Цитология, 2023, T. 65, № 6, стр. 509-521
Частичное репрограммирование клеток как способ ревитализации живых систем
М. А. Шорохова *
Институт цитологии РАН
194064 Санкт-Петербург, Россия
* E-mail: shili-mariya@yandex.ru
Поступила в редакцию 01.07.2023
После доработки 06.08.2023
Принята к публикации 01.09.2023
- EDN: OTBHIL
- DOI: 10.31857/S0041377123060093
Аннотация
Старение и ассоциированные с ними заболевания – острая проблема современной биологии и медицины. Хотя старение в настоящее время невозможно предотвратить, его влияние на продолжительность жизни и здоровье пожилых людей потенциально может быть сведено к минимуму с помощью вмешательств, направленных на возвращение клеток к нормальному функционированию. Постоянный поиск путей омоложения и улучшения регенеративной способности клеток привел к открытию в 2016 г. метода частичного репрограммирования, основанного на краткосрочной экспрессии факторов репрограммирования (Oct4, Sox2, Klf4 и c-Myc). В результате происходит восстановление молодой эпигенетической сигнатуры стареющих клеток. Эффективность метода показана как в системе in vitro, так и в системе in vivo. В представленном обзоре обсуждаются основные успехи частичного репрограммирования, а также проблемы и нерешенные вопросы, с которыми столкнулись исследователи. Отдельно обсуждаются данные о молекулярных изменениях в процессе частичного репрограммирования. Метод частичного репрограммирования дает широкий спектр возможностей для фундаментальных исследований старения и омоложения. А дальнейшие работы в этом направлении могут привести к разработке терапевтических стратегий с целью облегчения возрастных заболеваний и, таким образом, к улучшению здоровья и долголетию.
Процесс старения представляет собой системное снижение функционирования от клеток до всего организма. Актуальным вопросом современной биологии и медицины является поиск эффективных стратегий омоложения, с целью придания состарившимся клеткам или органам более молодых характеристик. На данный момент разработано несколько стратегий направленных на омоложение клеток и всего организма.
Настоящий обзор посвящен одной из них – омоложению с помощью частичного репрограммирования. Метод был разработан в 2016 г. В обзоре предпринята попытка максимально полно охватить результаты исследований частичного репрограммирования старых клеток с момента первой публикации по этой тематике до настоящего врмени. Отдельно в обзоре рассматриваются выявленные молекулярные пути, участвующие в процессе омоложения. Глубокое понимание движущих сил процесса репрограммирования обеспечит дальнейшее постижение клеточного омоложения и оценку возможностей применения метода в лечении заболеваний человека, связанных со старением. Более того, в настоящем обзоре приводится обсуждение проблем, с которыми столкнулись исследователи и поставлены вопросы, на которые еще предстоит ответить.
СТАРЕНИЕ НА КЛЕТОЧНОМ И ОРГАНИЗМЕННОМ УРОВНЕ
Население Земли неуклонно растет. Благодаря развитию медицины и технологий удалось значительно увеличить длительность жизни людей, что значительно увеличило популяцию пожилых людей. По оценкам Всемирной организации здравоохранения (ВОЗ), к 2030 г. ожидается, что 1 из 6 человек, или 2.1 млрд, будет старше 60 лет. Старение – это процесс изменения живых систем во времени, вызывающий нарушения в их структуре и функциях. Большая часть возрастных изменений приводит к ухудшению функций органов чувств, снижению активности в повседневной жизни, к повышенной восприимчивости к инфекциям и увеличению частоты заболеваний и, как следствие, к слабости или инвалидности (López-Otín et al., 2013; Brett, Rando, 2014). Хотя исследования в этой области активно развиваются, и с каждым годом увеличивается коли-чество новой информации, в области биологии старения остается очень много нерешенных вопросов.
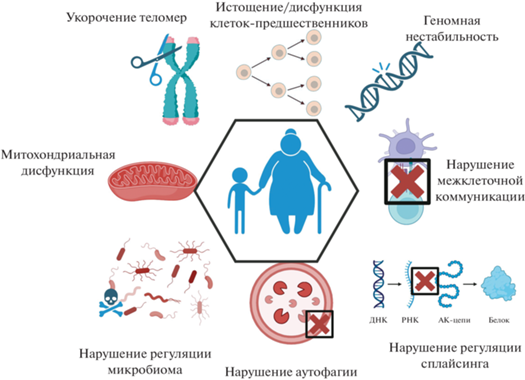
В ходе изучения процесса старения на клеточных моделях были определены его признаки, универсальные для любого клеточного типа (рис. 1). К ним относятся геномная нестабильность, истощение/дисфункция клеток-предшественников, теломерные и эпигенетические изменения, нарушение регуляции белкового гомеостаза, нарушение восприятия питательных веществ, митохондриальная дисфункция, нарушение межклеточной коммуникации, хроническое воспаление низкой степени выраженности (вызванное секреторным фенотипом, связанным со старением – SASP), фиброз, нарушение регуляции микробиома, нарушение аутофагии, изменение механических свойств клеток, нарушение регуляции сплайсинга и другие (Schmauck-Medina et al., 2022).
Рис. 1.
Схема ключевых признаков старения. Показаны: нестабильность генома, нарушение межклеточной коммуникации, нарушение регуляции сплайсинга, нарушение аутофагии, нарушение регуляции микробиома, митохондриальная дисфункция, укорочение теломер. АК – аминокислота.
До недавнего времени одной из важнейших проблем в изучении биологии старения и при разработке методов борьбы со старением было отсутствие универсального маркера старения, позволяющего уловить изменение биологического возраста во времени. Одно из важных требований заключалось в том, чтобы этот биомаркер был неинвазивным или, по крайней мере, нелетальным. Многие исследования на тканевом уровне ограничены этим критерием (органы могут быть извлечены только из умерщвленных мышей в определенный момент времени).
Более десяти лет назад был разработан метод определения биологического возраста на основе метилирования ДНК, названный эпигенетическими часами (Bocklandt et al., 2011; Koch, Wagner, 2011). Эпигенетические часы – это совокупность эпигенетических меток ДНК, позволяющая определить биологический возраст ткани, клетки или органа. Наиболее широко применимым и используемым является первый мультитканевый предиктор возраста – “часы Хорвата”. Они демонстрируют самую высокую корреляцию с хронологическим возрастом, предсказывая возраст (или эпигенетический возраст eAge) множества тканей со средней ошибкой 3.6 года (Horvath, 2013). Примечательно, что имеются доказательства того, что отклонение прогнозируемого и хронологического возраста связано со смертностью и распространенностью возрастных особенностей, что указывает на то, что eAge обеспечивают измерение биологического возраста, а не хронологического (Lin et al., 2016; Bell et al., 2019).
РАЗЛИЧНЫЕ СТРАТЕГИИ ОМОЛОЖЕНИЯ
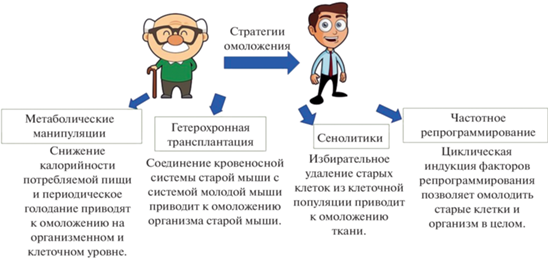
Старение многих видов млекопитающих, в том числе людей, ранее рассматривали как необратимый процесс (Galkin et al., 2020). Однако изучение механизмов старения и более глубокое понимание этого процесса позволили разработать несколько стратегий замедления старения и обращения его вспять (рис. 2).
Рис. 2.
Схема основных стратегий омоложения организма с кратким описанием сути метода: метаболические манипуляции, гетерохронная трансплантация, использование сенолитиков, частичное репрограммирование.
Согласно геронтологии, любые вмешательства, нацеленные на один из признаков старения, перечисленных в предыдущем разделе, могут опосредованно воздействовать и на другие признаки (Chini et al., 2019; Lewis-McDougall et al., 2019; Vizioli et al., 2020; Dungan et al., 2022; Zhu et al., 2022) Существует ряд стратегий омоложения организма.
Омоложение с помощью диеты. Это одна из самых изученных и самых старых манипуляций, направленных на борьбу с признаками старения организма (Brandhorst et al., 2015). Состав рациона питания и уровень калорийности являются ключевыми факторами, влияющими на старение и возрастные заболевания (Blagosklonny, 2013; Gems, Partridge, 2013; López-Otín et al., 2013). Во многих исследованиях было выявлено, что снижение калорийности употребляемой пищи, а так же периодическое голодание оказывает значительное влияние на репарацию окислительного повреждения тканей, оптимизируя энергетический обмен и усиливая клеточную защиту, улучшая общее состояние как на клеточном уровне, так и на уровне организма в целом (Haigis, Yankner, 2010; Lee et al., 2012; Brandhorst et al., 2015) .
Циклы полного голодания, длящиеся 2 или более дней, но разделенные, по крайней мере, неделей обычного питания, становятся высокоэффективной стратегией защиты нормальных клеток и органов от различных токсинов и токсических состояний (Raffaghello et al., 2008; Verweij et al., 2011). Получены замечательные результаты при изучении влияния полного голодания. Было обнаружено, что периодическое полное голодание вызывает снижение уровня глюкозы в крови, инсулина и инсулиноподобного фактора роста 1 (IGF-1) (Lee et al., 2010) и сопровождается аутофагией (Madeo et al., 2010).
Таким образом, во многих исследованиях показано, что изменения рациона питания и периодическое голодание способно очень благоприятно влиять как клеточном уровне, так на организменном, нивелируя или уменьшая признаки старения органов и тканей. В некоторых исследования удалось увеличить срок жизни экспериментальных животных с помощь различных диет.
Омоложение с помощью гетерохронной трансплантации. Другим методом омоложения, результаты которого заставляют исследователей оптимистично смотреть в будущее, является гетерохронный парабиоз. Это хирургическая процедура, при которой соединяют кровеносную систему старой мыши с системой молодой мыши. В результате таких хирургических манипуляций продолжительность жизни старой мыши увеличивается (Ludwig, Elashoff, 1972; Conboy et al., 2005). Было обнаружено, что при кратковременном соединении системы кровообращения молодых и пожилых мышей у старых мышей проявлялись признаки молодых мозга, мышц и печени, улучшались когнитивные функции, пополнялся пул стволовых клеток и повышались регенеративные способности (Conboy et al., 2005). В последствии исследователи сосредоточились на переливании крови, форменных элементов крови и трансплантации костного мозга экспериментальным животным для изменения биологического возраста подопытных.
Удивительные данные были получены при трансплантации костного мозга: эпигенетический возраст реципиентов крови начинал соответствовать возрасту доноров (Stölzel et al., 2017). А в другом исследовании было показано, что трансплантация костного мозга увеличивала продолжительности жизни мышей на 12% (Guderyon et al., 2020). При исследовании трансплантации форменных элементов крови достигнуты обнадеживающие результаты (Yousefzadeh et al., 2021).
Многочисленные исследования показали, что кровь от молодого организма может обратить вспять процессы старения в старом организме. Хотя переливание молодой крови эффективно для омоложения у пожилых мышей, остается неясным, имеет ли клинические преимущества переливание молодой крови пожилым людям.
Сенолитики. Другой подход к омоложению связан с клеточным фенотипом SASP и с избирательным удалением накапливающихся старых клеток из клеточной популяции. Была выдвинута гипотеза, что удаление стареющих клеток из тканей и органов может приводить к омоложению в целом (Childs et al., 2015).
Для этого используют так называемые сенолитики, которые избирательно убивают стареющие клетки, или сеноморфные средства, подавляющие фенотип SASP. К настоящему времени идентифицировано несколько классов сенолитических и сеноморфных средств, включая природные соединения (например, кверцетин и куркумин). И хотя убедительных доказательств их терапевтического потенциала пока нет, можно полагать, что на их основе смогут появиться терапевтические препараты для лечения возрастных заболеваний.
Частичное репрограммирование старых клеток. Это стратегия омоложения совсем новая. Исследования индуцированных плюрипотентных клеток дают основания полагать, что возрастные клеточные фенотипы, включающие морфологические параметры, количество митохондрий и целостность ядерной мембраны, могут быть обратимы (Miller et al., 2013; Mahmoudi et al., 2019). Однако в процессе репрограммирования клетки переходят в плюрипотентное состояние и теряют свою клеточную идентичность (Evans, Kaufman, 1981; Takahashi, Yamanaka, 2016). Следовательно, индукция плюрипотентности или прямой перенос плюрипотентных клеток в систему in vivo неизменно приводит к канцерогенезу (Abad et al., 2013; Ohnishi et al., 2014; Moradi et al., 2019). Чтобы использовать эффект омоложения в процессе репрограммирования, необходимо отделить этот процесс от дедифференцировки и потери клеточной идентичности, что, несомненно, крайне сложная задача. В 2016 г. было предложено элегантное решение этой дилемы (Ocampo et al., 2016). Авторы предложили метод циклического частичного репрограммирования, что позволило добиться эффекта омоложения на прогерийных мышах без образования тератом (Ocampo et al., 2016). Эта работа стала прорывной в биологии старения и открыла новое направление в области омоложении соматических клеток.
ИДЕЯ И ПРИНЦИПЫ МЕТОДА ЧАСТИЧНОГО РЕПРОГРАММИРОВАНИЯ СТАРЫХ КЛЕТОК
В 2006 г. были выявлены четыре транскрипционных фактора (Oct4, Sox2, Klf4 и c-Myc), названные факторами Яманаки, которые, будучи экспрессированными в соматических клетках, могут эффективно возвращать их к состоянию ранних эмбриональных клеток (Takahashi, Yamanaka, 2006). Такие клетки назвали индуцированными плюрипотентными стволовыми клетками (iPSCs). Интересно, что при репрограммировании в iPSCs практически все возрастные изменения ДНК обращаются вспять (Koch et al., 2013; Weidner et al., 2014).Таким образом, прогнозируемый eAge клеток близок к нулю, даже если перепрограммирование проводилось на клетках от пожилых доноров. Однако в процессе полного репрограммирования клетки теряют свою клеточную идентичность. При применении метода клеточного репрограммирования в системе in vivo на экспериментальных животных (мышах) неизбежно возникали опухоли (Abad et al., 2013; Ohnishi et al., 2014). Таким образом, репрограммирование с целью омоложения оказалось неприменимо к системе in vivo.
Первая идея использования цикла репрограммирования не до конца, а только частично, появилась в 2014 г. при изучении ранних этапов репрограммирования старых фибробластов. Было обнаружено (Manukyan, Singh, 2014), что подвижность эссенциального эпигенетического модификатра HP1β одинакова в эмбриональных стволовых клетках человека и в клетках iPSС. HP1β менее подвижен в стареющих фибробластах, чем в молодых делящихся фибробластах (Manukyan, Singh, 2014); после введения факторов Яманаки в стареющие фибробласты подвижность HP1β в стареющих клетках увеличивается и через 9 сут становится такой же, как и в молодых фибробластах.
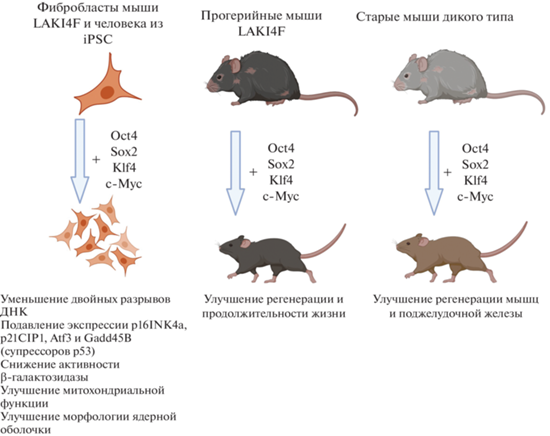
Эта работа подготовила почву для работы других авторов (Ocampo et al., 2016), которая заслуженно считается прорывной и основополагающей в области омоложения с помощью частичного репрограммирования (рис. 3). Эти авторы сообщили, что частичное репрограммирование, достигаемое путем периодической индукции OSKM (несколько повторов, по схеме: 2 сут включено, 5 сут выключено), уменьшает признаки старения без потери клеточной идентичности (Ocampo et al., 2016). Было проведено частичное репрограммирование сначала на фибробластах прогерийных мышей, в результате чего снизились возрастные признаки, такие как повреждение ДНК, изменения ядерной оболочки, дисрегуляция модификаций гистонов, синтез активных форм кислорода в митохондриях. После этого метод частичного репрограммирования применили in vivo к прогерийным мышам, в результате чего продолжительность их жизни увеличилась, а образования тератом не наблюдали. Метод частичного репрограммирования испытали также на естественно состарившихся мышах и показали улучшенную регенеративную способность мышц и поджелудочной железы после травм и возросшую толерантность к глюкозе (рис. 3).
Рис. 3.
Схема частичного репрограммирования путем кратковременной циклической экспрессии OSKM: улучшение характеристик старых клеток в культуре, уменьшение физиологических признаков старения и, как следствие, удлинение продолжительности жизни в мышиной модели преждевременного старения (LAKI4F) и улучшение регенеративных способностей у старых мышей дикого типа (по: Ocampo et al., 2016).
За этой работой (Ocampo et al., 2016) последовал шквал работ по омоложению с помощью частичного репрограммирования клеток. К сожалению, анализ с помощью метода эпигенетических часов для мышей был недоступен авторам (Ocampo et al., 2016), поэтому точную степень омоложения путем частичного перепрограммирования in vivo нельзя было определить количественно.
Однако метод стал доступен для последующих исследований. В исследовании Olova с соавторами (Olova et al., 2019) было обнаружено, что снижение eAge началось между 3- и 7-ми сут после трансдукции OSKM в частично репрограммированных клетках TRA-1-60 (+). Начиная с 20-ых сут, eAge в репрограммируемых клетках стабильно обнулялся. Популяции клеток TRA-1-60 (+) на 7- и 11-е сут ранее характеризовались как “частично перепрограммированные” из-за их высокой экспрессии маркеров плюрипотентности, а также высокой скорости реверсии к соматическому состоянию (Tanabe et al., 2013). Следовательно, наблюдаемое снижение eAge на 7- и 11-е сут предполагает, что частичное репрограммирование действительно можно рассматривать как механизм омоложения в клетках человека.
Применение неинтегративного протокола частичного репрограммирования с коктейлем мРНК, несущих OSKM и LIN28, продемонстрировало многогранное уменьшение клеточного старения фибробластов человека и эндотелиальных клеток от пожилых доноров: сброс eAge, снижение воспалительных реакций в хондроцитах и восстановление юношеских регенеративных реакций с возрастом, в каждом случае без изменения клеточной идентичности (Sarkar et al., 2020). Таким образом, было показано, что использование метода частичного репрограммирования (в различных его модификациях) приводит к снижению eAge.
БЕЗОПАСНОСТЬ И НАДЕЖНОСТЬ ЧАСТИЧНОГО РЕПРОГРАММИРОВАНИЯ
Полный цикл репрограммирования до уровня iPSCs сопровождается рядом опасностей, ограничивающих широкое использование цикла дедифференцировки в целях регенеративной медицины (Manukyan, Singh, 2014). Более того, было показано, что индукция iPSCs in vivo посредством непрерывной экспрессии OSKM увеличивает частоту тератом (Abad et al., 2013). В связи с этим особенно остро встает вопрос, насколько безопасно частичное репрограммирование старых клеток?
Метод омоложения с помощью техники частичного репрограммирования показал себя очень многообещающе для обращения вспять старения без потери клетками их идентичности как in vivo (Ocampo et al., 2016; Y. Lu et al., 2020; Alle et al., 2021), так и in vitro (Olova et al., 2019; Sarkar et al., 2020; Gill et al., 2022). Однако точные механизмы этого метода омоложения еще предстоит выявить и изучить. В связи с этим отслеживание любых следов плюрипотентности в частично репрограммированных клетках (особенно in vivo) является необходимой мерой предосторожности, чтобы свести к минимуму долгосрочный риск канцерогенеза.
В плане безопасности использования метода частичного репрограммирования идет большая работа по подбору оптимального коктейля для репрограммирования. В наборе OSKM большие опасения вызывает фактор c-Myc из-за того, что он является онкогеном. Поэтому в ряде работ исследовали эффект комбинации без фактора c-Myc (коктейль OSK: Oct4, Sox2, Klf4). Показано, что использование только Oct4 и Sox2 (OS) восстанавливало молодой профиль транскрипции в мезенхимных клетках без потери клеточной идентичности (Roux et al., 2022). Таким образом, риск потери клеточной идентичности и образования неопластической трансформации получается свести к минимуму (Roux et al., 2022). Экспрессия набора OSK (без c-Myc) восстанавливала остроту зрения у 11-месячных мышей и без канцерогенеза (Y. Lu et al., 2020); канцерогенеза не наблюдали даже через 10–18 мес непрерывной экспрессии OSK. Также было показано, что временная экспрессия неканонического фактора репрограммирования Nanog омолаживает стареющие миобласты без возникновения тератом (Shahini et al., 2021). Несмотря на достигнутые успехи, поисковые работы по выявлению оптимального по эффективности и безопасности коктейля репрограммирования продолжаются.
Другой важный момент, который необходимо учитывать в вопросе безопасности омоложения, это способ введения факторов репрограммирования. Наиболее изученным и распространенным методом является доставка факторов с помощью ретровирусов (Takahashi, Yamanaka, 2006; Ohnuki et al., 2014). Однако этот метод несет риски, такие как инсерционный мутагенез, остаточная экспрессия, повторная активация факторов репрограммирования (Hu, 2014; Klawitter et al., 2016). Сейчас появились альтернативные методы доставки – трансфекция РНК, транзиентная трансфекция, неинтегрирующиеся вирусные векторы, которые считаются более безопасными (Sarkar et al., 2020). Отдельное направление – это репрограммирование на основе химических веществ. Такой тип репрограммирования основан на прямом преобразовании соматической клетки в плюрипотентное состояние с помощью малых молекул и факторов роста (Hou et al., 2013; Ye et al., 2016; Kim et al., 2020). Такой способ также позволяет избежать использования c-Myc (Hofmann et al., 2015).
Перспективными выглядят методы доставки транскрипционных факторов с помощью электропорации (Sarkar et al., 2020), а также с помощью липофильных соединений. В той связи особенно актуальна недавняя работа, посвященная разработке липофильных соединений, способных проникать через клеточные мембраны и регулировать факторы репрограммирования (Guan et al., 2022). Местное применение таких соединений к состарившимся тканям (например, коже) может обеспечить омоложение путем частичного репрограммирования клеток in vivo у людей. Однако надо помнить о необходимости соблюдать осторожность, чтобы избежать необратимой дедифференцировки с сопутствующим риском неопластической трансформации.
В отношении частичного репрограммирования очень важны временные рамки этого процесса. В ряде исследований были определены общие закономерности для разных типов соматических клеток. Эффект омоложения в процессе репрограммирования наблюдается только в определенном временном окне. Далее клетки теряют свою клеточную идентичность и начинают дедифференцироваться.
Хорошо известно, что частичное репрограммирование происходит в рамках ранней, обратимой фазы во время репрограммирования iPSC во времени, которое включает стохастическую активацию генов плюрипотентности. За ней следует более детерминированная фаза созревания с предсказуемым порядком изменений экспрессии генов, когда судьба клеток прочно связана с плюрипотентностью. Действительно, было показано, что фибробласты мыши не могут стать iPSC и вернуться к своему исходному соматическому состоянию, если экспрессия OSKM прекращается во время начальной стохастической фазы. Было показано, что клетки TRA-1-60 (+) при перепрограммировании на 7- и 11-е сут еще не достигли созревания и частично перепрограммированы (Tanabe et al., 2013). Однако в другом исследовании было продемонстрировано, что большая часть маркерных генов фибробластов поддерживает относительно высокий уровень экспрессии до 15-х сут (Olova et al., 2019). Интересно, что пошаговое снижение экспрессии генов фибробластов совпадает с пиком экспрессии генов, характерных для старых клеток, и таким образом задерживает потерю соматической идентичности, но не экспрессию генов плюрипотентности. Вместе эти факты (разная динамика ступенчатой экспрессии генов фибробластов и снижения (линейное) eAge дополнительно указывает на то, что дедифференцировка и эпигенетическое омоложение могут быть не связаны.
Итак, большинство клеток на 15-е сут репрограммирования проходят точку невозврата и окончательно теряют свою клеточную идентичность (Tanabe et al., 2013). Т.е. безопасное в плане дедифференцировки состояние заканчивается в период 11–15 сут от начала процесса репрограммирования. Таким образом, частичное репрограммирование может обеспечить во времени “безопасное окно”, в котором клетки способны достичь существенного эпигенетического омоложения при сохранении их изначальной клеточной идентичности.
Кроме того, стоит учитывать этиологию репрограммируемых клеток, т. к. различные ткани организма обладают различной пластичностью. Так, гепатоциты взрослого организма более пластичны по сравнению с кардиомиоцитами. Гепатоциты демонстрируют спонтанное клеточное репрограммирование во время регенерации печени, а специфичная для гепатоцитов экспрессия OSKM всего за 2 сут приводит к летальному исходу (Hishida et al., 2022). А кардиомиоциты требуют 6 сут экспрессии OSKM для проявления признаков дедифференцировки и 12 сут для проявления признаков болезни и гибели животных (Chen et al., 2021). В связи с этим, один из способов адаптировать метод репрограммирования – это использовать циклическую экспрессию OSKM локально, в так называемой “оптимальной зоне возрастного репрограммирования” (Singh et al., 2019); только в пределах этой зоны происходит репрограммирование с целью омоложения(Abad et al., 2013). Таким образом, можно варьировать по длительности циклы репрограммирования с учетом пластичности репрограммируемой ткани.
Еще один интересный вопрос заключается в том, будут ли омолаживаться с помощью репрограммирования постмитотические терминально дифференцированные клетки, такие как нейроны, кардиомиоциты или адипоциты. В ряде работ было показано, что сначала в репрограммируемых клетках восстанавливалась клеточная пролиферация, а уже позже наблюдали эффект омоложения (Manukyan, Singh, 2014; Sarkar et al., 2020). Эти результаты позволяют предположить, что клеточная пролиферация является необходимым условием омоложения.
Другой важный вопрос, касающийся временных параметров, относится к стабильности достигнутых эффектов омоложения в процессе частичного репрограммирования. Недавно была предпринята попытка ответить на этот вопрос: при исследовании репрограммирования фибробластов от донора среднего возраста in vitro, экспрессию OSKM прекращали через 10, 13, 15 и 17 сут (Gill et al., 2022). Наблюдаемый эффект омоложения клетки сохраняли в течении как минимум 4 нед. после прекращения экспрессии OSKM. Таким образом, клетки приобрели состояние, соответствующему более молодому возрасту согласно eAge. Профиль экспрессии изменился и приблизился к профилю более молодых клеток.
МОЛЕКУЛЯРНЫЕ ИЗМЕНЕНИЯ В ПРОЦЕССЕ ЧАСТИЧНОГО РЕПРОГРАММИРОВАНИЯ КЛЕТОК
Изменение идентичности соматических клеток взрослого человека в сторону эмбриональных клеток in vitro путем сверхэкспрессии четырех транскрипционных факторов Яманаки, связанных с плюрипотентностью, произвело революцию в биологии более 15 лет назад (Takahashi, Yamanaka, 2006). С тех пор многие работы были направлены на изучение молекулярных основ клеточной пластичности, лежащей в основе изменения клеточной судьбы. При этом раскрываются многие метаболические, эпигенетические, транскриптомные и протеомные аспекты этого процесса.
Большая часть экспериментов, основанных на анализе клеточных популяций, не давали четкого представления о клеточной судьбе во время репрограммирования. Только недавно с помощью мультиомных технологий в нескольких исследованиях начали изучать судьбы единичных клеток во время OSKM-индуцированного репрограммирования (Schiebinger et al., 2019; X. Liu et al., 2020). Открытием этих работ было то, что в одной популяции репрогаммируемых клеток есть клетки с различными направлениями развития, включая клетки экстраэмбриональные (эндодермальные и трофэктодермальные), нейрональные и мезодермальные. До недавнего времен подобных исследований в системе in vivo не было.
В 2022 г. впервые клеточное репрограммирование с помощью OSKM in vivo проследили в динамике (Chondronasiou et al., 2022). Используя поджелудочную железу в качестве модели, авторы охарактеризовали клеточные состояния, возникающие на ранней стадии репрограммирования (через 7 сут после экспрессии OSKM), с помощью scRNA-seq (исследования транскриптома на единичных клетках) и выявили неоднородную реакцию на индукцию OSKM клеток одного и того же органа. Кроме того, авторы (Chondronasiou et al., 2022) определили сигнатуру генов, характерную для промежуточного репрограммирования в поджелудочной железе, которая также наблюдалась в других частично репрограммированных тканях (в желудке и толстой кишке). Подобные данные представляют особый интерес с точки зрения начальных этапов репрограммирования.
Данные о молекулярных изменениях в процессе частичного репрограммированния старых клеток стали появляться только в последние годы. В связи с этим хотелось бы остановиться подробнее на работах, посвященных этой тематике.
Изначально стирание признаков старения наблюдали в процессе полного репрограммирвания. Так, несколькими исследовательскими группами было показано, что iPSC, полученные от молодых и старых доноров, в значительной степени неотличимы, и это сходство сохраняется после дифференцировки полученных iPSC в различных направлениях (Nishimura et al., 2013; Mertens et al., 2015). Впоследствии было обнаружено, что частичное репрограммирование уменьшает транскрипционные признаки старения во многих типах клеток человека и мыши (Gill et al., 2022; Lu et al., 2019; Sarkar et al., 2020; Shahini et al., 2021) и улучшает регенеративную способность клеток органов и тканей ( Lu et al., 2019; Rodríguez-Matellán et al., 2020; Sarkar et al., 2020; Chen et al., 2021). Была сделана оценка влияния временной экспрессии факторов репрограммирования (OSKLMN) на транскриптом фибробластов и эндотелиальных клеток от пожилых людей и транскриптомом тех же типов клеток, выделенных от молодых доноров (Sarkar et al., 2020). При сравнении частично репрограммированных старых клеток и контрольных (старых) клеток обнаружили, что в фибробластах 1042 гена (734 активированных и 308 подавленных), а в эндотелиальных клетках 992 гена (461 активированных и 531 подавленных) экспрессировались дифференцированно. При анализе генных модулей дифференциально экспрессируемых генов было обнаружено, что экспрессия OSKLMN способствует очень быстрой активации профиля экспрессии генов молодых клеток, специфичного для клеточного типа, при этом экспрессия генов клеточной идентичности была неизменной.
В работе по изучению связи между изменением eAge и активности генов плюрипотентности в процессе репрограммирования (Olova et al., 2019) было выделено 2 группы паттернов экспрессии генов – ранних (кластер 1) и поздних (кластер 2) активированных генов плюрипотентности соответственно. В кластере 1 гены плюрипотентности включали NANOG, SALL4, ZFP42, TRA-1-60, UTF1, DPPA4 и LEFTY2, их экспрессия резко увеличивалась в течение первых 10 сут, а затем к 20 сут устанавливалась на стабильном уровне. В кластере 2 гены плюрипотентности (поздно экспрессирующиеся, такие как LIN28, ZIC3 и DNMT3B) повышали экспрессию медленнее и достигали стабильного уровня плюрипотентности к 28 сут. Интересно, что eAge сбрасывался до нуля в то время, когда гены в кластере 1 достигали уровней своего плюрипотентного состояния, которое по времени предшествует полной плюрипотентности. Это также совпало с пиком экспрессии ряда генов эмбрионального развития между 15 и 20 сут и может свидетельствовать о том, что перезагрузка отмечает точку, в которой клетки достигают эмбрионоподобного состояния, но еще не являются полностью плюрипотентными. Таким образом, снижение eAge наблюдается хорошо в пределах первой волны экспрессии гена плюрипотентности. Кроме того, исследователи изучили динамику подавления генов, характерных для фибробластов в качестве показателя потери идентичности соматических клеток. 19 часто используемых маркерных генов фибробластов (кластер1: REX1 (ZFP42), TRA-1-60/81 (PODXL, UTF1, DPPA4, TDGF1 (CRIPTO), SALL4, LEFTY1, LEFTY2, DNMT3A, TFCP2L1, TERF1; DPPA5, TERT, ZIC3, LIN28A, LIN28B, LECT1, DNMT3B, COL3A1, FSP-1, TGFB3, TGFB2; кластер 2: COL1A2, ITGA1, DDR2, P4HA3, THY1, FAP; CD248, VIM; кластер 3: COL1A1, ITGA5, P4HA1, P4HA2, TGFB1, HSP47, CD34) были сгруппированы в три составных паттерна экспрессии, два из которых (кластеры 2 и 3) показывали быстрое снижение экспрессии после индукции OS-KM. Кластер 1 оставался со стабильной экспрессией в течение первых 15 сут. Через 15 сут экспрессия генов фибробластов быстро снижалась во всех трех кластерах и только к 35-м сут все они достигали уровня экспрессии эмбриональных стволовых клеток (ЭСК), а фибробласты полностью теряли соматическую идентичность. Кластер 1 (содержащий маркеры идентичности фибробластов FSP1, COL3A1 и TGFB2/3) демонстрировал самое медленное снижение и был последним, достигшим уровней экспрессии ЭСК. Таким образом, было обнаружено, что ряд генов, специфичных для фибробластов, поддерживал высокие уровни экспрессии до 15-х сут, когда наблюдалось значительное снижение eAge.
Исследования изменения экспрессии генных модулей продолжились и далее с помощью технологии RNA-seq единичных клеток для определения изменений экспрессии генов, вызванных частичным репрограммированием (Roux et al., 2022). К настоящему времени это первое и единственное исследование геномов частично репрограммированных клеток с помощью технологии RNA-sec единичных клеток, в связи с чем хотелось бы остановиться на результатах этой работы более подробно.
Авторы (Roux et al., 2022) выполнили частичное репрограммирование первичных адипогенных и мезенхимных стволовых клеток (МСК) молодого и пожилого возраста и профилировали экспрессию генов одиночных клеток в различные моменты времени после отмены фактора репрограммирования. Было обнаружено, что частичное репрограммирование адипоцитов индуцировало набор новых состояний экспрессии генов по сравнению с контрольными клетками. При дифференциальном анализе экспрессии генов эти авторы обнаружили, что репрограммирование значительно изменило уровень экспрессии в сторону молодости в 3485 генах из общего числа 5984 генов, измененных с возрастом, при этом репрограммирование противодействовало возрастным изменениям во многих наборах генов. Наиболее яркими были усиление адипогенного метаболизма жирных кислот, регуляция которого снижается с возрастом, и подавление генов воспалительной реакции, усиливающейся с возрастом. В МСК частичное репрограммирование индуцировало новый профиль экспрессии генов, а именно подавление регуляции программы эпиталиально-мезенхимного перехода (EMT), восстановление экспрессии генов, характерных для молодых клеток (712 генов), а программа индуцированных старением фиброзных генов была подавлена. При этом исследование отдельных генов показало, что идентичность соматических клеток определяет эффекты частичного репрограммирования. Таким образом, авторы пришли к выводу, что в некоторых соматических клетках экспрессия генов, характерных для молодых клеток может быть восстановлена более эффективно, чем в других (Roux et al., 2022).
Эти же авторы исследовали влияние частичного репрограммирования на отдельные гены-маркеры идентичности соматических клеток. На адипогенных клетках они обнаружили, что гены Lpl и Fabp4 (адипогенные) были значительно подавлены, а Nanog, Snca и Fgf13 были активированы. В МСК аналогично: гены Acta2, Thy1 и Col1a1 (мезенхимные) были подавлены, в то время как Nanog, Snca и Fgf13 были активированы. Авторы приходят к выводу, что клеточные идентичности после частичного репрограммирования значительно подавляются (Roux et al., 2022). Важно заметить, что эти результаты контрастируют с предыдущими сообщениями о том, что частичное репрограммирование не влияет на идентичность соматических клеток и не активирует гены плюрипотентности. Требуется продолжение исследований, чтобы понять влияние частичного репрограммирования на экспрессию генов соматических клеток и механизмы ревитализации.
ЗАКЛЮЧЕНИЕ
Существует представление, что накопление молекулярных повреждений в клетках и тканях с течением времени играет центральную роль в процессе старения (Gladyshev et al., 2021). В разных тканях скорость накопления молекулярных повреждений разная, в связи с этим некоторые органы и ткани могут стареть быстрее, чем весь организм. С этим эффектом можно связать появление заболеваний различных органов, ассоциированных с возрастом, таких как сердечная недостаточность и ишемическая болезнь сердца, старческая астения, глаукома, диабетическая ангиоретинопатия, заболевания сетчатки и другие. Предложенный подход омоложения (Guan et al., 2022) дает возможность локально частично репрограммировать клетки, таким образом омолодить и регенерировать поврежденный орган. В связи с этим метод имеет перспективы для использования его в медицинской практике.
Метод частичного репрограммирования открывает широкий спектр возможностей для фундаментальных исследований. Изучение молекулярных особенностей процесса омоложения путем частичного репрограммирования позволит более глубоко понять не только сам процесс ревитализации, но и молекулярные и клеточные механизмы, лежащие в основе процесса старения. А возможность омолодить клетки и снова запустить процесс старения может стать очень удобной модельной системой для изучения старения.
Метод частичного репрограммирования появился сравнительно недавно и сейчас активно изучается и модернизируется. К настоящему времени доказана его эффективность как в системе in vitro, так и in vivo, разработаны несколько способов доставки факторов репрограммирования и регуляции их экспрессии; появились вариации состава коктейля факторов репрограммирования, обнаружены оптимальные зоны возрастного репрограммирования и оптимальное временное окно репрограммирования.
Мы еще далеки от широкого использования этого метода в медицинской практике. Но работа в этом направлении продолжаются и в конечном итоге может привести к разработке терапевтических стратегий с целью уменьшения заболеваемости, связанной с возрастом и, таким образом, улучшения здоровья и долголетия.
Список литературы
Abad M., Mosteiro L., Pantoja C., Cañamero M., Rayon T., Ors I., Graña O., Megías D., Domínguez O., Martínez D., Manzanares M., Ortega S., Serrano M. 2013. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. V. 502. P. 340. https://doi.org/10.1038/nature12586
Alle Q., Le Borgne E., Bensadoun P., Lemey C., Béchir N., Gabanou M., Estermann F., Bertrand-Gaday C., Pessemesse L., Toupet K., Vialaret J., Hirtz C., Noël D., Jorgensen C., Casas F., Milhavet O., Lemaitre J.-M. 2021. A single short reprogramming early in life improves fitness and increases lifespan in old age. BioRxiv. V. 21. P. e13714. https://doi.org/10.1111/acel.13714
Bell C.G., Lowe R., Adams P.D., Baccarelli A.A., Beck S., Bell J.T., Christensen B.C., Gladyshev V.N., Heijmans B.T., Horvath S., Ideker T., Issa J.P.J., Kelsey K.T., Marioni R.E., Reik W. et al. 2019. DNA methylation aging clocks: challenóges and recommendations. Genome Biol. V. 20. P. 249. https://doi.org/10.1186/s13059-019-1824-y
Blagosklonny M.V. 2013. TOR-centric view on insulin resistance and diabetic complications: Perspective for endocrinologists and gerontologists. Cell Death Disease. V. 4: e964. https://doi.org/10.1038/cddis.2013.506
Bocklandt S., Lin W., Sehl M.E., Sánchez F.J., Sinsheimer J.S., Horvath S., Vilain E. 2011. Epigenetic predictor of age. PLoS One V. 6: e14821. https://doi.org/10.1371/journal.pone.0014821
Brandhorst S., Choi I.Y., Wei M., Cheng C.W., Sedrakyan S., Navarrete G., Dubeau L., Yap L.P., Park R., Vinciguerra M., Di Biase S., Mirzaei H., Mirisola M.G., Childress P., Ji L., Groshen S. et al. 2015. A Periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. V. 22. P. 86.
Brett J.O., Rando T.A. 2014. Alive and well? Exploring disease by studying lifespan. Curr. Opin. Genet. Dev. V. 26. P. 33.
Chen Y., Lüttmann F.F., Schoger E., Schöler H.R., Zelarayán L.C., Kim K.P., Haigh J.J., Kim J., Braun T. 2021. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science. V. 373. P. 80.
Childs B.G., Durik M., Baker D.J., Van Deursen J.M. 2015. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nature Medicine. V. 21. P. 1424.
Chondronasiou D., Gill D., Mosteiro L., Urdinguio R.G., Berenguer-Llergo A., Aguilera M., Durand S., Aprahamian F., Nirmalathasan N., Abad M., Martin-Herranz D.E., Stephan-Otto Attolini C., Prats N. et al. 2022. Multi-omic rejuvenation of naturally aged tissues by a single cycle of transient reprogramming. Aging Cell V. 21: e13578. https://doi.org/10.1111/acel.13578
Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weismann I.L., Rando T.A. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature V. 433. P. 760.
Cuervo A.M., Bergamini E., Brunk U.T., Dröge W., Ffrench M., Terman A. 2005. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. V. 1. P. 131. https://doi.org/10.4161/auto.1.3.2017
Dungan C.M., Figueiredo V.C., Wen Y., VonLehmden G.L., Zdunek C.J., Thomas N.T., Mobley C.B., Murach K.A., Brightwell C.R., Long D.E., Fry C.S., Kern P.A., McCarthy J.J., Peterson C.A. 2022. Senolytic treatment rescues blunted muscle hypertrophy in old mice. GeroScience V. 44. P. 1925. https://doi.org/10.1007/s11357-022-00542-2
Galkin F., Mamoshina P., Aliper A., de Magalhães J.P., Gladyshev V.N., Zhavoronkov A. 2020. Biohorology and biomarkers of aging: current state-of-the-art, challenges and opportunities. ARR. V. 60: e 60:101050. https://doi.org/10.1016/j.arr.2020.101050
Gems D., Partridge L. 2013. Genetics of longevity in model organisms: debates and paradigm shifts. Annu. Rev. Physiol. V. 75. P. 621.
Gill D., Parry A., Santos F., Okkenhaug H., Todd C.D., Hernando-Herraez I., Stubbs T.M., Milagre I., Reik W. 2022. Multi-omic rejuvenation of human cells by maturation phase transient reprogramming. Elife V. 11: e71624. https://doi.org/10.7554/eLife.71624
Gladyshev V.N., Kritchevsky S.B., Clarke S.G., Cuervo A.M., Fiehn O., de Magalhães J.P., Mau T., Maes M., Moritz R.L., Niedernhofer L.J., Van Schaftingen E., Tranah G.J., Walsh K., Yura Y., Zhang B., Cummings S.R. 2021. Molecular damage in aging. Nature Aging. V.1: 1096. https://doi.org/10.1038/s43587-021-00150-3
Guan J., Wang G., Wang J., Zhang Z., Fu Y., Cheng L., Meng G., Lyu Y., Zhu J., Li Y., Wang Y., Liuyang S., Liu B., Yang Z., He H., Zhong X., Chen Q. et al. 2022. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature. V. 605. P. 325.
Guderyon M.J., Chen C., Bhattacharjee A., Ge G., Fernandez R.A., Gelfond J.A.L., Gorena K.M., Cheng C.J., Li Y., Nelson J.F., Strong R.J., Hornsby P.J., Clark R.A., Li S. 2020. Mobilization-based transplantation of young-donor hematopoietic stem cells extends lifespan in mice. Aging Cell. V. 19: e13110. https://doi.org/10.1111/acel.13110
Haigis M.C., Yankner B.A. 2010. The aging stress response. Mol. Cell. V. 40. P. 333.
Hishida T., Yamamoto M., Hishida-Nozaki Y., Shao C., Huang L., Wang C., Shojima K., Xue Y., Hang Y., Shokhirev M., Memczak S., Sahu S.K., Hatanaka F., Ros R.R., Maxwell M. et al. 2022. In vivo partial cellular reprogramming enhances liver plasticity and regeneration. Cell Rep. V. 39: 110730. https://doi.org/10.1016/j.celrep.2022.110730
Hofmann J.W., Zhao X., De Cecco M., Peterson A.L., Pagliaroli L., Manivannan J., Hubbard G.B., Ikeno Y., Zhang Y., Feng B., Li X., Serre T., Qi W., Van Remmen H., Miller R.A., Bath K.G. et al. 2015. Reduced expression of MYC increases longevity and enhances healthspan. Cell. V. 160. P. 477.
Horvath S. 2013. DNA methylation age of human tissues and cell types. Genome Biol. V. 14. P. R115.
Hou P., Li Y., Zhang X., Liu C., Guan J., Li H., Zhao T., Ye J., Yang W., Liu K., Ge J., Xu J., Zhang Q., Zhao Y., Deng H. 2013. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. V. 341. P. 651.
Hu K. 2014. All roads lead to induced pluripotent stem cells: the technologies of iPSC generation. Stem Cells Dev. V. 23. P. 1285.
Kim Y., Jeong J., Choi D. 2020. Small-molecule-mediated reprogramming: a silver lining for regenerative medicine. Exp. Mol. Med. V. 52. P. 213.
Klawitter S., Fuchs N.V., Upton K.R., Muñoz-Lopez M., Shukla R., Wang J., Garcia-Cañadas M., Lopez-Ruiz C., Gerhardt D.J., Sebe A., Grabundzija I., Merkert S., Gerdes P., Pulgarin J.A., Bock A., et al. 2016. Reprogramming triggers endogenous L1 and Alu retrotransposition in human induced pluripotent stem cells. Nat. Commun. V. 7: 10286. https://doi.org/10.1038/ncomms10286
Koch C.M., Reck K., Shao K., Lin Q., Joussen S., Ziegler P., Walenda G., Drescher W., Opalka B., May T., Brummendorf T., Zenke M., Saric T., Wagner W. 2013. Pluripotent stem cells escape from senescenceassociated DNA methylation changes. Genome Res. V. 23. P. 248.
Koch C.M., Wagner W. 2011. Epigenetic-aging-signature to determine age in different tissues. Aging (Albany. NY). V. 3. P. 1018.
Lapasset L., Milhavet O., Prieur A., Besnard E., Babled A., Ät-Hamou N., Leschik J., Pellestor F., Ramirez J.M., De Vos J., Lehmann S., Lemaitre J.M. 2011. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. V. 25. P. 2248. https://doi.org/10.1101/gad.173922.111
Lee C., Raffaghello L., Brandhorst S., Safdie F.M., Bianchi G., Martin-Montalvo A., Pistoia V., Wei M., Hwang S., Merlino A., Emionite L., De Cabo R., Longo V.D. 2012. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. V. 4: 124ra27. https://doi.org/10.1126/scitranslmed.3003293
Lee C., Safdie F.M., Raffaghello L., Wei M., Madia F., Parrella E., Hwang D., Cohen P., Bianchi G., Longo V.D. 2010. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. V. 70. P. 1564.
Lewis-McDougall F.C., Ruchaya P.J., Domenjo-Vila E., Shin Teoh T., Prata L., Cottle B.J., Clark J.E., Punjabi P.P., Awad W., Torella D., Tchkonia T., Kirkland J.L., Ellison-Hughes G.M. 2019. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell. V. 18: e12931. https://doi.org/10.1111/acel.12931
Lin Q., Weidner C.I., Costa I.G., Marioni R.E., Ferreira M.R.P., Deary I.J., Wagner W. 2016. DNA methylation levels at individual age-associated CpG sites can be indicative for life expectancy. Aging (Albany. NY). V. 8. P. 394.
Liu X., Ouyang J.F., Rossello F.J., Tan J.P., Davidson K.C., Valdes D.S., Schröder J., Sun Y.B.Y., Chen J., Knaupp A.S., Sun G., Chy H.S., Huang Z., Pflueger J., Firas J. et al. 2020. Reprogramming roadmap reveals route to human induced trophoblast stem cells. Nature. V. 586. P. 101.
Longo V.D., Finch C.E. 2003. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. V. 299. P. 1342.
López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. 2013. The hallmarks of aging. Cell. V. 153. P. 1194.
Lu A.T., Quach A., Wilson J.G., Reiner A.P., Aviv A., Raj K., Hou L., Baccarelli A.A., Li Y., Stewart J.D., Whitsel E.A., Assimes T.L., Ferrucci L., Horvath S. 2019. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany. NY). V. 11. P. 303.
Lu Y., Brommer B., Tian X., Krishnan A., Meer M., Wang C., Vera D.L., Zeng Q., Yu D., Bonkowski M.S., Yang J.H., Zhou S., Hoffmann E.M., Karg M.M., Schultz M.B., Kane A.E., Davidsohn N. et al. 2020. Reprogramming to recover youthful epigenetic information and restore vision. Nature. V. 588. P. 124.
Ludwig F.C., Elashoff R.M. 1972. Mortality in syngeneic rat parabionts of different chronological age. Trans. N.Y. Acad. Sci. V. 34. P. 582.
Madeo F., Tavernarakis N., Kroemer G. 2010. Can autophagy promote longevity? Nat. Cell Biol. V. 12. P. 842.
Mair W., Dillin A. 2008. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. V. 77. P. 727.
Manukyan M., Singh P.B. 2014. Epigenome rejuvenation: HP1β mobility as a measure of pluripotent and senescent chromatin ground states. Sci. Rep. V. 4. P. 4789.
Marioni R.E., Shah S., McRae A.F., Chen B.H., Colicino E., Harris S.E., Gibson J., Henders A.K., Redmond P., Cox S.R., Pattie A., Corley J., Murphy L., Martin N.G., Montgomery G.W. et al. 2015. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. V. 16. P. 25.
Mertens J., Paquola A.C.M., Ku M., Hatch E., Böhnke L., Ladjevardi S., McGrath S., Campbell B., Lee H., Herdy J.R., Gonçalves J.T., Toda T., Kim Y., Winkler J., Yao J. et al. 2015. Directly reprogrammed human neurons retain aging-associated transcriptomic signatures and reveal age-related nucleocytoplasmic defects. Cell Stem Cell. V. 17. P. 705.
Miller J.D., Ganat Y.M., Kishinevsky S., Bowman R.L., Liu B., Tu E.Y., Mandal P.K., Vera E., Shim J.W., Kriks S., Taldone T., Fusaki N., Tomishima M.J., Krainc D., Milner T.A. et al. 2013. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell V. 13. P. 691.
Narasimhan S.D., Yen K., Tissenbaum H.A. 2009. Converging pathways in lifespan regulation. Curr. Biol. V. 19: R657. https://doi.org/10.1016/j.cub.2009.06.013
Nishimura T., Kaneko S., Kawana-Tachikawa A., Tajima Y., Goto H., Zhu D., Nakayama-Hosoya K., Iriguchi S., Uemura Y., Shimizu T., Takayama N., Yamada D., Nishimura K., Ohtaka M. et al. 2013. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. V. 12. P. 114.
Ocampo A., Reddy P., Martinez-Redondo P., Platero-Luengo A., Hatanaka F., Hishida T., Li M., Lam D., Kurita M., Beyret E., Araoka T., Vazquez-Ferrer E., Donoso D., Roman J. L. et al. C. 2016. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. V. 167. P. 1719.
Ohnishi K., Semi K., Yamamoto T., Shimizu M., Tanaka A., Mitsunaga K., Okita K., Osafune K., Arioka Y., Maeda T., Soejima H., Moriwaki H., Yamanaka S., Woltjen K., Yamada Y. 2014. Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation. Cell. V. 156. P.663.
Ohnuki M., Tanabe K., Sutou K., Teramoto I., Sawamura Y., Narita M., Nakamura M., Tokunaga Y., Nakamura M., Watanabe A., Yamanaka S., Takahashi K. 2014. Dynamic regulation of human endogenous retroviruses mediates factor-induced reprogramming and differentiation potential. Proc. Natl. Acad. Sci. USA. V. 111. P. 12426.
Olova N., Simpson D.J., Marioni R.E., Chandra T. 2019. Partial reprogramming induces a steady decline in epigenetic age before loss of somatic identity. Aging Cell. V. 18: e12877. https://doi.org/10.1111/acel.12877
Raffaghello L., Lee C., Safdie F.M., Wei M., Madia F., Bianchi G., Longo V.D. 2008. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. USA. V. 105. P. 8215.
Rodríguez-Matellán A., Alcazar N., Hernández F., Serrano M., Ávila J. 2020. In vivo reprogramming ameliorates aging features in dentate gyrus cells and improves memory in mice. Stem Cell Reports V. 15. P. 1056.
Roux A.E., Zhang C., Paw J., Zavala-Solorio J., Malahias E., Vijay T., Kolumam G., Kenyon C., Kimmel J.C. 2022. Diverse partial reprogramming strategies restore youthful gene expression and transiently suppress cell identity. Cell Syst. V. 13. P. 574.
Sarkar T.J., Quarta M., Mukherjee S., Colville A., Paine P., Doan L., Tran C.M., Chu C.R., Horvath S., Qi L.S., Bhutani N., Rando T.A., Sebastiano V. 2020. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat. Commun. V. 11. P. 1545.
Schiebinger G., Shu J., Tabaka M., Cleary B., Subramanian V., Solomon A., Gould J., Liu S., Lin S., Berube P., Lee L., Chen J., Brumbaugh J., Rigollet P., Hochedlinger K. et al. 2019. Optimal-Transport analysis of single-cell gene expression identifies developmental trajectories in reprogramming. Cell. V. 176. P. 928.
Schmauck-Medina T., Molière A., Lautrup S., Zhang J., Chlopicki S., Madsen H.B., Cao S., Soendenbroe C., Mansell E., Vestergaard M.B., Li Z., Shiloh Y., Opresko P.L., Egly J.M., Kirkwood T. et al. 2022. New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary. Aging (Albany. NY). V. 14: 6829. https://doi.org/10.18632/aging.204248
Shahini A., Rajabian N., Choudhury D., Shahini S., Vydiam K., Nguyen T., Kulczyk J., Santarelli T., Ikhapoh I., Zhang Y., Wang J., Liu S., Stablewski A., Thiyagarajan R., Seldeen K. et al. 2021. Ameliorating the hallmarks of cellular senescence in skeletal muscle myogenic progenitors in vitro and in vivo. Sci. Adv. V. 7: eabe5671. https://doi.org/10.1126/sciadv.abe5671
Singh P.B., Laktionov P.P., Newman A.G. 2019. Deconstructing age reprogramming. J. Biosci. V. 44. P. 106.
Smith E.D., Kaeberlein T.L., Lydum B.T., Sager J., Welton K.L., Kennedy B.K., Kaeberlein M. 2008. Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans. BMC Dev. Biol. V. 8. P. 49.
Stölzel F., Brosch M., Horvath S., Kramer M., Thiede C., Von Bonin M., Ammerpohl O., Middeke M., Schetelig J., Ehninger G., Hampe J., Bornhäuser M. 2017. Dynamics of epigenetic age following hematopoietic stem cell transplantation. Haematologica. V. 102: e321. https://doi.org/10.3324/haematol.2016.160481
Takahashi K., Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. V. 126. P. 663.
Tanabe K., Nakamura M., Narita M., Takahashi K., Yamanaka S. 2013. Maturation, not initiation, is the major roadblock during reprogramming toward pluripotency from human fibroblasts. Proc. Natl. Acad. Sci. USA. V. 110. P. 12172.
Verweij M., Van Ginhoven T.M., Mitchell J.R., Sluiter W., Den Engel S. Van, Roest H.P., Torabi E., Ijzermans J.N.M., Hoeijmakers J.H.J., De Bruin R.W.F. 2011. Preoperative fasting protects mice against hepatic ischemia/reperfusion injury: mechanisms and effects on liver regeneration. Liver Transplant. V. 17. P. 695.
Vizioli M.G., Liu T., Miller K.N., Robertson N.A., Gilroy K., Lagnado A.B., Perez-Garcia A., Kiourtis C., Dasgupta N., Lei X., Kruger P.J., Nixon C., Clark W., Jurk D., Bird T.G. et al. 2020. Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev. V. 34. P. 428.
Weidner C.I., Lin Q., Koch C.M., Eisele L., Beier F., Ziegler P., Bauerschlag D.O., Jöckel K.H., Erbel R., Mühleisen T.W., Zenke M., Brümmendorf T.H., Wagner W. 2014. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. V. 15. P. R24.
Ye J., Ge J., Zhang X., Cheng L., Zhang Z., He S., Wang Y., Lin H., Yang W., Liu J., Zhao Y., Deng H. 2016. Pluripotent stem cells induced from mouse neural stem cells and small intestinal epithelial cells by small molecule compounds. Cell Res. V. 26. P. 34.
Yousefzadeh M.J., Flores R. R., Zhu Y., Schmiechen Z.C., Brooks R.W., Trussoni C.E., Cui Y., Angelini L., Lee K.A., McGowan S.J., Burrack A.L., Wang D., Dong Q., Lu A., Sano T., O’Kelly R.D. et al. 2021. An aged immune system drives senescence and ageing of solid organs. Nature. V. 594. P. s41586.
Дополнительные материалы отсутствуют.
Инструменты
Цитология


