Цитология, 2023, T. 65, № 5, стр. 490-498
Влияние перинатальной гипоксии (асфиксии) на распределение субъединицы α1 GABAA-рецептора в неокортексe новорожденных крыс
Л. И. Хожай *
Институт физиологии им. И.П. Павлова РАН
199034 Санкт-Петербург, Россия
* E-mail: astarta0505@mail.ru
Поступила в редакцию 14.04.2023
После доработки 15.05.2023
Принята к публикации 21.06.2023
- EDN: DIJGRD
- DOI: 10.31857/S004137712305005X
Аннотация
Цель работы заключалась в исследовании распределения субъединицы α1 GABAA-рецептора в слоях неокортекса крыс в неонатальный период после воздействия гипоксии. Воздействие гипоксии на мозг новорожденных крыс осуществляли на 2-е неонатальные сут в течение 1 ч при содержании кислорода в дыхательной смеси 7.8%. Для выявления субъединицы α1 GABAA-рецептора использовали иммуногистохимическую реакцию. Содержание белка оценивали по плотности иммунного окрашивания продукта реакции в цитоплазме и отростках нейронов. Изучали соматосенсорную область неокортекса на 5 и 10 неонатальные сутки (П5, П10). Установлено, что на ранних сроках неонатального периода в неокортексе присутствует значительная популяция молодых нейронов, в цитоплазме которых иммуногистохимически выявляется субъединица α1, входящая в состав GABAA-рецептора. К концу неонатального периода у контрольных животных плотность окрашивания продукта иммуногистохимического выявления GABAAα1 в слоях неокортекса существенно повышается. Воздействие перинатальной гипоксии вызывает сокращение числа нейронов, содержащих субъединицу α1 GABAA-рецептора и значительное снижение плотности иммуногистохимического окрашивания во всех слоях неокортекса.
Известно, что в центральной нервной системе (ЦНС) нейротрансмиссия GABA (ɣ-аминомасляная кислота) опосредуется GABAA-рецепторами, при этом рецепторы, содержащие субъединицу α1, являются основными при быстрой синаптической тормозной нейропередаче (Farrant, Nusser, 2005; Mohler, 2006; Rudolph, Mohler, 2006). Этот подтип рецептора GABAAα1 составляет 40–60% всех кортикальных GABAA-рецепторов, локализующихся преимущественно на телах и дендритах главных пирамидных нейронов (Klausberger et al., 2002).
GABAA-рецепторы представлены различными структурно-функциональными подтипами, каждый из которых является гетеропентамерным протеином, чаще всего состоящим из пяти субъединиц: двух α-, двух β- и пятой субъединицы, которой может быть одна из β-, γ-, δ- или ε-субъединиц (Baumann et al., 2001; Klausberger et al., 2001). Более того, разнообразие α-, β- и γ-субъединиц дополняется несколькими их изоформами (Hevers, Lüddens, 1998).
Отличительной особенностью GABAA-рецепторов у взрослых животных является их широкая распространенность в разных отделах мозга, что, в общем, обуславливает их значительную структурно-функциональную гетерогенность. Клинические наблюдения показали, что качественные и количественные изменения состава субъединиц в GABAA-рецепторах меняют их свойства и могут лежать в основе развития неврологических и психических расстройств (Pirker et al., 2000). При патологических состояниях, таких как эпилепсия, тревожность, аутизм, а также при невротических расстройствах, связанных со стрессом, было отмечено изменение уровня экспрессии разных субъединиц GABAA-рецептора (Blatt et al., 2001; Hales et al., 2006; Fatemi et al., 2009; Delahanty et al., 2011; Hernandez, Macdonald, 2019; Feng et al., 2022; Qin et al., 2022).
В развивающемся мозге млекопитающих GABAA-рецепторы выявлены на достаточно ранних стадиях, еще до появления функциональных тормозных связей и синаптической нейротрансмиссии. В это время в переднем мозге было отмечено присутствие разных подтипов GABAA-рецептора, содержащих субъединицы α2, α4, α5, β1, β2, β3 и γ1, при этом преобладающими были α3, β2/3 и γ3. Было показано, что интенсивность экспрессии разных субъединиц GABAA-рецептора в различных структурах мозга различается и может меняться в процессе их развития (Owens et al., 1999; Pirker et al., 2000).
Известно, что воздействие гипоксии (ишемии) на ранних этапах онтогенеза оказывает существенное повреждающее действие на состояние центральной нервной системы, проявляющееся в отдаленные сроки, вызывая различные нарушения в цитоархитектонике, клеточной организации нейронов, утрате части клеток, потере нервных проекций, изменении синтеза нейротрансмиттеров и их трансмиссии (Dean et al., 2013; McClendon et al., 2014).
Вопрос о воздействии гипоксии (асфиксии) во время родов на экспрессию различных субъединиц, входящих в состав GABAA-рецептора, и в частности субъединицы α1, в литературе практически не освещен. Тем не менее детальное знание временного распределения отдельных субъединиц GABAA-рецептора в неокортексе как в норме, так и при воздействии повреждающих факторов, необходимо для правильного понимания влияния нейротрансмиссии GABA на развитие и функционирование коры в неонатальный период.
В связи с этим, задача настоящего исследования состояла в изучении распределения субъединицы α1 GABAA-рецептора и изменения ее содержания в разных слоях неокортекса у крыс в неонатальный период после воздействия гипоксии.
МАТЕРИАЛ И МЕТОДИКА
Работа выполнена на лабораторных крысах линии Wistar из ЦКП “Биоколлекция ИФ РАН” (Санкт-Петербург).
Работа проведена на модели недоношенной беременности человека, т.е. модели общей гипоксии, которую испытывают преждевременно родившиеся дети, как правило, с недоразвитой респираторной системой. Эта модель, с одной стороны, исключает смертность животных, а с другой, воспроизводит широкий спектр повреждений разных отделов мозга, возникающих при асфиксии во время преждевременных родов (Otellin et al., 2021). В работе была исследована соматосенсорная область неокортекса.
Воздействие гипоксии осуществляли на 2-е неонатальные сут. Животных помещали на 1 ч в камеру с проточной газовой смесью, содержащей 7.8% кислорода, 0.4% углекислого газа и 91.8% азота, при температуре 21.5–23.0°C и нормальном давлении (760 мм рт. ст.). Исследование проводили в неонатальный период (период новорожденности) на 5 (П5) и 10 (П10) постнатальные сутки. Животных делили на 2 группы: 1) экспериментальную (П5 и П10, n = 10 в каждом случае), подвергавшихся в специальной камере воздействию гипоксии и 2) контрольную (интактные крысы; П5 и П10, n = 10 в каждом случае).
Иммуногистохимическое выявление субъединицы α1 GABAA-рецептора. У новорожденных крыс мозг извлекали и обрабатывали по общепринятой методике: фиксировали в цинк-этанолформальдегиде на фосфатно-солевом буфере (рН 7.4), обезвоживали, заливали в парафин и готовили фронтальные серийные срезы толщиной 5–7 мкм на уровне брегмы – 0.60mm–0.40mm (по стереотаксическому атласу координатных таблиц мозга крыс в возрасте П5–П10 (Khazipov et al., 2015). Срезы помещали на предметные стекла Super frost plus gold (Menzel-Glasser, Германия).
Иммуногистохимическое выявление субъединицы α1 ионотропного GABAA-рецептора проводили с использованием кроличьих первичных поликлональных антител (anti-GABAA-receptor alpha 1 antibody; Abcam, Великобритания) в разведении 1 : 300. После проведения процедуры демаскирования белков в цитратном буфере (рН 6.1) (Dako, Дания) в течение 25 мин, срезы инкубировали в первичных антителах при температуре 4°C в течение 16 ч. В качестве вторичных антител использовали Goat anti-rabbit IgG H&L (HRP; Abcam, Великобритания). Срезы помещали во вторичные антитела на 30 мин при комнатной температуре. Для визуализации продукта реакции использовали хромоген DAB+ (Dako, Дания). Специфичность иммунной реакции проверяли при помощи негативного контроля (без первичных антител). Общий морфологический анализ проводили на срезах мозга, окрашенных по Нисслю по общепринятой методике. Срезы заключали в синтетическую среду Permaunt (Termo, США).
Статистическая оценка результатов. При проведении иммуногистохимической реакции все процедуры были стандартизированы и осуществлялись одновременно для гистологических срезов мозга, полученных от контрольных и подопытных животных обеих возрастных групп. Материал анализировали на цифровых изображениях фронтальных серийных срезов, полученных при помощи светового микроскопа Leica DME и цифровой камеры Leica EC3 (Leica, Германия).
Количество клеток, содержащих GABAAα1, оценивали на стандартной площади 0.1 мм2 (усл. ед. площади) при увеличении объектива микроскопа 100×. Анализ данных осуществляли на цифровых изображениях 17–20 гистологических срезов мозга животных каждой исследуемой возрастной группы при помощи пакетов компьютерных программ Im-ageJ (NIH, США), Origin 5.0. Статистически обработанные данные представлены как средние значения и стандартная ошибка среднего (M ± SEM).
Оценку плотности окрашивания продукта иммунной реакции (или иммунореактивности) осуществляли при помощи системы анализа изображений, включающей световой микроскоп Olympus 1 (Япония), цветную цифровую камеру Video/Zavr Standart VZ-C31Sr и программное обеспечение (для денситометрии) Видеозавр Мультиметр 2.3 (ООО АТМ-практика, Санкт-Петербург). Анализ плотности окрашивания продукта иммунной реакции проводили при увеличении объектива микроскопа 60×. Цифровые изображения срезов мозга контрольных и экспериментальных животных были получены при одинаковых параметрах освещения, насыщенности цвета и контраста. Для оценки плотности окрашивания продукта иммунной реакции выделяли участки окрашенной цитоплазмы клеток, участки проксимальных отделов окрашенных отростков, крупных гранул, которые считаются терминальными синаптическими структурами (Guthmann et al., 1998). Количественный анализ данных осуществляли на цифровых изображениях, полученных с 20 гистологических срезов мозга животных каждой исследуемой возрастной группы, при этом на каждом срезе изучали по 7–8 участков в каждом из слоев II–VI неокортекса. Обработанные данные, выраженные в усл. ед. (D – плотность окраски продукта иммунной реакции), представлены как средние значения и стандартная ошибка среднего (M ± SEM).
Для анализа и сравнения полученных результатов между разными группами животных использовали t-критерий Стьюдента и oneway ANOVA (Statistica 8.0, Statsoft Inc. США), различия считали достоверными при Р < 0.05.
РЕЗУЛЬТАТЫ
Количество нейронов, содержащих субъединицу α1 GABAА-рецептора, в неокортексе крыс в контроле и после воздействия гипоксии на стадии П5 и П10. У контрольных животных на П5 во всех слоях неокортекса число клеток, содержащих α1 GABAА-рецептора, на условной единице площади высокое. Наибольшее количество таких клеток присутствует в верхних слоях II–III (34.5 ± 0.2) (табл. 1), а в глубоких слоях IV, V и VI их количество меньше, при этом числовые значения в этих слоях очень близкие (25.5 ± 0.4, 23.1 ± 0.1 и 21.5 ± 0.7 соответственно) (табл. 1).
Таблица 1.
Число нейронов, содержащих субъединицу α1 GABAА-рецептора в слоях соматосенсорной области неокортекса крыс на 5- и 10-е неонатальные сут в контроле и после воздействия гипоксии
| Слои | Число нейронов, содержащих GABAАα1, на 1 усл. ед. площади | |||
|---|---|---|---|---|
| срок развития | ||||
| П5, контроль |
П5, гипоксия |
П10, контроль |
П10, гипоксия |
|
| II–III | 34.4 ± 0.2 | 35.0 ± 0.8 | 25.4 ± 0.9 | 28.7 ± 0.6 |
| IV | 25.5 ± 0.4 | 34.8 ± 0.6а | 22.7 ± 0.6 | 17.3 ± 0.8а |
| V | 23.1 ± 0.1 | 25.3 ± 0.2 | 18.3 ± 0.4 | 14.5 ± 0.6а |
| VI | 21.5 ± 0.7 | 20.1 ± 0.6 | 22.1 ± 0.8 | 13.5 ± 0.5а |
После воздействия гипоксии количество клеток, содержащих субъединицу α1 GABAА-рецептора, в верхних II–III и глубоких слоях V и VI остается высоким, а числовые показатели соответствуют контрольным, за исключением слоя IV, где их численность значимо выше (табл. 1).
На стадии П10 контрольных животных в глубоких слоях неокортекса IV, V и VI число клеток, содержащих α1 GABAА-рецептора, оказывается близким по значению таковому у контрольных животных на П5 (табл. 1). В верхних слоях II–III количество таких клеток значимо снижается (в 1.4 раза).
После воздействия гипоксии в верхних слоях II–III число клеток, содержащих α1 GABAА-рецептора, почти соответствует таковому в контроле (28.7 ± 0.6) (табл. 1), а в глубоких слоях IV, V и VI значимо снижается (в 1.3, 1.3 и 1.6 раза соответственно).
Таким образом, у контрольных животных на П5 во всех слоях неокортекса количество клеток, содержащих субъединицу α1 GABAА-рецептора, достаточно высокое, и они примерно равномерно распределяются по слоям неокортекса. После воздействия гипоксии численность таких клеток в слоях неокортекса не изменяется и находится в пределах контрольных значений, кроме слоя IV. К концу неонатального периода (П10) у контрольных животных в глубоких слоях количество клеток, содержащих субъединицу α1, соответствует контрольным значениям на П5, однако в верхних слоях II–III существенно снижается по сравнению с П5. Воздействие гипоксии приводит к снижению количества этих клеток в глубоких слоях в 1.3 раза.
Плотность окрашивания продукта иммунной реакции (D) при выявлении субъединицы α1 GABAA-рецептора в слоях неокортекса крыс в контроле и после воздействия гипоксии на стадии П5 и П10. У контрольных животных на П5 в слоях I, II–III, IV нейропиль развит слабо, присутствуют отдельные отростки с варикозными расширениями, имеющие иммунное окрашивание, встречаются единичные гранулы (синаптические структуры). В этих слоях в цитоплазме нейронов и отростков плотность окрашивания продукта иммунной реакции относительно высокая и равна 0.240 ± 0.003, 0.205 ± 0.006 и 0.188 ± 0.004 соответственно (табл. 2). В глубоких слоях V и VI нейропиль представлен рыхлой сетью отростков с варикозными расширениями, имеющих иммунное окрашивание. В этих слоях в цитоплазме нейронов и отростков плотность окрашивания продукта иммунной реакции ниже, чем в верхних слоях (0.152 ± 0.007 и 0.071 ± 0.005 соответственно).
Таблица 2.
Показатели оптической плотности (D) продукта иммунной реакции при выявлении субъединицы α1 G-ABAA-рецептора в слоях соматосенсорной области неокортекса крыс на 5-е и 10-е неонатальные сут в контроле и после воздействия гипоксии
| Слои неокортекса |
D, усл. ед. | |||
|---|---|---|---|---|
| сроки развития | ||||
| П5, контроль |
П5, гипоксия |
П10, контроль |
П10, гипоксия |
|
| I | 0.240 ± 0.003 | 0.166 ± 0.004а | 0.220 ± 0.003 | 0.158 ± 0.005а |
| II–III | 0.205 ± 0.006 | 0.130 ± 0.003а | 0.180 ± 0.008 | 0.116 ± 0.004а |
| IV | 0.188 ± 0.004 | 0.112 ± 0.008а | 0.206 ± 0.006 | 0.088 ± 0.007а |
| V | 0.152 ± 0.007 | 0.096 ± 0.006а | 0.178 ± 0.004 | 0.074 ± 0.008 а |
| VI | 0.071 ± 0.005 | 0.048 ± 0.008а | 0.092 ± 0.006 | 0.067 ± 0.004а |
После воздействия гипоксии во всех слоях неокортекса – I, II–III, IV, V и VI – в цитоплазме нейронов и отростков плотность окрашивания продукта иммунной реакции значительно снижается по сравнению с контролем (в 1.4, 1.6, 1.7, 1.6 и 1.5 раза соответственно) (табл. 2).
Плотность окрашивания продукта иммунной реакции (D) при выявлении субъединицы α1 GABAA-рецептора в слоях неокортекса на 10 неонатальные сутки у крыс в контроле и после воздействия гипоксии. У контрольных животных на П10 (к концу неонатального периода) в верхних слоях I, II–III в цитоплазме клеток и отростков показатели плотности окрашивания продукта иммунной реакции при выявлении субъединицы α1 по сравнению с предыдущим сроком исследования незначительно снижаются (в 1.1 раза), а в глубоких слоях IV, V и VI повышаются (в 1.1, 1.2 и 1.3 раза соответственно) (табл. 2).
После воздействия гипоксии во всех слоях неокортекса I, II–III, IV, V и VI показатели плотности окрашивания продукта иммунной реакции значимо снижаются в 1.4, 1.6, 2.3, 2.4 и 1.3 раза соответственно по сравнению с показателями в контроле (табл. 2, рис. 1).
Рис. 1.
Соматосенсорная область неокортекса крыс на 10-е неонатальные сутки в контроле (а, б) и после воздействия перинатальной гипоксии (в, г). Иммуногистохимическое выявление субъединицы α1 GABAA-рецептора. a, в – Слои II–III; б, г – слой V. Отмечается снижение оптической плотности продукта иммунной реакции в слоях неокортекса II—III и V после воздействия гипоксии по сравнению с контролем. Стрелки – иммуномеченые нейроны. Увел. об. 100×.
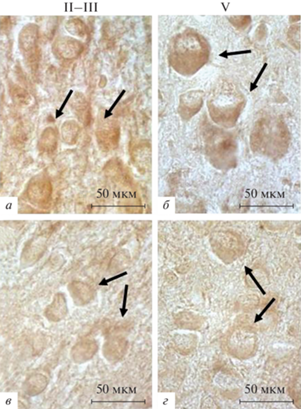
Таким образом, на П5 у контрольных животных в цитоплазме нейронов и отростков во всех слоях неокортекса определяются достаточно высокие показатели плотности окрашивания продукта реакции при иммуногистохимическом выявлении GABAAα1, а после воздействия гипоксии во всех слоях коры они заметно снижаются. На П10 у контрольных животных показатели плотности окрашивания продукта реакции во всех слоях неокортекса постепенно повышаются по сравнению с П5. Воздействие гипоксии приводит к существенному снижению показателей плотности окрашивания продукта реакции на GABAAα1 по сравнению с контролем. К концу неонатального периода тенденция к снижению этих показателей продолжается.
ОБСУЖДЕНИЕ
Проведенная работа показала, что у контрольных крыс в неокортексе, преимущественно в верхних слоях, на ранних сроках неонатального периода (П5) присутствует значительное число нейронов, содержащих субъединицу α1 GABAА-рецептора. В глубоких слоях этих нейронов меньше и они примерно равномерно распределяются по слоям. Плотность окрашивания продукта иммунной реакции при выявлении субъединицы α1 GABAA-рецептора в цитоплазме и отростках клеток на этом раннем сроке развития в верхних слоях I–IV также выше, чем в глубоких слоях V, VI. К концу неонатального периода (П10) в верхних слоях II–III число клеток, содержащих субъединицу α1 GABAА-рецептора, существенно снижается, в остальных слоях их число не изменяется и соответствует таковому на П5. При этом плотность окрашивания продукта иммунной реакции при выявлении GABAАα1 в верхних слоях снижается, а в более глубоких слоях неокортекса с увеличением возраста постепенно повышается. Полученные результаты дают основание предполагать, что в глубоких слоях, которые формируются раньше, определенное число нейронов, содержащих GABAАα1, появляется в первые дни после рождения и сохраняется до конца неонатального периода. Колебание численности таких нейронов в верхних слоях, образующихся последними, объясняется тем, что процессы миграции, дифференцировки и созревания нейронов, их окончательное расположение в этих слоях, а также формирование нейропиля завершаются только к концу неонатального периода (Marin-Padila, 1992; Ignacio et al., 1995; Hou et al., 2011).
У крыс перед рождением в переднем мозге было отмечено присутствие субъединиц α2, α5, β2 и β3 рецептора GABAА, при этом субъединица α1 не выявлялась иммуногистохимическим методом. Было показано, что в процессе развития существует временнáя смена экспрессии некоторых субъединиц. Так, экспрессия субъединицы α2 к концу пренатального периода значительно снижается, а уровень экспрессии субъединицы α1 после рождения, напротив, начинает повышаться, т.е. на самых ранних сроках неонатального периода (П1–П3) происходит смена интенсивности экспрессии субъединицы α2 и субъединицы α1 в большинстве отделов мозга и неокортексе (Fritschy et al., 1994; Owens et al., 1999). Существует утверждение, что смена состава субъединиц, входящих в основные подтипы GABAА-рецепторов, совпадает с началом активации синаптической нейропередачи (Owens et al., 1999; Dvorzhak et al., 2010)
Выявленное в нашей работе присутствие популяции нейронов, содержащих GABAАα1, и постепенное повышение уровня содержания субъединицы α1 во время неонатального периода (от П5 до П10) согласуется с этими данными.
Следует отметить, что у взрослых крыс рецепторы к нейротрансмиттерам, как к возбуждающим, так и тормозным могут синтезировать астроциты (Schousboe, 2016). Поэтому необходимо остановиться на этом вопросе. У грызунов после рождения миграция нейронов в верхние слои неокортекса продолжается и заканчивается только к П7. Одновременно с этим в первую постнатальную неделю предшественники нейронов меняют программу дифференцировки и начинают продуцировать глиальные клетки, которые пролиферируют до конца первого месяца жизни, увеличиваясь в количестве (Namihira et al., 2009). Созревание астроцитов начинается в конце второй (П12–14) и в течение третьей постнатальной недели (П21), совпадая с процессами активного синаптогенеза. В это время астроциты в контакте с нейронами синтезируют ряд белков, связанных с транспортом и высвобождением пресинаптических пузырьков, с формированием постсинаптического уплотнения, секретируют синапс-регулирующие белки и рецепторы к нейротрансмиттерам (Blue, Parnavelas, 1983; Bandeira et al., 2009; Li et al., 2010; Buosi et al., 2017). К концу третьей постнатальной недели каждый астроцит занимает определенное положение в слоях коры (Bushong et al., 2004; Buosi et al., 2017).
Необходимо подчеркнуть, что в процессе развития в неокортексе созревание тормозных сетей происходит позже возбуждающих, поскольку на ранних стадиях развития высвобождение GABA приводит к возбуждению нейронов из-за сдвига равновесного потенциала ионов хлора, и только в течение второй постнатальной недели происходит переход функции GABA от возбуждения к торможению. В это время устанавливается баланс между возбуждением и торможением, являющегося, как известно, важным условием для нормального развития мозга. Кроме того, роль астроцитов в формировании и функционировании тормозных синапсов изучена не так широко, как формирование возбуждающих синапсов (Zhang et al., 2011; Ge et al., 2012; Farhy-Tselnicker, Allen, 2018).
В связи с выше изложенным, можно полагать, что все визуализируемые нами GABAAα1-иммунопозитивные клетки представляют собой только нейроны, поскольку выявление астроцитов, экспрессирующих GABAAα1, на сроках П5–10 является маловероятным.
Результаты показали, что после воздействия гипоксии в течение первой неонатальной недели в слоях неокортекса количество нейронов, содержащих GABAАα1, по сравнению с контролем почти не изменяется, за исключением слоя IV, где их число значимо повышается (в 1.4 раза). К концу неонатального периода в верхних слоях II–III число этих нейронов соответствует контрольному значению, а в остальных слоях существенно сокращается (в 1.3–1.6 раза). Что касается плотности иммунного окрашивания продукта реакции при выявлении GABAАα1 после воздействия гипоксии, то на П5 она во всех слоях значительно снижается, несмотря на то, что количество нейронов, содержащих GABAАα1, на этом сроке развития соответствует контрольным значениям. К концу неонатального периода тенденция к снижению плотности продукта реакции в слоях неокортекса продолжается и значения этого показателя становятся еще ниже.
Установлено, что в ранние сроки неонатального периода активация GABAΑ-рецепторов, опосредуемая GABA, приводит к сильной деполяризации мембраны незрелых молодых нейронов посредством открытия ионных каналов, оттока ионов хлора и значительного снижения его относительно высокой внутриклеточной концентрации, которая поддерживается в незрелых клетках. Эти события приводят к возникновению возбуждающего потенциала действия в нейронах. После чего в неокортексе у новорожденных грызунов и человека появляются коррелированные паттерны спонтанной сетевой активности нейронов, которая является ключевым регулятором процессов, происходящих на субклеточном уровне, таких как высвобождение хлоридов, экспрессия транспортеров и рецепторов к GABA, изменение функциональных свойств GABAА-рецептора и GABAергический синаптогенез. Спонтанная нейронная активность также способствует росту и дифференцировке отростков – дендритов и аксонов – и необходима для формирования зрелых функциональных нейронных сетей (Gulledge, Stuart, 2003; Morita et al., 2006; Holmgren et al., 2010; Deidda et al., 2015; Marchetti et al., 2015).
Вероятно, воздействие перинатальной гипоксии на ранних этапах развития может вызывать нарушение активации GABAА-рецепторов, снижение деполяризующего и возбуждающего эффекта GABA на ранних сроках, изменения спонтанной нейронной активности, что в результате и может приводить к резкому снижению экспрессии субъединицы α1 GABAА-рецептора и сокращению в глубоких слоях коры числа нейронов, содержащих GABAАα1. Последнее может быть вызвано и нарушением миграции молодых нейронов в глубокие слои, которая еще продолжается на начальных сроках неонатального периода, а также задержкой формирования аксональных и дендритных отростков, способствующих миграции, или частичной утратой клеток (Inada et al., 2011). Не исключено, что воздействие гипоксии на ранних этапах неонатального развития может изменять в нейронах внутреннюю программу появления и созревания разных подтипов GABAА-рецепторов (Paysan, Fritschy, 1998; Bartolini et al., 2013). Выявленное в данной работе существенное снижение содержания GABAАα1 на ранних сроках неонатального периода, вызванное воздействием перинатальной гипоксии, имеет отдаленный характер действия.
В предыдущей нашей работе, выполненной на взрослых половозрелых животных (П90), переживших воздействие перинатальной гипоксии, было показано, что число нейронов, содержащих GABAАα1, к этому возрасту сокращалось на 37% по сравнению с контролем, а в слоях неокортекса в цитоплазме нейронов имело место уменьшение содержания субъединицы α1 (Хожай, Отеллин, 2020), т.е. после воздействия перинатальной гипоксии изменение синтеза рецепторного белка сохраняется в течение продолжительного периода времени в онтогенезе. Такие нарушения, как правило, приводят к изменению нейротрансмиссии GABA и снижению тормозных эффектов.
Все больше данных свидетельствует о важности GABAергической тормозной дисфункции в патогенезе абсансной детской эпилепсии (Hassan et al., 2022), являющейся сложным генетическим неврологическим расстройством, характеризующимся генерализованными приступами. Одну из возможных причин развития этого типа эпилепсии связывают с изменением нейротрансмиссии GABA и нарушением тормозных эффектов. У грызунов на модели абсансной эпилепсии было обнаружено значительное снижение количества несинаптических и синаптических GABAAα1-рецепторов (на 18 и 12.2% соответственно) в неокортексе и гиппокампе (Hassan et al., 2022). Считают, что в основе одного из механизмов развития эпилепсии лежит нарушение экспрессии GABAА-рецептора, вызванное мутациями генов, кодирующих субъединицы α1 и γ2 (Baulac et al., 2001; Adotevi et al., 2021; Jafarian et al., 2021).
Мутации вызывают не только изменение экспрессии субъединиц GABAА-рецептора, но и нарушение динамики нейротрансмиссии GABA на ранних сроках, что, в свою очередь, может влиять на развитие и созревание нейронов, синапсов и экспрессию самих GABAА-рецепторов (Owens, Kriegstein, 2002). Эти нарушения считаются первичными генетическими дефектами. Однако часто возникают вторичные изменения трансмиссии GABA, происходящие после воздействия ряда повреждающих факторов, в том числе гипоксии, и играющие важную роль в патогенезе эпилепсии. Более того, показано, что в соматосенсорной области коры у грызунов и человека значительное снижение синтеза субъединицы α1 GABAА-рецептора или ее отсутствие приводит к изменению экспрессии других коэкспрессирующихся субъединиц GABAA-рецептора (Kralic et al., 2002). Это предполагает неизбежную перестройку пентамерной структуры самого рецептора, что также будет приводить к изменению динамики процесса торможения.
Таким образом, исследование показало, что у контрольных животных на ранних сроках неонатального периода присутствует значительная популяция нейронов, содержащих субъединицу α1 GABAA-рецептора, с появлением которой связывают начало процессов созревания и становления неокортекса. К концу неонатального периода в цитоплазме нейронов всех слоев неокортекса существенно повышается содержание GABAAα1. Воздействие перинатальной гипоксии вызывает сокращение как числа нейронов, содержащих GABAAα1, так и значительное снижение содержания рецепторного белка. Тормозные эффекты, опосредованные GABAA-рецептором, содержащим субъединицу α1, являются неотъемлемой частью физиологического баланса возбуждения–торможения в нейронных сетях, и любое изменение в структуре или функционировании комплексов субъединиц GABAA-рецептора может быть основой патогенеза многих заболеваний центральной нервной системы (Crunelli et al., 2020; Hassan et al., 2022). Полученные нами данные могут быть полезными для дальнейших исследований специфических изменений в нейротрансмиссии GABA, имеющих важное значение для разработки новых методов профилактики и лечения неврологических расстройств, возникающих у детей, переживших асфиксию во время родов.
Список литературы
Хожай Л.И., Отеллин В.А. 2020. Экспрессия субъединицы α1 ионотропного ГАМКА рецептора в неокортексе крыс после перинатальной гипоксии. Журн. эвол. Биохим. физиол. Т. 56. № 2. С. 75. (Khozhai L.I., Otellin V.A. 2020. Expression of the α1 subunit of the ionotropic GABAA-receptor in the neocortex of rats after perinatal hypoxia. J. Evol. Biochem. Physiol. 2020. V. 56. № 2. P. 75.) https://doi.org/10.31857/S0044452920020072
Adotevi N., Su A., Peiris D., Hassan M., Leitch B. 2021. Altered neurotransmitter expression in the corticothalamocortical network of an absence epilepsy model with impaired feedforward inhibition. Neurosci. V. 467. P. 73. https://doi.org/10.1016/j.neuroscience.2021.05.024
Bartolini G., Ciceri G., Marin O. 2013. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron. V. 79. P. 849. https://doi.org/10.1016/j.neuron.2013.08.014
Baulac S., Huberfeld G., Gourfinkel-An I., Mitropoulou G., Beranger A., Prud’homme J.-F., Baulac M., Brice A., Bruzzone R., Le Guern E. 2001. First genetic evidence of GABAA-receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nat. Genet. V. 28. P. 46. https://doi.org/10.1038.4267/10608/2027
Baumann S.W., Baur R., Sigel E. 2001. Subunit arrangement of γ-aminobutyric acid type A receptors. J. Biol. Chemi. V. 276. P. 36275. https://doi.org/10.1074/jbc.M105240200
Blatt G.J., Fitzgerald C.M., Guptill J.T., Booker A.B., Kemper T.L., Bauman M.L. 2001. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J. Autism Dev. Disorders. V. 31. P. 537. https://doi.org/10.1023/a:1013238809666
Bandeira F., Lent R., Herculano-Houzel S. 2009. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc. Natl. Acad. Sci. USA. V 106. P. 14108. https://doi.org/10.1073/pnas.0804650106
Blue M.E., Parnavelas J.G. 1983. The formation and maturation of synapses in the visual cortex of the rat. I. Qualitative analysis. Neurocytol. V. 12. P. 599. https://doi.org/10.1007/BF01181526
Buosi A.S., Matias I., Araujo A.P.B., Batista C., Gomes F.C.A. 2017. Heterogeneity in synaptogenic profile of astrocytes from different brain regions. Mol. Neurobiol. V. 55. P. 751. https://doi.org/10.1007/s12035-016-0343-z
Bushong E.A., Martone M.E., Ellisman M.H. 2004. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int. J. Dev. Neurosci. V. 22. 73. https://doi.org/10.1016/j.ijdevneu.2003.12.008
Crunelli V., Lorincz M.L., McCafferty C., Lambert R.C., Leresche N., Di Giovanni G., David F. 2020. Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures. Brain. V. 143. P. 2341. https://doi.org/10.1093/brain/awaa072
Dean J., McClendon E., Hansen K., Azimi-Zonooz A., Chen K., Riddle A., Gong X., Sharifnia E., Hagen M., Ahmad T., Leigland L., Hohimer A., Kroenke C., Back S. 2013. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci. Transl. Med. V. 5: 168ra7. https://doi.org/10.1126/scitranslmed.3004669
Deidda G., Allegra M., Cerri C., Naskar S., Bony G., Zunino G., Bozzi Y., Caleo M., Cancedda L. 2015. Early depolarizing GABA controls critical-period plasticity in the rat visual cortex. Nat. Neurosci. V. 18. P. 87. https://doi.org/10.1038/nn.3890
Delahanty R.J., Kang J.Q., Brune C.W., Kistner E.O., Courchesne E., Cox N.J., Cook E.H., Macdonald R.L., Sutcliffe J.S. 2011. Maternal transmission of a rare GABRB3 signal peptide variant is associated with autism. Mol. Psychiatry. V. 16. P. 86. https://doi.org/10.1038/mp.2009.118
Dvorzhak A., Myakhar O., Unichenko P., Kirmse K., Kirischuk S. 2010. Estimation of ambient GABA levels in layer I of the mouse neonatal cortex in brain slices. J. Physiol. V. 588. P. 2351. https://doi.org/10.1113/jphysiol.2010.187054
Farhy-Tselnicker I., Allen N.J. 2018. Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural. Dev. V. 13. P. 7. https://doi.org/10.1186/s13064-018-0104-y
Farrant M., Nusser Z. 2005. Variations on an inhibitory theme: Phasic and tonic activation of GABAA-receptors. Nat. Rev. Neurosci. V. 6. P. 215. https://doi.org/10.1038/nrn1625
Fatemi S.H., Reutiman T.J., Folsom T.D., Thuras P.D. 2009. GABAA-receptor down regulation in brains of subjects with autism. J. Autism Dev. Disorders. V. 39. P. 223. https://doi.org/10.1007/s10803-008-0646-7
Feng Y., Wei Z.-H., Liu C., Li G.-Y., Qiao X.-Z., Gan Y.-J., Zhang C.-C., Deng Y.-C. 2022. Genetic variations in GABA metabolism and epilepsy. Seizure. V. 101. P. 22. https://doi.org/10.1016/j.seizure.2022.07.007
Fritschy J.-M., Paysan J., Enna A., Mohler H. 1994. J. Neurosci. V. 14. P. 5302. https://doi.org/10.1523/JNEUROSCI.14-09-05302.1994
Ge W.-P., Miyawaki A., Gage F.H., Jan Y.N., Jan L.Y. 2012. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. V. 484. P. 376. https://doi.org/10.1038/nature10959
Gulledge A.T., Stuart G.J. 2003. Excitatory actions of GABA in the cortex. Neuron. V. 37. P. 299. https://doi.org/10.1016/s0896-6273(02)01146-7
Guthmann A., Fritschy J.M., Ottersen O.P., Torp R., Herbert H. 1998. GABA, GABA transporters, GABAA-receptor subunits and GAD mRNAs in the rat parabrachial and Kolliker-Fuse nuclei. J. Comp. Neurol. V. 400. P. 229.
Hales T.G., Deeb T.Z., Tang H., Bollan K.A., King D.P., Jhnson S.J., Connolly C.N. 2006. An asymmetric contribution to gamma-aminobutyric type A receptor function of a conserved lysine within TM2-3 of alpha1, beta2, and gamma2 subunits. J. Biol. Chem. V. 281. P. 17034. https://doi.org/10.1074/jbc.M603599200
Hassan M., Adotevi N.K., Leitch B. 2022. Altered GABAA-receptor expression in the primary somatosensory cortex of a mouse model of genetic absence epilepsy. Intern. J. Mol. Sci. V. 23. P. 15685. https://doi.org/10.3390/ijms232415685
Hernandez C.C., Macdonald R.L. 2019. A structural look at GABAA-receptor mutations linked to epilepsy syndromes. Brain Res. V. 1714. P. 234. https://doi.org/10.1016/j.brainres.2019.03.004
Hevers W., Lüddens H. 1998. The diversity of GABAA-receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol. Neurobiol. V. 18. P. 35. https://doi.org/10.1007/BF02741459
Holmgren C.D., Mukhtarov M., Malkov A.E., Popova I.Y., Bregestovski P., Zilberter Y. 2010. Energy substrate availability as a determinant of neuronal resting potential, GABA signaling and spontaneous network activity in the neonatal cortex in vitro. J. Neurochem. V. 112. P. 900. https://doi.org/10.1111/j.1471-4159.2009.06506.x
Hou J., Eriksen N., Pakkenberg B. 2011. The temporal pattern of postnatal neurogenesis found in the neocortex of the Göttingen minipig brain. Neuroscience. V. 195. P. 176. https://doi.org/10.1016/j.neuroscience.2011.08.025
Ignacio M.P., Kimm E.J., Kageyama G.H., Yu J., Robertson R.T. 1995. Postnatal migration of neurons and formation of laminae in rat cerebral cortex. Anat. Embryol. (Berl). V. 191. P. 89. https://doi.org/10.1007/BF00186782
Inada H., Watanabe M., Uchida T., Ishibash H., Wake H., Nemoto T., Yanagawa Y., Fukuda A., Nabekura J. 2011. GABA regulates the multidirectional tangential migration of GABAergic interneurons in living neonatal mice. PLoS One. V. 6: e27048. https://doi.org/10.1371/journal.pone.0027048
Jafarian M., Mousavi S.M.M., Rahimi S., Ghaderi P.F., Lotfinia A.A., Lotfinia M., Gorji A. 2021. The effect of GABAergic neurotransmission on the seizure-related activity of the laterodorsal thalamic nuclei and the somatosensory cortex in a genetic model of absence epilepsy. Brain Res. V. 1757. P. 47 304. https://doi.org/10.1016/j.brainres.2021.147304
Khazipov R., Zaynutdinova D., Ogievetsky E., Valeeva G., Mitrukhina O., Manent J.-B., Represa A. 2015. Atlas of the postnatal rat brain in stereotaxic coordinates. Front. Neuroanat. V. 9. P. 161. https://doi.org/10.3389/fnana.2015.00161
Klausberger T., Roberts J.D., Somogyi P. 2002. Cell type- and input-specific differences in the number and subtypes of synaptic GABAA-receptors in the hippocampus. J. Neurosci. V. 22. P. 2513. https://doi.org/10.1523/JNEUROSCI.22-07-02513.2002
Klausberger T., Sarto I., Ehya N., Fuchs K., Furtmuller R., Mayer B., Huck S., Sieghart W. 2001. Alternate use of distinct intersubunit contacts controls GABAA-receptor assembly and stoichiometry. J. Neurosci. V. 21. P. 9124. https://doi.org/10.1523/JNEUROSCI.21-23-09124.2001
Kralic J.E., Korpi E.R., O’Buckley T.K., Homanics G.E., Morrow A.L. 2002. Molecular and pharmacological characterization of GABAA-receptor alpha1 subunit knockout mice. J. Pharmacol. Exp. Ther. V. 302. P. 1037. https://doi.org/10.1124/jpet.102.036665
Li M., Cui Z., Niu Y., Liu B., Fan W., Yu. D., Deng J. 2010. Synaptogenesis in the developing mouse visual cortex. Brain Res Bull. V. 81. P. 107. https://doi.org/10.1016/j.brainresbull.2009.08.028
Marchetti C., Tabak J., Chub N., O’Donovan M.J., Rinzel J. 2015. Modeling spontaneous activity in the developing spinal cord using activity-dependent variations of intracellular chloride. J. Neurosci. V. 25. P. 3601. https://doi.org/10.1523/JNEUROSCI.4290-04.2005
Marín-Padilla M. 1992. Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitectonics: a unifying theory. J. Comp. Neurol. V. 321. P. 223. https://doi.org/10.1002/cne.903210205
McClendon E., Kevin C., Gong X., Sharifnia E., Hagen M., Cai V., Shaver D., Riddle A., Dean J.M., Gunn A.J., Mohr C., Kaplan J.S., Rossi D.J., Kroenke C.D., Hohimer A.R., Back S.A. 2014. Prenatal cerebral ischemia triggers dysmaturation of caudate projection neurons. Ann. Neurol. V. 75. P. 508. https://doi.org/10.1002/ana.24100
Mohler H. 2006. GABAA-receptor diversity and pharmacology. Cell Tissue Res. V. 26. P. 505. https://doi.org/10.1007/s00441-006-0284-3
Morita K., Tsumoto K., Aihara K. 2006. Bidirectional modulation of neuronal responses by depolarizing GABAergic inputs. J. Biophys. V. 90. P. 1925. https://doi.org/10.1529/biophysj.105.063164
Namihira M., Kohyama J., Semi K., Sanosaka T., Deneen B., Taga T., Nakashima K. 2009. Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev. Cell. V. 16. P. 245. https://doi.org/10.1016/j.devcel.2008.12.014
Owens D.F., Liu X., Kriegstein A.R. 1999. Changing properties of GABAA-receptor-mediated signaling during early neocortical development. J. Neurophysiol. V. 82. P. 570. https://doi.org/10.1152/jn.1999.82.2.570
Owens D.F., Kriegstein A.R. 2002. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. V. 3. P. 715. https://doi.org/10.1038/nrn919
Otellin V.A., Khozhai L.I., Shishko T.T., Vershinina E.A. 2021. Nucleolar ultrastructure in neurons of the rat neocortical sensorimotor area during the neonatal period after perinatal hypoxia and its pharmacological correction. J. Evol. Biochem. Physiol. V. 57. P. 1251. https://doi.org/10.1134/S0022093021060053
Paysan J., Fritschy J.M. 1998. GABAA-receptor subtypes in developing brain. Actors or spectators? Perspect. Dev. Neurobiol. V. 5. P. 179.
Pirker S., Schwarzer C., Wieselthaler A., Sieghart W., Sperk G. 2000. GABAA-receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neurosci. V. 101. P. 815. https://doi.org/10.1016/s0306-4522(00)00442-5
Qin X., Pan X.-Q., Huang S.-H., Zou J.-X., Zheng Z.-H., Liu X.-X., You W.-J., Liu Z.-P., Cao J.-L., Zhang W.-H., Pan B.-X. 2022. GABAA-receptor hypofunction in the amygdala-hippocampal circuit underlies stress-induced anxiety. Sci. Bull. (Beijing). V. 67. P. 97. https://doi.org/10.1016/j.scib.2021.09.007
Rudolph U., Mohler H. 2006. GABA-based therapeutic approaches: GABAA-receptor subtype functions. Curr. Opin. Pharmacol. V. 6. P. 18. https://doi.org/10.1016/j.coph.2005.10.003
Schousboe A. 2016 Metabolic signaling in the brain and the role of astrocytes in control of glutamate and GABA neurotransmission. Neurosci. Lett. V. 689. P. 11. https://doi.org/10.1016/j.neulet.2018.01.038
Zhang Z., Jiao Y.-Y., Sun Q.-Q. 2011. Developmental maturation of excitation and inhibition balance in principal neurons across four layers of somatosensory cortex. Neurosci. V. 74. P. 10.
https://doi.org/10.1016/j.neuroscience.2010.11.045
Дополнительные материалы отсутствуют.


