для исключения послеоперационных осложнений фоновая запись до и после стимуляции была исключена. Для фоновой записи были выбраны участки межстимульных интервалов (они составили от 1500 до 2000 мс).
ЭЭГ-мониторинг
Нами была рассчитана доминирующая частота скальповой ЭЭГ у всех пациентов. Для этого два опытных нейрофизиолога выбрали два свободных от артефактов участка (весь анализируемый фрагмент ЭЭГ делился пополам, и тот или иной фрагмент рандомизированно предлагался экспертам), которые соответствовали критериям при регистрации паттерна пачек/подавления. Расчет частоты доминирующего пика проводился двумя способами: 1) ЭЭГ подвергалась быстрому преобразованию Фурье и далее подсчитывалась частота центра тяжести спектра ЭЭГ в диапазоне 0.6–30 Гц при помощи ПО Matlab (MathWorks, США); 2) доминирующая частота оцениваемого участка ЭЭГ подсчитывалась рутинным способом специалистами в области функциональной диагностики. Полученные результаты сравнивались между собой. Расхождения в результатах подсчетов составили не более 5% для каждого пациента. Доминирующая частота колебалась от 2.3 до 4.5 Гц (медиана 3.1 Гц), что соответствует состоянию глубокого медикаментозного сна.
Анализ данных
При анализе данных скальповой ЭЭГ был применен полосовой фильтр 4–30 Гц. Артефакты были удалены при помощи метода независимых компонент (ICA) с помощью EEGLAB 14.1.1b (MATLAB, MathWorks, США). Дальнейший анализ данных включал следующие этапы:
1. Сегментация ЭЭГ на эпохи в соответствии с метками о начале стимула (от начала стимула до 1000 мс после начала стимула) для всех типов стимулов (собственное имя, чужое имя и шум). Таким образом, мы проанализировали 35 односекундных фрагментов ЭЭГ для каждого типа стимулов. Также были отобраны 35 односекундных интервалов фоновой записи (среди интерстимульных интервалов) для сравнения.
2. В результате для анализа были подготовлены четыре типа состояний: собственное имя, другое имя, фон и шум, – которые анализировались для каждого пациента отдельно. Для каждого фрагмента ЭЭГ рассчитывались спектральная мощность ЭЭГ и пиковая частота альфа-ритма (ПЧА).
Спектральная мощность ЭЭГ
Спектральный анализ мощности проводили с использованием быстрого преобразования Фурье. Рассчитывались логарифмированные мощности ЭЭГ в диапазоне 4–30 Гц с шагом 1 Гц. Анализировались следующие спектральные полосы: 4–8 Гц (тета), 8–10 Гц (альфа1), 10–12 (альфа2) и 12–30 Гц (бета). Отдельно был проанализирован диапазон альфа-ритма: 8–9, 9–10, 10–11, 11–12, 12–13 Гц и суммарный диапазон 8–13 Гц. Анализ проводился в среде EEGLAB 14.1.1b (MATLAB, MathWorks, США).
Пиковая частота альфа-ритма (ПЧА). Расчет ПЧА проводился путем анализа спектров мощности скальпового и глубинного электродов с использованием быстрого преобразования Фурье, как частоты центра тяжести в диапазоне 8–13 Гц, чтобы исключить ПЧА, лежащую за пределами фиксированных полос частот (Haegens et al., 2014), у пациентов в состоянии общей анестезии. Частота центра тяжести определяется как “взвешенная сумма спектральных оценок, деленная на мощность”, альфа-ритма:
где a(f) – спектральная мощность на частоте f в альфа-диапазоне (Klimesch, 1999). Использование данного метода анализа частоты альфа-ритма имеет ряд преимуществ при работе с данными ЭЭГ у различных групп пациентов (Klimesch, 1999), (Klimesch et al., 1993). Анализ проводился в среде EEGLAB 14.1.1b (MATLAB, MathWorks, США).Статистический анализ
Анализ данных проводился методом дисперсионного анализа для показателей ЭЭГ с учетом типа стимулов как для скальповых, так и для глубинных электродов. В исследовании мы использовали как однофакторный, так и многофакторный дисперсионный анализ (ANOVA для повторных измерений) для каждого электрода в отдельности: как для скальповых электродов (F3, F4, C3, C4, P3, P4, T3, T4, O1, O2), так и для глубинных, оценивался общий эффект различия между двумя типами стимулов. Групповой эффект оценивали при помощи факторного дисперсионного анализа с учетом поправки на множественное сравнение (Post-hoc Bonferroni test).
Статистический анализ проводился как на индивидуальном уровне, когда для каждого пациента отдельно рассчитывались значимые различия между показателями ЭЭГ (по 35 фрагментов для четырех условий (свое имя, чужое имя, шум, фон), так и на групповом уровне, когда статистический анализ проводился с учетом индивидуальных показателей ЭЭГ для группы из 10 пациентов. Пациент P005 не участвовал в групповом анализе, но его данные были использованы для качественного анализа результатов.
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЙ
Спектральная мощность ЭЭГ
В ходе клинического анализа данных ЭЭГ в ходе мониторинга было обнаружено, что у всех пациентов, как при предъявлении звуковых раздражителей, так и при хирургических манипуляциях, реактивность ЭЭГ отсутствовала и электроэнцефалограмма соответствовала уровню глубокого наркоза (табл. 2). При этом у восьми пациентов зарегистрирован паттерн “вспышка-подавление”.
Мы не обнаружили каких-либо значимых различий между стимулами ни в одном из анализируемых частотных диапазонов: тета, альфа1, альфа2, бета, 8–9, 9–10, 10–11, 11–12, 12–13, 8–13 Гц (p > 0.64).
Индивидуальные различия
ПЧА была значимо больше при предъявлении своего имени по сравнению с чужим (рис. 2) для глубинных электродов у 9 из 11 пациентов (результаты статистического анализа представлены в табл. 4). Аналогичные различия обнаружены между собственным именем и шумом, а также фоном. Значения ПЧА значимо не различались между чужим именем, шумом и фоном (p > 0.63).
Рис. 2.
Индивидуальные значения ПЧА и их различия; построены для каждого 1-секундного фрагмента ЭЭГ для следующих условий: собственное имя (свое) и чужое имя (чужое), измеренные и усредненные по двум глубинным электродам. Пунктирной линией выделен пациент, не участвующий в групповом анализе. Fig. 2. The individual values Peak alpha frequency for each 1 second EEG fragment for self-own name (SON) and other name (ON) measured and averaged over two depth electrodes.
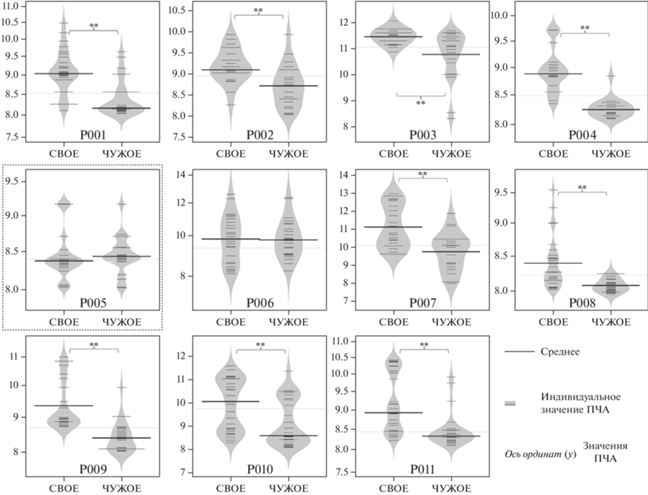
Таблица 4.
Описательная статистика: индивидуальные показатели ПЧА и результаты статистического анализа различий между своим и чужим именем. * – пациент, исключенный из группового анализа Table 4. Descriptive statistics: individual indications of the peak frequency of the alpha rhythm and the results of statistical analysis of the differences between patient’s own and someone else’s name. * – the patient excluded from the group analysis
| Пациент | Свое имя | Чужое | Результаты дисперсионного анализа по скальповым электродам | Результаты дисперсионного анализа по глубинным электродам | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cкальп | cредний мозг | cкальп | cредний мозг | |||||||
| cред. | cт. откл. | cред. | cт. откл. | cред. | cт. откл. | cред. | cт. откл. | |||
| P001 | 8.46 | 1.15 | 9.08 | 0.95 | 8.43 | 1.03 | 8.12 | 0.42 | F(1, 73) = 0.677, p = 0.413 | F(1, 73) = 13.193, p ≤ 0.001 |
| P002 | 9.21 | 0.46 | 9.18 | 0.66 | 8.52 | 1.66 | 8.63 | 0.64 | F(1, 73) = 8.994, p = 0.003 | F(1, 73) = 8.106, p = 0.005 |
| P003 | 11.06 | 1.02 | 11.42 | 0.89 | 10.77 | 1.14 | 10.73 | 1.22 | F(1, 73) = 5.302, p = 0.033 | F(1, 73) = 11.570, p = 0.001 |
| P004 | 8.82 | 1.59 | 8.88 | 0.72 | 8.31 | 1.36 | 8.24 | 0.45 | F(1, 73) = 7.518, p = 0.008 | F(1, 73) = 12.004, p ≤ 0.001 |
| P005* | 8.42 | 1.5 | 8.34 | 0.51 | 8.31 | 1.64 | 8.41 | 0.42 | F(1, 73) = 1.008, p = 0.621 | F(1, 73) = 1.111, p = 0.475 |
| P006 | 9.93 | 1.16 | 8.22 | 1.1 | 9.89 | 0.16 | 9.91 | 1.04 | F(1, 73) = 1.237, p = 0.291 | F(1, 73) = 12.213, p ≤ 0.001 |
| P007 | 10.13 | 2.25 | 11.08 | 1.76 | 9.48 | 2.25 | 9.69 | 1.05 | F(1, 73) = 10.383, p = 0.002 | F(1, 73) = 8.347, p = 0.005 |
| P008 | 8.41 | 1.33 | 8.43 | 0.62 | 8.05 | 1.86 | 8.06 | 0.59 | F(1, 73) = 8.567, p = 0.004 | F(1, 73) = 15.374, p ≤ 0.001 |
| P009 | 8.84 | 1.85 | 9.21 | 1.1 | 8.17 | 1.94 | 8.37 | 0.74 | F(1, 73) = 8.641, p = 0.004 | F(1, 73) = 15.740, p ≤ 0.001 |
| P010 | 10.42 | 0.85 | 10.03 | 1.12 | 8.95 | 1.22 | 8.56 | 1.03 | F(1, 73) = 12.497, p = 0.008 | F(1, 73) = 14.225, p ≤ 0.001 |
| P011 | 8.51 | 1.05 | 8.94 | 0.91 | 8.48 | 0.95 | 8.39 | 1.18 | F(1, 73) = 1.088, p = 0.357 | F(1, 73) = 13.913, p ≤ 0.001 |
Аналогичное увеличение ПЧА на собственное имя по сравнению с чужим именем, шумом или фоном было обнаружено в височных областях билатерально (рис. 3) у 8 из 11 пациентов. Чужое имя, шум и фон также не различались между собой (p > 0.39).
Рис. 3.
Индивидуальные значения ПЧА и их различия; построены для каждого 1-секундного фрагмента ЭЭГ для следующих условий: собственное имя (свое) и чужое имя (чужое), для усредненных значений (F3, F4, C3, C4, Т3, Т4, P3, P4, O1, O2). Топография различий представлена также по индивидуальным данным и построена для каждого электрода и каждого пациента индивидуально. Пунктирной линией выделен пациент, не участвующий в групповом анализе. Fig. 3. The individual values Peak alpha frequency for each 1 second EEG fragment for self-own name (SON) and other name (ON) measured over scalp electrodes and the topography of significant differences of PAFs between SON and ON.
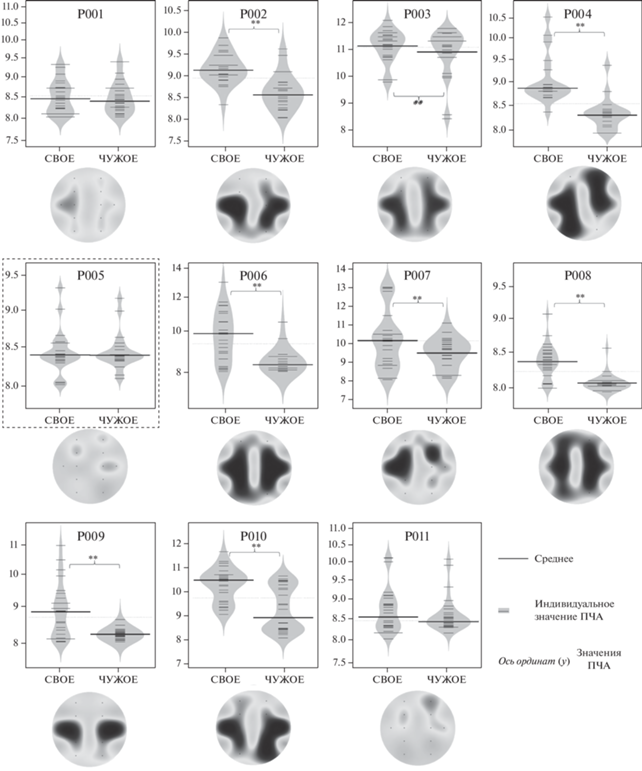
Таким образом, у 10 из 11 пациентов наблюдалось значимое увеличение ПЧА для звуков своего имени по сравнению с чужим либо на уровне скальповых электродов, либо на уровне глубинных электродов.
Групповые различия
ПЧА была значимо выше при прослушивании собственного имени по сравнению с чужим именем, шумом и фоновой записью как при регистрации со скальповых электродов (теменная и височная области), так и при регистрации с глубинных электродов (табл. 5). Дисперсионный анализ подтвердил значимое групповое увеличение ПЧА для собственного имени по сравнению с чужим именем и другими условиями.
Таблица 5.
Групповые различия ПЧА между собственным именем и другими состояниями, рассчитанные с использованием дисперсионного анализа (основной эффект, различия между стимулами с учетом каждого анализируемого фрагмента ЭЭГ каждого пациента) Table 5. Group differences in the peak frequency of the alpha rhythm between the patient’s own name and other conditions, calculated using variance analysis (main effect, differences between stimuli, taking into account each analyzed fragment of the EEG of each patient)
| Электроды | Сравнение между типами стимулов/ фоном | № | Результаты дисперсионного анализа | Partial eta-squared |
|---|---|---|---|---|
| Внутричерепные электроды (среднее) | Свое vs Чужое | 10 | F(1, 324) = 33.27, p < 0.0001 | 0.109 |
| Свое vs Шум | 10 | F(1, 324) = 47.22, p < 0.0001 | 0.142 | |
| Свое vs Фон | 10 | F(1, 324) = 74.51, p < 0.0001 | 0.149 | |
| Скальповые электроды (среднее по всем электродам) | Свое vs Чужое | 10 | F(1, 324) = 25.99, p < 0.0001 | 0.094 |
| Свое vs Шум | 10 | F(1, 324) = 40.88, p < 0.0001 | 0.128 | |
| Свое vs Фон | 10 | F(1, 324) = 91.29, p < 0.0001 | 0.099 |
Результаты корреляционного анализа
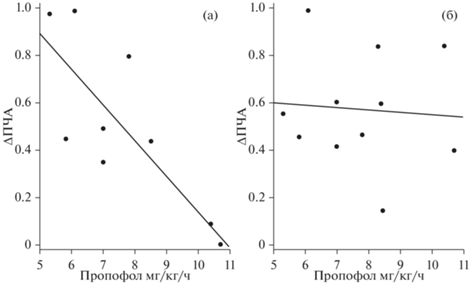
Мы обнаружили значимую отрицательную корреляцию между дозой пропофола и увеличением ПЧА для звука собственного имени по сравнению с другими условиями (чужое имя, шум, фон) для скальповых электродов. Для глубинных электродов значимой корреляции не обнаружено (табл. 6, рис. 4).
Таблица 6.
Корреляции по Спирмену между количеством препаратов для анестезии и различиями ПЧА между собственным именем и другими состояниями Table 6. Spearman’s correlations between the number of drugs for anesthesia and differences in the peak frequency of the alpha rhythm between the patient’s own name and other conditions
| Пары переменных | Количество | Внутричерепные электроды (среднее) | Скальповые электроды (среднее по всем электродам) | ||||
|---|---|---|---|---|---|---|---|
| Spearman – R | t(N – 2) | p-level | Spearman – R | t(N – 2) | p-level | ||
| Пропофол в мг/кг/час × ΔПЧА (Свое – Чужое) | 10 | –0.132 | –0.414 | 0.689 | –0.799 | –3.988 | 0.002 |
| Пропофол в мг/кг/час × ΔПЧА (Свое – Шум) | 10 | –0.162 | –0.537 | 0.632 | –0.831 | –4.223 | 0.001 |
| Пропофол в мг/кг/час × ΔПЧА (Свое – Фон) | 10 | –0.101 | –0.307 | 0.769 | –0.850 | –4.358 | 0.0002 |
Рис. 4.
Диаграмма рассеяния отображает корреляцию между количеством вводимого пропофола в мг/кг/ч во время стимуляции и разницей ПЧА между своим и чужим именем, измеренной на скальповых электродах (а) и на глубоких электродах (б). Fig. 4. The scatter-plot depicts the correlation between Propofol mg/kg/h during stimulation and difference of PAF between SON and ON measured over scalp electrodes (а) and in deep electrodes (б).
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
Результаты исследования показали, что при прослушивании собственного имени наблюдалось значимое увеличение ПЧА по сравнению с другими состояниями у десяти из одиннадцати пациентов. Согласно данным литературы, увеличение ПЧА является нейрофизиологическим маркером повышения активности у человека в состоянии активного бодрствования и свидетельствует о его более высоком уровне возбуждения, а также о наличии более интенсивных физических нагрузок (di Fronso et al., 2019), (Gutmann et al., 2015) и готовности к решению задач (Badalova, 2020) и умственным нагрузкам (Gutmann et al., 2018). При этом уменьшение ПЧА, согласно данным различных исследований, оказалось связанным как с состояниями релаксации или медитации, так и с некоторыми патологическими состояниями, например, онкологическими заболеваниями, а также синдромом эмоционального выгорания (Gutmann et al., 2018; Zimmer et al., 2015; Banquet, 1973; Portnova et al., 2020). Таким образом, мы предположили, что увеличение ПЧА свидетельствует об активирующем действии звука собственного имени на пациента, находящегося в состоянии глубокого наркоза. Полученные результаты согласуются с данными о наличии специфических изменений ЭЭГ у пациентов в бессознательном состоянии при предъявлении им звука собственного имени, сопровождающихся динамикой ЭЭГ на частоте альфа-ритма, а также изменениями поздних компонентов ВП, связанных с повышением уровня активности пациентов (Holler et al., 2011; Kempny et al., 2018).
Наличие или отсутствие реакции пациентов в виде увеличения ПЧА в ответ на звук своего имени, согласно нашей гипотезе, должно было зависеть от глубины анестезии и дозы применяемых препаратов. По классификации глубины анестезии она во всех случаях была адекватной нейрохирургическому вмешательству: клинических признаков преждевременного пробуждения не отмечалось, а на ЭЭГ выявлялись признаки глубокого наркоза (Musizza, Ribaric, 2010). По действию пропофол хорошо известен как ингибитор корковой активности, в то же время в более высоких дозах он может воздействовать на подкорковые структуры, в том числе на ретикулярную формацию среднего мозга (Rudolph, Antkowiak, 2004). Согласно нашим результатам, между дозой вводимых препаратов и реакцией на звук своего имени была обнаружена обратная корреляция, и только для скальпового ЭЭГ. Между изменением ПЧА на уровне среднего мозга и дозой вводимых препаратов значимой корреляции обнаружено не было, что свидетельствует об адекватности применяемых доз общей анестезии (Rudolph, Antkowiak, 2004). При условии клинически адекватного уровня анестезии и доз пропофола, рекомендованных для использования инструкцией препарата и клиническими рекомендациями, следует отметить широкую вариабельность дозирования препарата и реакций на собственное имя коры головного мозга. С одной стороны, это указывает на то, что мишенью действия пропофола является кора головного мозга. С другой, что необходимы более точные инструменты подбора дозы препарата для достижения адекватного уровня анестезии.
Несмотря на сильную корреляционную зависимость между изменениями ЭЭГ и глубиной анестезии, у пациента Р005, несмотря на среднюю дозировку пропофола, отсутствовала реакция, как на уровне среднего мозга, так и на уровне коры. В соответствии с нейрохирургическим протоколом отсутствие у данного пациента реакции на звук собственного имени нельзя было объяснить ни тяжестью заболевания, ни течением оперативного вмешательства. Однако, согласно анамнестическим данным, владение пациента русским языком ограничивалось пониманием и употреблением отдельных слов или словосочетаний, а его родным языком был узбекский. Учитывая тот факт, что фонетические системы русского и узбекского языков имеют ряд принципиальных отличий (Badalova, 2020), речевые стимулы, в том числе имена, предъявляемые носителем русского языка (одинаковым для всех пациентов), могли быть не распознаны (Weber, Scharenborg, 2012), особенно во время анестезии, когда когнитивная активность, необходимая для узнавания слова с нетипичным произношением, была снижена. Таким образом, данные пациента Р005 подтверждают основную исследовательскую гипотезу о возможности реакции во время глубокого наркоза только на высоко значимые стимулы, для которых в ходе жизненного опыта выработан устойчивый условный рефлекс.
Результаты данного исследования также поднимают вопрос об участии речевой коры для распознавания звука своего имени во время глубокого наркоза. Согласно альтернативной гипотезе, активирующее влияние собственного имени не требует подробного речевого анализа и может быть связано с областями мозга, которые обеспечивают возбуждение и активацию коры и среднего мозга за счет восходящих и нисходящих нервных волокон. К таким областям мозга могут относиться например, гиппокамп или таламус (Schiff, 2008; Paus, 2000). В то же время мы не можем полностью исключить функцию коры головного мозга и ее участие, пусть и измененное, в анализе звуков. Что касается функции среднего мозга, то, согласно полученным результатам, реакция на уровне среднего мозга на звук своего имени по сравнению с корой наблюдалась у большего количества пациентов и значимости различий были в среднем выше. Эти данные позволяют предположить что, даже в том случае, если активация была обусловлена его связями с другими структурами мозга, в том числе гиппокампом или таламусом, мы не можем исключить значение собственно среднего мозга для формирования реакции пациента с глубоким угнетением сознания на звук собственного имени.
ЗАКЛЮЧЕНИЕ
Наши результаты показали, что пациенты, находящиеся в бессознательном состоянии под общей анестезией во время нейрохирургического вмешательства, способны реагировать на звук своего имени, что сопровождается увеличением пиковой частоты альфа-ритма. Изменения ПЧА были зарегистрированы как на уровне скальповых электродов, так и на мезоэнцефальном уровне, что свидетельствует о вовлечении различных мозговых структур в процесс восприятия собственного имени у пациента в состоянии глубокого наркотического сна. Обнаружена обратная корреляция между количеством вводимого пропофола и увеличением ПЧА на звук собственного имени. Индивидуальная вариабельность реакций пациентов свидетельствуют о том, что нетипичное произнесение собственного имени не вызывает реакции возбуждения. Однако на основании одного наблюдения можно говорить лишь о предположении, и требуется дальнейшая исследовательская работа в этом направлении. Полученные данные косвенно свидетельствуют о недостаточности клинического и стандартного электрофизиологического подходов для оценки уровня глубины анестезии.
Список литературы
Badalova B. Modern Methods of Teaching Russian and Uzbek as a Foreign Language to Students. Science and Education. 2020. 1 (Special Issue 2).
Banquet J.P. Spectral analysis of the EEG in meditation. Electroencephalography and clinical neurophysiology. 1973. 35 (2): 143–151. https://doi.org/10.1016/0013-4694(73)90170-3
Barry R.J., De Blasio F.M., Fogarty J.S., Clarke A.R. Natural alpha frequency components in resting EEG and their relation to arousal. Clinical neurophysiology: official J. International Federation of Clinical Neurophysiology. 2020. 131 (1): 205–212. https://doi.org/10.1016/j.clinph.2019.10.018
Bastuji H., Perrin F., Garcia-Larrea L. Semantic analysis of auditory input during sleep: studies with event related potentials. International j. psychophysiology: official journal of the International Organization of Psychophysiology. 2002. 46 (3): 243–255. https://doi.org/10.1016/s0167-8760(02)00116-2
Boldyreva G.N. The hyppocampal alpha-rhythm of the human brain. Electroencephalography and clinical neurophysiology. 1997. 1 (103): 199.
Cheng L., Gosseries O., Ying L., Hu X., Yu D., Gao H., He M., Schnakers C., Laureys S., Di H. Assessment of localisation to auditory stimulation in post-comatose states: use the patient’s own name. BMC neurology. 2013. 13: 27. https://doi.org/10.1186/1471-2377-13-27
De Gennaro L., Ferrara M., Curcio G., Cristiani R. Antero-posterior EEG changes during the wakefulness-sleep transition. Clinical neurophysiology: official j. International Federation of Clinical Neurophysiology. 2001. 112 (10): 1901–1911. https://doi.org/10.1016/s1388-2457(01)00649-6
di Fronso S., Fiedler P., Tamburro G., Haueisen J., Bertollo M., Comani, S. Dry EEG in Sports Sciences: A Fast and Reliable Tool to Assess Individual Alpha Peak Frequency Changes Induced by Physical Effort. Frontiers in neuroscience. 2019. 13: 982. https://doi.org/10.3389/fnins.2019.00982
Feige B., Scheffler K., Esposito F., Di Salle F., Hennig J., Seifritz E. Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J. neurophysiology. 2005. 93 (5): 2864-2872. https://doi.org/10.1152/jn.00721.2004
Fischer C., Dailler F., Morlet D. Novelty P3 elicited by the subject’s own name in comatose patients. Clinical neurophysiology: official J. International Federation of Clinical Neurophysiology. 2008. 119 (10): 2224–2230. https://doi.org/10.1016/j.clinph.2008.03.035
Freitas S., Simoes M.R., Maroco J., Alves L., Santana I. Construct Validity of the Montreal Cognitive Assessment (MoCA). J. International Neuropsychological Society. 2012. 18 (2): 242–250. https://doi.org/10.1017/S1355617711001573
Gutmann B., Mierau A., Hulsdunker T., Hildebrand C., Przyklenk A., Hollmann W., Struder H.K. Effects of physical exercise on individual resting state EEG alpha peak frequency. Neural plasticity. 2015. 717312. https://doi.org/10.1155/2015/717312
Gutmann B., Zimmer P., Hulsdunker T., Lefebvre J., Binnebossel S., Oberste M., Bloch W., Struder H.K., Mierau A. The effects of exercise intensity and post-exercise recovery time on cortical activation as revealed by EEG alpha peak frequency. Neuroscience letters. 2018. 668: 159–163. https://doi.org/10.1016/j.neulet.2018.01.007
Haegens S., Cousijn H., Wallis G., Harrison P.J., Nobre A.C. Inter- and intra-individual variability in alpha peak frequency. NeuroImage. 2014. 92 (100): 46–55. https://doi.org/10.1016/j.neuroimage.2014.01.049
Holeckova I., Fischer C., Morlet D., Delpuech C., Costes N., Mauguiere F. Subject’s own name as a novel in a MMN design: a combined ERP and PET study. Brain research. 2008. 1189: 152–165. https://doi.org/10.1016/j.brainres.2007.10.091
Holler Y., Kronbichler M., Bergmann J., Crone J.S., Ladurner G., Golaszewski S. EEG frequency analysis of responses to the own-name stimulus. Clinical neurophysiology: official J. International Federation of Clinical Neurophysiology. 2011. 122 (1): 99–106. https://doi.org/10.1016/j.clinph.2010.05.029
Jensen O., Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Frontiers in human neuroscience. 2010. 4: 186. https://doi.org/10.3389/fnhum.2010.00186
Kannabiran N., Bidkar P.U. Total intravenous anesthesia in neurosurgery. J. Neuroan-aesthesiology and Critical Care. 2018. 5 (3): 141–149. https://doi.org/10.1055/s-0038-1673544
Kempny A.M., James L., Yelden K., Duport S., Farmer S.F., Playford D.E., Lef A.P. Patients with a severe prolonged Disorder of Consciousness can show classical EEG responses to their own name compared with others' names. NeuroImage: Clinical. 2018. 19: 311–319. https://doi.org/10.1016/j.nicl.2018.04.027
Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain research reviews. 1999. 29 (2–3): 169–195. https://doi.org/10.1016/s0165-0173(98)00056-3
Klimesch W., Schimke H., Pfurtscheller G. Alpha frequency, cognitive load and memory performance. Brain topography. 1993. 5 (3): 241–251. https://doi.org/10.1007/BF01128991
Li K., Fan L., Cui Y., Wei X., He Y., Yang J., Lu Y., Li W., Shi W., Cao L., Cheng L., Li A., You B., Jiang T. The human mediodorsal thalamus: Organization, connectivity, and function. NeuroImage. 2022. 249: 118876. https://doi.org/10.1016/j.neuroimage.2022.118876
Luria A.R. Higher cortical function in man. N.Y.: Springer New York, 1980. https://doi.org/10.1007/978-1-4615-8579-4
Manshanden I., De Munck J.C., Simon N.R., Lopes da Silva F.H. Source localization of MEG sleep spindles and the relation to sources of alpha band rhythms. Clinical neurophysiology: official J. International Federation of Clinical Neurophysiology. 2002. 113 (12): 1937–1947. https://doi.org/10.1016/s1388-2457(02)00304-8
Moretti D.V., Prestia A., Fracassi C., Geroldi C., Binetti G., Rossini P.M., Zanetti O., Frisoni G.B. Volumetric differences in mapped hippocampal regions correlate with increase of high alpha rhythm in Alzheimer’s disease. International J. Alzheimer’s disease. 2011. 208218. https://doi.org/10.4061/2011/208218
Musizza B., Ribaric S. Monitoring the depth of anaesthesia. Sensors. 2010. 10 (12): 10896–10935. https://doi.org/10.3390/s101210896
Omata K., Hanakawa T., Morimoto M., Honda M. Spontaneous Slow Fluctuation of EEG Alpha Rhythm Reflects Activity in Deep-Brain Structures: A Simultaneous EEG-fMRI Study. PloS one. 2013. 8 (6): e66869. https://doi.org/10.1371/journal.pone.0066869
Oswald I., Taylor A.M., Treisman M. Discriminative responses to stimulation during human sleep. Brain: a journal of neurology. 1960. 83 (3): 440–453. https://doi.org/10.1093/brain/83.3.440
Patel A.K., Reddy V., Shumway K.R., Araujo J.F. Physiology, Sleep Stages. Treasure Island (FL): StatPearls Publishing. 2023.
Paus T. Functional anatomy of arousal and attention systems in the human brain. Progress in brain research. 2000. 126: 65–77. https://doi.org/10.1016/S0079-6123(00)26007-X
Perrin F., Garcia-Larrea L., Mauguiere F., Bastuji H. A differential brain response to the subject’s own name persists during sleep. Clinical neurophysiology: official J. International Federation of Clinical Neurophysiology. 1999. 110 (12): 2153–2164. https://doi.org/10.1016/s1388-2457(99)00177-7
Portas C.M., Krakow K., Allen P., Josephs O., Armony J.L., Frith C.D. Auditory processing across the sleep-wake cycle: simultaneous EEG and fMRI monitoring in humans. Neuron. 2000. 28 (3): 991–999. https://doi.org/10.1016/s0896-6273(00)00169-0
Portnova G.V., Proskurnina E.V., Sokolova S.V., Skorokhodov I.V., Varlamov A.A. Perceived pleasantness of gentle touch in healthy individuals is related to salivary oxytocin response and EEG markers of arousal. Experimental brain research. 2020. 238 (10): 2257–2268. https://doi.org/10.1007/s00221-020-05891-y
Ruby P., Blochet C., Eichenlaub J.B., Bertrand O., Morlet D., Bidet-Caulet A. Alpha reactivity to complex sounds differs during REM sleep and wakefulness. PloS one. 2013a. 8 (11): e79989. https://doi.org/10.1371/journal.pone.0079989
Ruby P., Blochet C., Eichenlaub J.B., Bertrand O., Morlet D., Bidet-Caulet A. Alpha reactivity to first names differs in subjects with high and low dream recall frequency. Frontiers in psychology. 2013b. 4: 419. https://doi.org/10.3389/fpsyg.2013.00419
Rudolph U., Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nature reviews Neuroscience. 2004. 5 (9): 709–720. https://doi.org/10.1038/nrn1496
Sadato N., Nakamura S., Oohashi T., Nishina E., Fuwamoto Y., Waki A., Yonekura Y. Neural networks for generation and suppression of alpha rhythm: a PET study. Neuroreport. 1998. 9 (5): 893–897. https://doi.org/10.1097/00001756-199803300-00024
Schiff N.-D. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Annals of the New York Academy of Sciences. 2008. 1129: 105–118. https://doi.org/10.1196/annals.1417.029
Schreckenberger M., Lange-Asschenfeldt C., Lochmann M., Mann K., Siessmeier T., Buchholz H. G., Bartenstein P., Grunder G. The thalamus as the generator and modulator of EEG alpha rhythm: a combined PET/EEG study with lorazepam challenge in humans. NeuroImage. 2004. 22 (2): 637–644. https://doi.org/10.1016/j.neuroimage.2004.01.047
Schwabedal J.T., Riedl M., Penzel T., Wessel N. Alpha-wave frequency characteristics in health and insomnia during sleep. J. sleep research. 2016. 25 (3): 278–286. https://doi.org/10.1111/jsr.12372
Shaw J.C. The brain’s alpha rhythms and the mind. BV Elsevier Science. 2003.
Scheeringa R., Petersson K.M., Oostenveld R., Norris D.G., Hagoort P., Bastiaansen M.C. Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage. 2009. 44 (3): 1224–1238. https://doi.org/10.1016/j.neuroimage.2008.08.041
Vanhaudenhuyse A., Laureys S., Perrin F. Cognitive event-related potentials in comatose and post-comatose states. Neurocritical care. 2008. 8 (2): 262–270. https://doi.org/10.1007/s12028-007-9016-0
Voss U., Harsh J. Information processing and coping style during the wake/sleep transition. J. sleep research. 1998. 7 (4): 225–232. https://doi.org/10.1046/j.1365-2869.1998.00117.x
Weber A., Scharenborg O. Models of spoken-word recognition. Wiley interdisciplinary reviews Cognitive science. 2012. 3 (3): 387–401. https://doi.org/10.1002/wcs.1178
Zimmer P., Mierau A., Bloch W., Struder H.K., Hulsdunker T., Schenk A., Fiebig L., Baumann F.T., Hahn M., Reinart N., Hallek M., Elter T. Post-chemotherapy cognitive impairment in patients with B-cell non-Hodgkin lymphoma: a first comprehensive approach to determine cognitive impairments after treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone or rituximab and bendamustine. Leukemia & lymphoma. 2015. 56 (2): 347–352. https://doi.org/10.3109/10428194.2014.915546
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


