Агрохимия, 2023, № 9, стр. 28-36
Совместное воздействие штамма PGPB Pseudomonas plecoglossicida 2,4-D и гуминовых веществ на рост, содержание фотосинтетических пигментов и фитогормонов в растениях пшеницы в условиях засухи
А. В. Феоктистова 1, *, М. Д. Тимергалин 1, Т. В. Рамеев 1, С. П. Четвериков 1
1 Уфимский институт биологии – обособленное структурное подразделение
Уфимского федерального исследовательского центра РАН
450054 Уфа, просп. Октября, 69, Россия
* E-mail: feoktistova.arisha@yandex.ru
Поступила в редакцию 01.03.2023
После доработки 08.04.2023
Принята к публикации 14.06.2023
- EDN: VEZILD
- DOI: 10.31857/S0002188123090065
Аннотация
Показано накопление сырой массы растений пшеницы при обработке штаммом бактерий Pseudomonas plecoglossicida 2,4-D и гуминовыми веществами при дефиците почвенной влаги. Стимуляция роста растений связана с активацией роста корня, что приводило к увеличению индекса азотного баланса и концентрации хлорофилла в побегах обработанных растений. Обнаруженное увеличение концентрации хлорофилла в растениях, обработанных P. plecoglossicida 2,4-D, коррелировало со снижением содержания абсцизовой кислоты в побегах, а у растений, обработанных гуматами – с увеличением цитокининов в побегах. Более высокая эффективность обработки растений комбинацией бактерий и гуминовых веществ, чем любым из них в отдельности, может быть связана с аддитивным эффектом этих обработок на гормональный баланс.
ВВЕДЕНИЕ
Ростстимулирующие бактерии (PGPВ) стали очень популярными благодаря своей способности активировать рост растений и повышать их продуктивность [1–3]. Ростостимулирующее действие бактерий проявляется не только в благоприятных условиях произрастания растений, а чаще всего при эдафических стрессах [4–6]. Многочисленные работы посвящены повышению засухоустойчивости растений за счет поддержания их роста под влиянием PGP-бактерий [7]. Поиск путей повышения продуктивности растений в условиях засухи имеет фундаментальное и практическое значение, поскольку засушливые регионы широко распространены, а недостаток влаги приводит к критическим потерям урожая [8]. В недавних работах показано, что PGP-бактерии из рода Bacillus улучшали прорастание семян, активировали рост проростков и усвоение калия растениями сои [9], Azospirillum lipoferum усиливали ветвление корней растений кукурузы при избытке почвенной влаги [10], Bacillus cereus смягчали тепловой стресс у томатов [11], Rhizobium leguminosarum и Paenibacillus polymyxa повышали продуктивность растений пшеницы при засолении почвы [12].
Наряду с PGPB для повышения продуктивности растений в условиях засухи также применяются гуминовые вещества [13]. Гуминовые вещества (ГВ), состоящие из гумусовых кислот (гуминовые и фульвокислоты), являются продуктами разложения органического вещества, их извлекают из бурого угля, торфа и других источников [14–16]. Применение гумата калия при подкормке семян и внесении в почву повышало продуктивность и качество волокна хлопчатника [17], гуминовые кислоты стимулировали рост побегов огурца за счет усиления регуляции генов, кодирующих аквапорины, тем самым увеличивая гидравлическую проводимость корней [18]. Многочисленные исследования рекомендуют использовать в сельском хозяйстве в качестве биостимуляторов либо PGP-бактерии, либо гуминовые вещества. Однако работ, рассматривающих действие PGPB в сочетании с ГВ, чрезвычайно мало. Недостаток информации по этой теме приводит к тому, что некоторые важные аспекты остаются нерешенными. Было показано, что ГВ могут стимулировать рост бактерий [19], но остается неясным, является ли этот эффект более важным для роста растений, чем прямое действие гуматов на сами растения.
Способность PGP-бактерий синтезировать растительные гормоны и влиять на концентрацию фитогормонов в растениях рассматривается как один из основных механизмов, стимулирующих рост растений [3, 20, 21]. Недавно было показано, что комбинация PGP-бактерий и ГВ увеличивала концентрацию ауксинов в корнях растений пшеницы, тем самым стимулируя их ветвление [22]. Однако влияние такой обработки на концентрацию других гормонов не изучали. Между тем цитокинины и абсцизовая кислота являются гормонами растений, которые чаще, чем ауксины, участвуют в реакции растений на засуху [23, 24]. Учитывая все сказанное выше, цель работы – изучение влияния бактерий, стимулирующих рост растений, и гуминовых веществ на содержание хлорофилла, индекс азотного баланса, концентрацию цитокининов и абсцизовой кислоты в растениях пшеницы, выращенных в условиях засухи.
МЕТОДИКА ИССЛЕДОВАНИЯ
Источником гуминовых веществ послужил бурый уголь Тюльганского месторождения в Оренбургской обл. РФ. Уголь смешивали с 0.1 М КОН в соотношении 1 : 10 и извлекали ГВ в течение 1 сут. Осадок удаляли центрифугированием при 12 000 об./мин в течение 10 минут. Затем к надосадочной жидкости по каплям добавляли 0.1 М HCl до достижения рН 3.0 и перемешивали в течение 1 мин. Фракции фульвовой кислоты (надосадочная жидкость) и гуминовой кислоты (осадок) отделяли центрифугированием при 12 000 об./мин в течение 10 мин, затем осадок промывали холодной дистиллированной водой. Образцы гуминовой и фульвовой кислот высушивали при 60°C. Для обработки растений использовали 0.1% водный раствор совместного экстракта гуминовой и фульвокислот.
В работе использовали штамм бактерий Pseudomonas plecoglossicida 2,4-D, описанный в статье [25], способный накапливать индолил-3-уксусную кислоту (ИУК) в питательных средах [26]. Бактерии для обработки растений культивировали в течение 3-х сут в жидкой питательной среде Кинг Б [22]. Количество клеток в культурах определяли путем последовательного разведения среды Кинг Б агар-агаром (15 г/л) и последующего подсчета количества колониеобразующих единиц (КОЕ). Бактериальную культуру разводили стерильной водой для получения раствора для опрыскивания растений, содержащего (1.0 ± 0.5) × × 108 КОЕ/мл.
Эксперименты проводили с мягкой яровой пшеницей (Triticum aestivum L., сорт Кинельская юбилейная). Растения выращивали на светоплощадке при 14-часовом фотопериоде, температуре 23–25/18°C день/ночь и освещении 400 мкмоль м–2 с–1 фитолампами Osram fluora L36/W77 (Мюнхен, Германия). Семена пшеницы стерилизовали в 2%-ном растворе гипохлорита натрия в течение 10 мин, затем семена промывали дистиллированной водой и проращивали в течение 3-х сут. Проростки сажали в горшки с песком. Песок использовали из-за отсутствия в нем ГВ. Контрольные варианты эксперимента (нормальные условия) поливали, ежедневно поддерживая уровень влажности 50–60% от общей влагоемкости песка, дефицит воды – на уровне 20–30%.
Обработку растений суспензией бактерий и/или гуматами проводили через 3 сут путем опрыскивания.
Содержание гормонов определяли методом иммуноферментного анализа через 3 сут после обработки. Для этого побеги и корни гомогенизировали и экстрагировали 80%-ным этиловым спиртом. Спиртовой экстракт выпаривали до водного остатка, центрифугировали и отбирали аликвоты надосадочной жидкости для анализа. Очистку и концентрирование АБК проводили по модифицированной схеме с уменьшением объема [5]. Цитокинины концентрировали на колонке (картридж С-18) и разделяли с помощью тонкослойной хроматографии [27, 28]. Гормоны подвергали иммуноанализу с использованием соответствующих специфических антител.
Концентрацию хлорофилла и индекс азотного баланса (NBI) измеряли с помощью портативного анализатора растений Dualex Scientific+ (Force-A, Париж, Франция) на 7-е сут после обработки растений, а показатели роста (масса побегов и корней, длина побега) оценивали через 2 нед.
Данные выражены в виде средних, которые были рассчитаны при всех обработках с использованием программы MS Excel. Значимые различия между средними были проанализированы с помощью однофакторного дисперсионного анализа ANOVA и критерия Дункана. Данные обработаны с использованием программного обеспечения Statistica 10 (Statsoft, Москва, Россия).
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
В отсутствие обработок засуха значительно подавляла рост растений пшеницы, что проявлялось в торможении роста побегов (рис. 1а) и в снижении массы как побегов, так и корней по сравнению с хорошо обеспеченными водой контрольными растениями (рис. 1б). В условиях дефицита воды инокуляция растений пшеницы бактерией, либо обработка ГВ, примененная по отдельности или в комбинации, стимулировали линейный рост побегов, так что их длина не отличалась от длины хорошо обеспеченных водой растений. Не было никакой разницы в длине побега между растениями, обработанными только бактерией или гуминовыми веществами, однако комбинация бактерий с ГВ была более эффективной, что проявлялось в наибольшей длине побега.
Рис. 1.
Длина побегов (а), масса побегов и корней (б) растений пшеницы через 14 сут после обработки штаммом бактерий Pseudomonas plecoglossicida 2,4-D (бактерия), гуминовыми веществами (ГВ) и их комбинацией (бактерия + ГВ) в условиях нормального полива (полив) и дефицита воды (засуха). Статистически отличающиеся средние отмечены разными буквами, p ≤ 0.05. То же на рис. 2, 3. n = 15 (ANOVA, Duncan’s test).
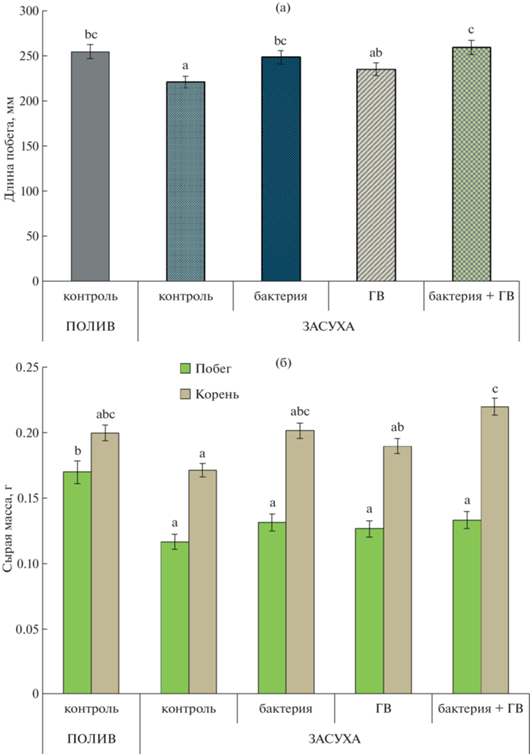
Масса побегов растений в условиях засухи была меньше, чем растений, не испытывавших недостаток влаги, и увеличение массы побегов во всех вариантах обработки было статистически незначимым. Вызванное засухой снижение массы корней было меньше, чем снижение массы побега. Тем не менее, масса корней контрольных растений, не обработанных ни бактериями, ни гуматами, снижалась в условиях засухи по сравнению с хорошо обеспеченными водой растениями. Была выявлена относительная активация роста корней, которая проявлялась в увеличении соотношения масс корень : побег, это хорошо известная адаптивная реакция на дефицит воды, позволяющая оптимизировать поиск воды растениями в почве и ее поглощение в условиях засухи.
Обработка растений пшеницы бактериями и ГВ повышала массу корней в условиях дефицита воды до уровня растений, не испытывавших ее дефицит. Ранее было показано, что ростстимулирующий эффект штамма бактерий P. plecoglossicida 2,4-D обусловлен его способностью продуцировать ауксины и увеличивать их концентрацию в корнях, тем самым увеличивая массу корней [22], поскольку известно, что этот гормон стимулирует рост боковых корней [29]. Гуминовые вещества также увеличивали концентрацию ауксинов в корнях [16]. Был обнаружен аддитивный эффект в комбинации ГВ и бактерий по сравнению с применением их по отдельности, и это могло быть связано с увеличением продукции бактериального ауксина, индуцируемого гуминовыми веществами, тем самым способствуя активации роста корня [22].
Концентрация хлорофилла снижалась при дефиците воды (рис. 2а), в то время как все обработки растений увеличивали концентрацию хлорофилла до уровня контрольных, хорошо обеспеченных водой растений. Не было никакой существенной разницы в концентрации хлорофилла между растениями, обработанными либо бактериями, либо ГВ, примененными отдельно, но их комбинация привела к значительно более высокой концентрации пигмента.
Рис. 2.
Концентрация хлорофилла (а) и индекс азотного баланса (б) в листьях растений пшеницы через 7 сут после обработки. n = 30 (ANOVA, Duncan’s test).
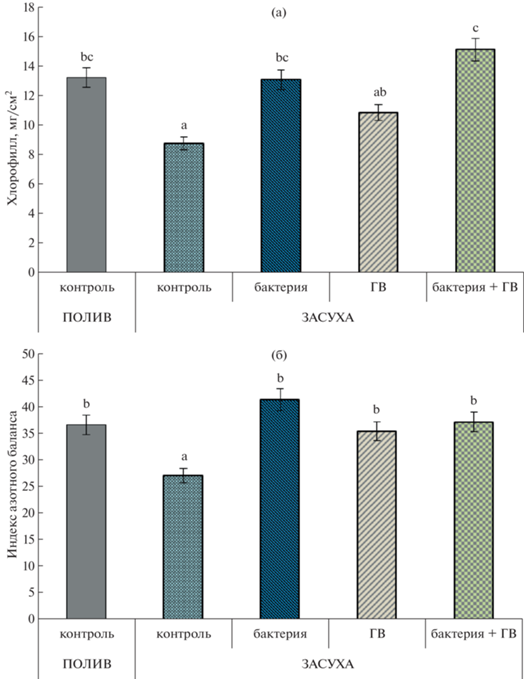
У растений, не обработанных ни ГВ, ни бактериями, индекс азотного баланса в листьях уменьшался в условиях засухи, в то время как каждая обработка и их комбинация увеличивали этот показатель до уровня контрольных растений (рис. 2б).
Тенденция к накоплению АБК в побегах растений, испытавших дефицит воды, по сравнению с контролем была статистически незначимой (рис. 3а). Концентрация АБК в побегах растений, обработанных ГВ, была значительно больше, чем в растениях, обработанных бактериями (отдельно или в комбинации с ГВ). Дефицит воды увеличивал концентрацию АБК в корнях контрольных растений (не обработанных ни бактериями, ни ГВ), а также в корнях растений, обработанных гуматами. Бактериальная обработка (отдельно или в комбинации с ГВ) предотвращала накопление АБК, вызванное засухой.
Рис. 3.
Концентрации АБК (а) и цитокининов (б) в побегах и корнях растений пшеницы через 3 сут после обработки. n = 9 (ANOVA, Duncan’s test).
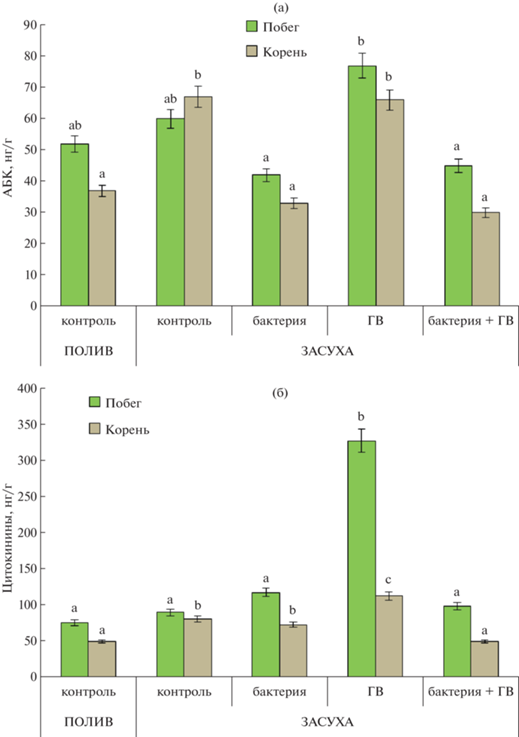
Концентрация цитокининов в побегах была значительно больше только при обработке гуматами (рис. 3б). Все остальные варианты (включая контроль в условиях засухи) существенно не влияли на концентрацию цитокининов в побегах. Засуха увеличила концентрацию цитокининов в корнях всех растений, за исключением тех, которые обрабатывали комбинацией гуматов с бактериями. Концентрация цитокининов была самой высокой в корнях растений, обработанных только гуминовыми веществами.
Повышенный индекс азотного баланса соотносился с концентрацией хлорофилла в листьях пшеницы под влиянием обработок бактерией и гуматами. Была обнаружена высокая корреляция между концентрацией хлорофилла и массой растения (r = 0.85). Увеличение концентрации хлорофилла было наиболее выраженным в растениях, обработанных только бактериями и их комбинацией с гуматами. Интересно, что больший эффект этих вариантов был связан со снижением концентрации АБК в побегах растений. Известно, что АБК участвует в ускоренной деградации хлорофилла [30], и следовательно, снижение концентрации этого гормона может способствовать повышению концентрации хлорофилла. Снижение концентрации АБК было обнаружено как в побегах, так и в корнях растений, обработанных бактериями либо отдельно, либо в комбинации с ГВ. Известно, что некоторые бактерии способны катаболизировать АБК, тем самым снижая концентрацию этого гормона в обработанных растениях [31]. Снижение концентрации АБК обнаружено в растениях, инокулированных P. plecoglossicida 2,4-D, что свидетельствовало о способности этого бактериального штамма катаболизировать АБК.
Содержание АБК в листьях растений, обработанных только гуматами, не снижалось по сравнению с контрольными растениями, подвергнутыми засухе, а концентрация хлорофилла при этом повышалась. Вероятно, это было связано с накоплением цитокининов в побегах растений, обработанных гуматами (рис. 3б). Цитокининоподобная активность была обнаружена у различных гуминовых веществ [32], и обработка растений пшеницы ГВ увеличила концентрацию цитокинина в побегах [16]. Известно, что цитокинины действуют как антагонисты АБК, предотвращая деградацию хлорофилла [33]. Этим объясняется повышенная концентрация хлорофилла в листьях растений, обработка которых гуматами повышала концентрацию цитокининов. Тем не менее, концентрация хлорофилла в растениях, обработанных ГВ, была меньше, чем в растениях, обработанных комбинацией ГВ с бактериями. Этот эффект может быть объяснен высоким содержанием АБК в побегах растений пшеницы, обработанных гуминовыми веществами. Тем не менее, возможно и другое объяснение. Корни растений, обработанных ГВ, характеризовались высокой концентрацией цитокининов, в то время как известно, что эти гормоны подавляют рост корней [34–36]. Таким образом, повышенная концентрация цитокининов в растениях, обработанных гуматами, является очевидной причиной обнаруженного снижения массы корней растений. Интересно, что концентрация цитокинина не была повышена в корнях растений, обработанных комбинацией гуминовых веществ и бактерий. Это может быть объяснено способностью этого штамма продуцировать ауксины [25, 26], которые, как известно, повышают активность цитокининоксидаз [37], тем самым подавляя накопление цитокининов.
ЗАКЛЮЧЕНИЕ
Таким образом, было выявлено накопление сырой массы растений пшеницы при обработке их P. plecoglossicida 2,4-D и гуминовыми веществами в условиях засухи. Это может найти применение в сельскохозяйственной практике, поскольку известно, что скорость роста коррелирует с продуктивностью растений. Было показано, что стимулирование роста растений связано с активацией роста корней, что приводило к увеличению индекса азотного баланса и концентрации хлорофилла в обработанных растениях. Повышение концентрации хлорофилла также было связано со снижением концентрации АБК в растениях, обработанных бактериями, и увеличением концентрации цитокининов в побегах, обработанных ГВ. Повышенная эффективность обработки растений комбинацией бактерий и гуматов, чем любым из них по отдельности, могла быть связана с дополнительным действием этих обработок на гормональный баланс растений пшеницы.
Список литературы
Ruzzi M., Aroca R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture // Sci. Hortic. 2015. V. 196. P. 124–134. https://doi.org/10.1016/j.scienta.2015.08.042
Backer R., Rokem J.S., Ilangumaran G., Lamont J., Praslickova D., Ricci E., Subramanian S., Smith D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture // Front. Plant Sci. 2018. V. 9. P. 1473. https://doi.org/10.3389/fpls.2018.01473
Kudoyarova. G., Arkhipova T., Korshunova T., Bakaeva M., Loginov O., Dodd I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses // Front. Plant Sci. 2019. V. 10. P. 1368. https://doi.org/10.3389/fpls.2019.01368
Richardson A.E., Barea J.M., Mc Neill A.M., Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms // Plant Soil. 2009. V. 321. P. 305–339. https://doi.org/10.1007/s11104-009-9895-2
Kudoyarova G.R., Vysotskaya L.B., Arkhipova T.N., Kuzmina L.Y., Galimsyanova N.F., Sidorova L.V., Gabbasova I.M., Melentiev A.I., Veselov S.Y. Effect of auxin producing and phosphate solubilizing bacteria on mobility of soil phosphorus, growth rate, and P acquisition by wheat plants // Acta Physiol. Plant. 2017. V. 39. P. 253. https://doi.org/10.1007/s11738-017-2556-9
Meena V.S., Mauryaa B.R., Verma J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? // Microbiol. Res. 2014. V. 169. P. 337–334. https://doi.org/10.1016/j.micres.2013.09.003
Islam M.R., Sultana T., Joe M.M., Yim W., Cho J.-C., Sa T. Nitrogen-fixing bacteria with multiple plant growth-promoting activities enhance growth of tomato and red pepper // J. Basic Microbiol. 2013. V. 53. P. 1004–1015. https://doi.org/10.1002/jobm.201200141
Asari S., Tarkowská D., Rolčík J., Novák O., David Palmero D.V., Bejai S., Meijer J. Analysis of plant growth-promoting properties of Bacillus amyloliquefaciens UCMB5113 using Arabidopsis thaliana as host plant // Planta. 2017. V. 245. P. 15–30.
Bakhshandeh E., Gholamhosseini M., Yaghoubian Y., Pirdashti H. Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress // Plant Growth Regul. 2020. V. 90. P. 123–136. https://doi.org/10.1007/s10725-019-00556-5
Czarnes S., Mercier P.-E., Lemoine D.G., Hamzaoui J., Legendre L. Impact of soil water content on maize responses to the plant growth-promoting rhizobacterium Azospirillum lipoferum CRT1 // J. Agro. Crop Sci. 2020. V. 206. P. 505–516. https://doi.org/10.1111/jac.12399
Mukhtar T., Rehman S., Smith D., Sultan T., Seleiman M.F., Alsadon A.A. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling // Sustainability. 2020. V. 12. P. 2159. https://doi.org/10.3390/su12062159
El-Sayed S.Y.S., Hagab R.H. Effect of organic acids and plant growth promoting rhizobacteria (PGPR) on biochemical content and productivity of wheat under saline soil conditions // Middle East J. Agric. Res. 2020. V. 9. P. 227–242. https://doi.org/10.36632/mejar/2020.9.2.2
Shen J., Guo M., Wang Y., Yuan X., Wen Y., Song X., Dong S., Guo P. Humic acid improves the physiological and photosynthetic characteristics of millet seedlings under drought stress // Plant Signal. Behav. 2020. V. 15. № 8. P. 1774212. https://doi.org/10.1080/15592324.2020.1774212
Canellas L.P., Olivares F.L., Aguiar N.O., Jones D.L., Nebbioso A., Mazzei P. Humic and fulvic acids as biostimulants in horticulture // Sci. Hortic. 2015. V. 196. P. 15–27. https://doi.org/10.1016/j.scienta.2015.09.013
Olaetxea M., De Hita D., Garcia C.A., Fuentes M., Baigorri R., Mora V. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root and shoot-growth // Appl. Soil Ecol. 2017. V. 123. P. 521–537. https://doi.org /https://doi.org/10.1016/j.apsoil.2017.06.007
Nazarov A.M., Garankov I.N., Tuktarova I.O., Salmanova E.R., Arkhipova T.N., Ivanov I.I., Feoktistova A.V., Prostyakova Z.G., Kudoyarova G.R. Hormone balance and shoot growth in wheat (Triticum durum Desf.) plants as influenced by sodium humates of the granulated organic fertilizer // Agricult. Biol. 2020. V. 55. P. 945–955.
Ullah A., Ali M., Shahzad K., Ahmad F., Iqbal S., Rahman M.H.U., Ahmad S., Iqbal M.M., Danish S., Fahad S., Alkahtani J. Impact of seed dressing and soil application of potassium humate on cotton plants productivity and fiber quality // Plants. 2020. V. 9. P. 1444. https://doi.org/10.3390/plants9111444
Olaetxea M., Mora V., Bacaicoa E., Garnica M., Fuentes M., Casanova E., Zamarreño A.M., Iriarte J.C., Etayo D., Ederra I. Abscisic acid regulation of root hydraulic conductivity and aquaporin gene expression is crucial to the plant shoot growth enhancement caused by rhizosphere humic acids // Plant Physiol. 2015. V. 169. P. 2587–2596. https://doi.org/10.1104/pp.15.00596
Tikhonov V.V., Yakushev A.V., Zavgorodnyaya Y.A., Byzov B.A., Demin V.V. Effects of humic acids on the growth of bacteria // Euras. J. Soil Sci. 2010. V. 43. P. 305–313. https://doi.org/10.1134/S1064229310030087
Verbon E.H., Liberman L.M. Beneficial microbes affect endogenous mechanisms controlling root development // Trends Plant Sci. 2016. V. 21. P. 218–229. https://doi.org/10.1016/j.tplants.2016.01.013
Cueva-Yesquén L.G., Goulart M.C., Attili de Angelis D., Nopper Alves M., Fantinatti-Garboggini F. Multiple plant growth-promotion traits in endophytic bacteria retrieved in the vegetative stage from passionflower // Front. Plant Sci. 2021. V. 11. P. 621740. https://doi.org/10.3389/fpls.2020.621740
Feoktistova A., Bakaeva M., Timergalin M., Chetverikova D., Kendjieva A., Rameev T., Hkudaygulov G., Nazarov A., Kudoyarova G., Chetverikov S. Effects of humic substances on the growth of Pseudomonas plecoglossicida 2,4-D and wheat plants inoculated with this strain // Microorganisms. 2022. V. 10. P. 1066. https://doi.org/10.3390/microorganisms10051066
Hai N.N., Chuong N.N., Tu N.H.C., Kisiala A., Hoang X.L.T., Thao N.P. Role and regulation of cytokinins in plant response to drought stress // Plants (Basel). 2020. V. 9. P. 422. https://doi.org/10.3390/plants9040422
Muhammad Aslam M., Waseem M., Jakada B.H., Okal E.J., Lei Z., Saqib H.S.A., Yuan W., Xu W., Zhang Q. Mechanisms of abscisic acid-mediated drought stress responses in plants // Inter. J. Mol. Sci. 2022. V. 23. P. 1084. https://doi.org/10.3390/10.3390/ijms23031084
Chetverikov S.P., Sharipov D.A., Korshunova T.Y., Loginov O.N. Degradation of perfluorooctanyl sulfonate by strain Pseudomonas plecoglossicida 2,4-D // Appl. Biochem. Microbiol. 2017. V. 53. P. 533–538. https://doi.org/10.1134/S0003683817050027
Bakaeva M., Kuzina E., Vysotskaya L., Kudoyarova G., Arkhipova T., Rafikova G., Chetverikov S., Korshunova T., Chetverikova D., Loginov O. Capacity of Pseudomonas strains to degrade hydrocarbons, produce auxins and maintain plant growth under normal conditions and in the presence of petroleum contaminants // Plants. 2020. V. 9. P. 379. https://doi.org/10.3390/plants9030379
Vysotskaya L.B., Korobova A.V., Veselov S.Y., Dodd I.C., Kudoyarova G.R. ABA mediation of shoot cytokinin oxidase activity: assessing its impacts on cytokinin status and biomass allocation of nutrient deprived durum wheat // Funct. Plant Biol. 2009. V. 36. P. 66–72.
Kudoyarova G.R., Melentiev A.I., Martynenko E.V., Arkhipova T.N., Shendel G.V., Kuzmina L.Y., Dodd I.C., Veselov S.Yu. Cytokinin producing bacteria stimulate amino acid deposition by wheat roots // Plant Physiol. Biochem. 2014. V. 83. P. 285–291. https://doi.org/10.1016/j.plaphy.2014.08.015
Nacry P., Canivenc G., Muller B., Azmi A., Onckelen H.V., Rossignol M., Doumas P. A role for auxin redistribution in the response of the root system architecture to phosphate starvation in Arabidopsis // Plant Physiol. 2005. V. 138. P. 2061–2074. https://doi.org/10.1104/pp.105.060061
Yang J., Worley E., Udvardi M. A NAP-AAO3 regulatory module promotes chlorophyll degradation via aba biosynthesis in Arabidopsis leaves // Plant Cell. 2014. V. 26. P. 4862–4874. https://doi.org/10.1105/tpc.114.133769
Belimov A.A., Dodd I.C., Safronova V.I., Dumova V.A., Shaposhnikov A.I., Ladatko A.G., Davies W.J. Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth // Plant Physiol. Biochem. 2014. V. 74. P. 84–91. https://doi.org/10.1016/j.plaphy.2013.10.032
Pizzeghello D., Francioso O., Ertani A., Muscolo A., Nardi S. Isopentenyladenosine and cytokinin-like activity of different humic substances // J. Geochem. Explor. 2013. V. 129. P. 70–75.
Hönig M., Plíhalova L., Husičkova A., Nisler J., Doležal K. Role of cytokinins in senescence, antioxidant defence and photosynthesis // Inter. J. Mol. Sci. 2018. V. 19. P. 4045. https://doi.org/10.3390/ijms19124045
Korobova A.V., Akhiyarova G.R., Veselov S.Y., Kudoyarova G.R., Fedyaev V.V., Farkhutdinov R.G. Participation of nitrate sensor NRT1.1 in the control of cytokinin level and root elongation under normal conditions and nitrogen deficit // Mosc. Univ. Biol. Sci. Bull. 2019. V. 74. P. 221–226. https://doi.org/10.3103/S0096392519040072
Werner T., Nehnevajova E., Köllmer I., Novak O., Strnad M., Krämer U., Schmülling T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and Tobacco // Plant Cell. 2010. V. 22. P. 3905–3920. https://doi.org/10.1105/tpc.109.072694
Liu S., Strauss S., Adibi M., Mosca G., Yoshida S., Ioio R.D., Runions A., Andersen T.G., Grossmann G., Huijser P., Smith R.S., Tsiantis M. Cytokinin promotes growth cessation in the Arabidopsis root // Curr. Biol. 2022. V. 32. P. 1974–1985. https://doi.org/10.1016/j.cub.2022.03.019
Jones B.J., Ljung K. Auxin and cytokinin regulate each other’s levels via a metabolic feedback loop // Plant Signal. Behav. 2011. V. 6. P. 901–904. https://doi.org/10.4161/psb.6.6.15323
Дополнительные материалы отсутствуют.


