Почвоведение, 2023, № 12, стр. 1522-1561
Супрагляциальные почвы и почвоподобные тела: разнообразие, генезис, функционирование (обзор)
Н. С. Мергелов a, *, С. В. Горячкин a, Э. П. Зазовская a, c, Д. В. Карелин a, Д. А. Никитин a, b, С. С. Кутузов a, d
a Институт географии РАН
119017 Москва, Старомонетный пер., 29, Россия
b Почвенный институт им. В.В. Докучаева
119017 Москва, Пыжевский пер., 7, стр. 2, Россия
c Центр прикладных изотопных исследований, Университет штата Джорджия-Атенс
GA30602 Атенс, Ривербенд роэд, 120, США
d Центр полярных и климатических исследований Берда, Университет штата Огайо
OH43210 Колумбус, Кармак роэд, 1090, США
* E-mail: mergelov@igras.ru
Поступила в редакцию 17.07.2023
После доработки 07.08.2023
Принята к публикации 07.08.2023
- EDN: DQOASG
- DOI: 10.31857/S0032180X23601494
Аннотация
В XXI в. ледники стали рассматривать как отдельный биом, который приобрел особое значение в современном мире отступающего льда. В обзоре систематизированы результаты последних исследований органо-минеральных образований на ледниках, их разнообразия, генезиса, функционирования и биосферной роли. Ставится вопрос о возможности вовлечения супрагляциальных (наледниковых) органо-минеральных образований в круг объектов почвоведения, а супрагляциальная зона рассматривается как область формирования почвенных и почвоподобных тел, биогеохимические процессы в которых влияют на ледниковый биом и окружающие его ландшафты. Типизация супрагляциальных органо-минеральных образований с позиции почвоведения выявила в них признаки почвенных процессов: аккумуляции и стабилизации органического вещества (ОВ), его гетеротрофной трансформации, образования темноцветного гумифицированного ОВ и накопления остаточных твердофазных продуктов функционирования in situ, агрегации мелкозема и его биогенного оструктуривания, биохимического выветривания. Среди супрагляциальных образований предложено выделять предпочвы, в том числе эфемерные образования, почвоподобные тела и почвы во льду и снеге, метастабильные почвоподобные тела на криоконите и почвы с микропрофилями под моховыми сообществами на льду, а также относительно стабильные почвы с макропрофилями на мелкоземисто-обломочных отложениях с подстиланием ледников и льда, уже прекратившего движение. Легкодоступное водорастворимое ОВ, накопившееся и трансформировавшееся, в том числе в супрагляциальных почвах и почвоподобных телах, оказывает существенное влияние на перигляциальную зону, приводя к резервуарному и прайминг эффектам. Фундаментальное значение исследований супрагляциальных органо-минеральных систем велико для понимания эволюции экосистем на Земле и для построения моделей поверхностных образований внеземных тел с обширной криосферой. Супрагляциальное почвообразование – это также модельный объект для исследования формирования почв вне ледников в условиях постоянного поступления внешних органических и минеральных компонентов, вклад которых вне ледников не менее значителен, но замаскирован полиминеральным субстратом самих почв и почвообразующих пород.
ВВЕДЕНИЕ
История ледников на Земле насчитывает как минимум 2.9 млрд лет. Об этом свидетельствуют древнейшие ископаемые морены – тиллиты кратона Каапвааль в Южной Африке [26, 249]. Ледники и скопления минерального материала на их поверхности, вероятно, возникли практически синхронно, так как дисперсные продукты выветривания пород с легкостью переносятся ветром или гравитационными процессами и аккумулируются на льду. Микроорганизмы стали осваивать терригенные породы как минимум 3.2–2.6 млрд л. н. [50, 52, 103, 241], поэтому с самого начала на поверхности ледников, в так называемой наледниковой или супрагляциальной зоне, наряду с минеральными могли накапливаться и биогенные компоненты. В связи с этим можно предположить, что супрагляциальные органо-минеральные системы могут являться очень древними образованиями. Уже на первых этапах существования ледников они могли влиять на их энергетический баланс за счет изменения альбедо и регулировать биогеохимические процессы в супрагляциальной зоне и за ее пределами. Значение супрагляциальных органо-минеральных систем существенно возрастало во времена масштабных похолоданий как в палеопротерозое (гуронская гляциоэра ~2.4–2.2 млрд л. н.), так и в неопротерозое (криогений ~0.72–0.64 млрд л. н.), когда оледенения охватывали все континенты и достигали уровня моря в тропических широтах [79, 186, 233]. Дискуссия о масштабах докембрийских оледенений продолжается [102, 113, 124], но последние палеоклиматические модели показывают, что даже в условиях, близких к Snowball Earth, на континентах никогда не было недостатка в свободных ото льда территориях [50, 101, 139], а аккумулировавшаяся на поверхности льда вулканическая и континентальная пыль могла существенно снижать альбедо [32]. Такие безледные пространства вероятно были схожи с Сухими долинами современной Антарктиды и служили источниками мелкозема. Этому есть свидетельства в осадочной летописи криогения в виде лёсситов – сохранившихся в консолидированном состоянии эоловых отложений перигляциальных обстановок [78].
Палеонтологические данные указывают на то, что несколько групп фотоавтотрофных эукариот, например, красные и зеленые водоросли [57, 88, 125, 126], появившиеся до криогения (т.е. ранее 0.72 млрд л. н.), пережили всепланетарные оледенения. В качестве рефугиумов при масштабных оледенениях наряду с гидротермальными ландшафтами, озерами и почвами безледных “оазисов” наподобие антарктических, также могли выступать абляционные ледниковые поверхности, покрытые органо-минеральными седиментами (см. ссылки в обзоре [101]). Супрагляциальная зона, по-видимому, была самым большим по площади рефугиумом для докембрийских организмов, включая протисты [62]. Организмы должны были адаптироваться к жизни в криосфере, циклам замораживания–оттаивания, высокой ультрафиолетовой нагрузке и крайне лимитированной первичной продукции органического вещества [232]. Авторы [253] обосновывают роль холодных ледниковых обстановок в эволюции наземных растений и их дальнейшей экспансии на суше, а также возможность возврата от многоклеточных форм к одноклеточным у некоторых водорослей в связи с адаптациями к условиям на ледниках. Ледники, покрытые мелкоземисто-обломочным материалом, могли выступать в качестве рефугиумов для сосудистых растений во время четвертичных оледенений [83]. Некоторые исследователи рассматривают супрагляциальные седименты как модельные объекты для понимания условий, в которых могли существовать микроорганизмы на древней Земле, а также в качестве потенциальных аналогов супрагляциальных образований на внеземных телах, включая Марс и спутник Сатурна Титан [106, 196, 231].
Таким образом, полученные в последние годы палеонтологические и палеоклиматические данные указывают на важное значение органо-минеральных седиментов на ледниках для эволюции как отдельных организмов, так и экосистем на Земле. Сейчас мы стали современниками драматического таяния ледников, усиленного, в том числе за счет темноцветного органо-минерального материала на их поверхности, что как никогда актуализирует исследование генезиса такого материала, его разнообразия и структуры, развития в нем почвенных процессов, а также влияния на почвы и почвенные покровы, возникающие после отступания ледников.
При площади оледенения в 18 горных регионах континентальной части России 3480 км2 большинство ледников ускоренно отступают, потеряв за XX и начало XXI вв. десятки процентов площади [123]. В частности, с 1960 по 2014 гг. на Кавказе освободилось ото льда 480 км2 [230], ледники Эльбруса за период 1997–2017 гг. потеряли 11% площади (14 км2) [129], на Алтае с 1950-х гг. до 2008 г. ледники сократились на 27% [21]. Ледники Российской Арктики занимают в общей сложности 51592 км2, и их ежегодная общая потеря массы в 2002–2016 гг. составляла около 11 Гт в год [245]. Такое отступание ледников уже давно привлекло почвоведов для изучения закономерностей и скоростей формирования почв на ледниковом материале различного возраста [9].
По нашей оценке, за последние два десятилетия международными коллективами опубликовано более 300 работ, посвященных супрагляциальным образованиям, включая криокониты (от греч. kryos – холод и konis – пыль) – темноцветные, тонкодисперсные и хорошо агрегированные органо-минеральные седименты на ледниках, которые были описаны А. Норденшельдом более 150 л. н. В это число входит несколько крупных обзоров [37, 64, 97, 98, 187].
Пионерные работы Глазовской [6, 7] по эоловым отложениям на ледниках Тянь-Шаня были выполнены в составе экспедиций Института географии АН СССР и Казахстанского филиала АН СССР за десятилетия до бума супрагляциальных исследований. Уже тогда была подтверждена возможность биогенного преобразования эолового наледникового мелкозема in situ, выявлено его обогащение органическим веществом за счет развития цианобактерий и водорослей, а также формирование прочных крупитчатых агрегатов. М.А. Глазовская указывала на переотложение такого мелкозема в ближней перигляциальной зоне и не исключала возможности происхождения лёссовидных суглинков, а также покровных суглинков Русской равнины из эоловых наледниковых отложений.
При пока еще значительной площади оледенений в России мы плохо представляем разнообразие и площади супрагляциальных органо-минеральных седиментов. Отсутствует их систематическое изучение, а такой термин, как “криоконит” до последнего времени в русскоязычной научной литературе употреблялся редко.
В настоящее время опубликованы данные о водорослевых сообществах и криоконитах на леднике Аккем на Алтае [225], леднике 31 на Сунтар-Хаята [224, 226], появились данные по микроморфологии, геохимии и биохимическим свойствам супрагляциального мелкозема [2, 29, 31, 127, 128, 131] и устойчивым к антибиотикам бактериям на ледниках Кавказа [147], гранулометрическому составу криоконита с о. Большевик архипелага Северная Земля [28], радиоэкологическим и геохимическим свойствам криоконита с архипелага Новая Земля [153], потокам парниковых газов с участков ледников Кавказа и Алтая, покрытых криоконитом и другим супрагляциальным материалом [14].
Супрагляциальные системы антарктических и низкоширотных ледников также стали активно изучаться. Установлено, что в отличие от горных и арктических областей супрагляциальный органо-минеральный материал в Антарктиде оттаивает не каждый год, а возраст органического вещества, состав микробных сообществ, первичная продукция, дыхание, прирост биомассы в криоконите существенно отличаются [3, 44, 45, 55, 68, 84, 85, 145, 173, 191, 192, 205, 232, 243]. Супрагляциальные системы в районах российских антарктических станций пока слабо изучены.
В последние годы некоторые исследователи [3, 24, 30, 151, 175] стали рассматривать супрагляциальные органо-минеральные системы как почвоподобные стратифицированные образования, а также как источник обогащенного биогенными элементами материала, который поступает с ледника в перигляциальную зону и играет роль локального “катализатора” первичного почвообразования на освободившихся ото льда олиготрофных субстратах [13, 27, 162].
Пока нет глобальных оценок, какие площади занимают почвоподобные системы в снеге и льду, но, учитывая, что ледники покрывают около 10% поверхности суши [37], а постоянный и сезонный снег до 35% [154], эти площади очень значительные, даже сейчас – в период сокращающейся криосферы. Супрагляциальные почвоподобные тела могут быть рассмотрены с позиций теории экстремального почвообразования [11, 12], так как они формируются при низких температурах с продолжительным темным периодом зимой (большая часть льда сосредоточена в полярных областях) и контрастных им относительно теплых условиях при интенсивном солнечном излучении летом. Населяющие их сообщества проходят через суточные, сезонные и годовые циклы замерзания–оттаивания, испытывают смену аэробных и анаэробных обстановок, высокий уровень ультрафиолетового излучения, существуют в условиях нестабильного субстрата, лимитированного пула питательных элементов, периодически высокой обводненности и могут быть погребены под снежным покровом даже в период таяния.
Считается, что там, где заканчивается ледник, начинаются почвы. Новые междисциплинарные исследования супрагляциальных систем позволили почвоведам осознать, что там, где начинается ледник, почвы не заканчиваются (точнее не обязательно заканчиваются). Конечно, на поверхности ледника нет полноценного почвенного покрова, но в настоящий момент известно, что это область, где реализуются некоторые процессы, характерные для почв, и формируются объекты, которые могут быть отнесены как минимум к почвоподобным телам. Пока концепция супрагляциального почвообразования не сформулирована: не описано разнообразие органо-минеральных систем на ледниках с позиции почвоведения, нет понимания какие объекты на ледниках следует относить именно к почвам или почвоподобным телам, а какие таковыми не являются, что отличает наледниковые почвы от почв вне ледников, каковы их морфотипы, процессы формирования, абсолютный возраст и время пребывания, траектории эволюции и биосферные функции.
Цель настоящего обзора – обобщить современные представления о происхождении, разнообразии и трансформации органо-минеральных тел на поверхности ледников, выявить среди них потенциальные объекты почвоведения, а также описать связи супрагляциальных процессов и начальных этапов почвообразования в ближних приледниковых обстановках.
УСЛОВИЯ ДЛЯ ПОЧВООБРАЗОВАНИЯ НА ЛЕДНИКЕ
Такие условия возникают с появлением твердофазных примесей на поверхности ледника и формированием здесь гетерогенных систем из льда, снега, фирна, талой воды и примесных органических, минеральных и органо-минеральных компонентов. Компоненты взаимодействуют между собой, и из-за специфики среды их существенная часть переходит в водорастворимую форму. По выражению классика изучения супрагляциальных образований, Н. Такеючи, у ледников существует “светлая” (лед–снег–фирн) и “темная” сторона. Сложность процессов на “темной” стороне ледников, т.е. в примесном темноцветном материале, по-видимому, сопоставима с таковой в других наземных системах, например в почвах. Поверхность ледника – это отнюдь не чистая зона, а так же, как почва – среда для развития организмов, фильтр для многочисленных привнесенных соединений и критическая зона на стыке взаимодействия гидросферы, атмосферы, литосферы и педосферы.
В гляциологии, в качестве наиболее важной функции наледниковых твердофазных примесей рассматривается их влияние на отражательную способность поверхности – альбедо. Такое воздействие отмечалось еще Норденшельдом [163, 164] и Нансеном [161]. Примеси усиливают прямое положительное приповерхностное радиационное воздействие, ускоряющее таяние [158, 159, 244], и называются “light-absorbing impurities” с разными вариантами перевода на русский как “светопоглощающие примеси” или “светопоглощающие частицы” [1]. Первый вариант представляется точнее, так как “частицы” могут включать крупные фрагменты и скопления органического и минерального материала, а также отдельные организмы. По сравнению с массой и объемом льда и снега эти компоненты являются именно примесными.
Светопоглощающие примеси по-разному меняют отражательные свойства снега или льда [73, 250]. Спектральные сигнатуры минеральной пыли [74, 75], водорослей [122, 214] и наледникового обломочного материала [59] различаются не только между собой, но и в пределах каждой группы в зависимости от минералогического и химического состава компонентов. Наиболее распространенными наледниковыми светопоглощающими примесями являются водоросли и цианобактерии, продукты их гетеротрофной трансформации [223], а также криокониты [73]. Особо эффективными в изменении альбедо являются частицы черного углерода [53, 92, 142] и, в меньшей степени, минеральная пыль [74, 166]. Последняя образуется либо из материалов плотных пород, либо из почв и привносится из местных и отдаленных ландшафтов, преимущественно из пустынь [132, 204].
В настоящем обзоре кратко рассматриваем неорганическое вещество на поверхности ледника, но основное внимание уделяем органическим и органо-минеральным компонентам, которые являются основой для биологического круговорота, биогеохимических преобразований и почвообразовательных процессов в супрагляциальной зоне.
Неорганическое вещество. Исследования Глазовской [6, 7] по плосковершинным приводораздельным ледникам Тянь-Шаня, на которых отсутствует поступление гравитационных масс с прилегающих горных склонов, показали, что основным материалом, накапливающимся на леднике эоловым путем, является крупная и тонкая пыль. В составе пыли преобладают кварц, полевые шпаты, в значительном количестве присутствуют глинистые минералы – гидрослюды (сегодня бы написали иллит, вермикулит), у которых тонкие легкие, но широкие зерна, что способствует их ветровому переносу.
Современные исследования, выполненные на глобальном уровне [187], показали, что отложения минеральных частиц на льду, в целом, соответствуют составу поверхностных отложений литосферы. Соотношение местных и дальнеприносных минеральных частиц сильно варьирует и зависит от абсолютной высоты и положения ледника (покровный, приводораздельный, горно-долинный). Как правило, чем ниже находится ледник, тем выше доля местного материала. На низкорасположенных ледниках минеральный материал может иметь песчаный состав или быть полидисперсным с преобладанием фракций мелкого песка и крупной пыли [28]. Геологические условия или географическая изоляция ледника определяют специфическую геохимию супрагляциального материала. Например, в криоконите ледника Керстен на стратовулкане Килиманджаро, высокое содержание редкоземельных элементов связано с обилием вулканического стекла [69]. Локально криоконит может быть обогащен кальцием или железом, при наличии поблизости карбонатных пород или экспонировании железорудных месторождений. На ледяной покров, окружающий безледные участки суши в Антарктике, эоловый материал поступает в основном из локальных источников [72].
Анализ глинистых минералов позволяет различать местный материал от дальнеприносного [131, 170, 228]. Особенно это касается минералов – индикаторов. Например, палыгорскит свидетельствует о формировании в аридных условиях. И конечно, по сравнению с пионерными исследованиями М.А. Глазовской набор глинистых минералов на ледниках мира был существенно расширен – были найдены смектиты, смешанно-слойные минералы, каолинит.
Так же, как в почве, экологически значимую часть вещества в криоконите составляют тяжелые металлы и радионуклиды [31, 41, 42, 111, 143, 144, 160]. Важным дополнительным индикатором источника минеральной пыли является ее изотопный состав, например, соотношение изотопов стронция указывает, откуда был привнесен материал на альпийские ледники [228].
Минеральные частицы, поступающие на поверхность ледника, часто предварительно преобразованы процессами физического и химического выветривания, что увеличивает удельную поверхность для взаимодействия с живым веществом, и способствуют дальнейшему биохимическому выветриванию уже в супрагляциальной зоне [255].
Органическое вещество (ОВ). В ледниках содержится около 6 Пг (петаграмм – 1015 г) органического С, из них большая часть в водорастворимой форме и примерно четверть в твердофазных частицах [104]. 96–98% глобальных запасов С приходятся на антарктический и гренландский ледяные щиты, 2–4% – на горные ледники. В глобальном масштабе резервуар органического С ледников существенен, хотя сильно уступает таким крупным резервуарам криосферы, как почвы мерзлотных областей (~1600 Пг). Тем не менее, при абляции ледник высвобождает большое количество легкодоступного водорастворимого ОВ, которое быстро поступает в окружающие почвы. Этот лабильный резервуар органического С пока слабо учитывается в углеродном балансе перигляциальных экосистем [104].
Супрагляциальное ОВ. На поверхности ледников ОВ [36, 91, 104, 141] накапливается за счет первичной продукции in situ, поступает из внешних источников при дальнем аэральном переносе, эрозии окружающих почв и осадочных пород, а также высвобождается из тела ледника при абляции [199, 212, 215]. По разнообразию форм и сложности химической структуры ОВ супрагляциальной зоны не уступает ОВ почвы.
В различных супрагляциальных органо-минеральных образованиях может содержаться от первых до десятков процентов органического С [187], а его распределение имеет пространственные закономерности в пределах одного ледника [137, 162] и между разными ледниками [91]. На поверхности ледников формируется динамичный пул органических соединений инситного микробного происхождения, привнесенных в составе растительного и почвенного материала, продуктов сжигания ископаемого топлива и биомассы, морских и микробных аэрозолей и многих других аллохтонных С-содержащих компонентов, включая, например, пылеватый материал пустынных областей [130], пестициды [81] и частицы микропластика [34].
Живое ОВ. Ледники – это отдельный биом в составе криосферы [37]. Организмы либо обитают в супрагляциальной зоне, либо используют ее как транзитную среду, все вместе вызывая эффект биоиндуцированного снижения альбедо [108], которым ранее пренебрегали на фоне влияния абиотических факторов. Живые компоненты супрагляциальной системы – это цианобактерии и эукариотические микроводоросли, мхи, сложные гетеротрофные микробные сообщества, беспозвоночные, птицы и даже крупные млекопитающие.
Прокариотические и эукариотические фотоавтотрофы составляют основу супрагляциальной экосистемы. Среди них преобладают цианобактерии, зеленые и диатомовые водоросли [119, 211]. Зеленые водоросли в снеге являются значимым поглотителем С. Например, в Антарктике накапливаются в количествах от 5 до 5800 г сухой массы/м2 [90], где верхний предел значений сопоставим с запасами органического С в почве. В обобщении [187] приведены наиболее часто встречающиеся таксоны фотоавтотрофов: среди цианобактерий распространены семейства Pseudanabaenaceae (Leptolyngbya, Pseudanabaena) и Phormidiaceae (Phormidium, Phormidesmis, Wilmottia, Microcoleus), среди зеленых водорослей – семейства Mesotaeniaceae (Cylindrocystis, Ancylonema) и Ulotrichaceae (Klebsormidium), а среди диатомовых водорослей – семейство Naviculaceae (Pinnularia, Navicula). Род зеленых водорослей Chlamydomonas семейства Chlamydomonadales является основной, но не единственной причиной такого распространенного явления как розовый, красный или “арбузный” снег. Например, род Sanguina этого же семейства также вызывает эффект красного и оранжевого снега [179].
Как и в любой наземной экосистеме автотрофные организмы существуют на ледниках в ассоциации с гетеротрофными сообществами бактерий, архей и грибов, а также протистами и вирусами [36–38, 119, 146]. Фотическая зона ледников содержит 1021–1026 клеток бактерий и архей [114, 115], а грибная биомасса достигает 104–105 КОЕ на 100 мл [70, 71, 108]. Среди гетеротрофов криоконитов преобладают бактерии [58, 76, 172]. Микобиоте криоконитов пока уделено мало внимания [77, 119, 149, 200], хотя известно, что обычно в криоконитах преобладают дрожжи, а не мицелиальные грибы [169, 200]. Среди микобиоты доминируют Cryptococcus gilvescens, Mrakia spp., Rhodotorula spp., Phialophora alba и Articulospora tetracladia. Большая часть их штаммов психрофильна и продуцирует гидралазы, активные при околонулевых температурах [200]. Этот факт доказывает, что микобиота может активно участвовать в разложении и трансформации ОВ криоконита.
Различные пигменты, например, каротиноид астаксантин [182] и фенол пурпурогаллин у водорослей [183], меланины у грибов [169], дополнительно поглощают энергию, регулируя температуру окружающей среды, высвобождая талую воду и растворенные в ней питательные элементы.
В последнее время появились данные [147, 148], что ледники являются средой обитания современных и хранилищем древних антибиотикорезистентных бактерий. По-видимому, супрагляциальная зона, так же как почва и водная среда [18], выступает в качестве резервуара генов устойчивости к антибиотикам, которые могут быть переданы клинически значимым бактериям путем горизонтального переноса – основной причины возникновения множественной лекарственной устойчивости.
Вирусы (отнесение их к живому ОВ здесь условно) на ледниках регулируют численность бактерий, оказывают косвенное влияние на микробную биомассу и количество ОВ в целом [38, 49, 206].
Растения и растительный материал. Наиболее известными обитателями ледников являются мхи родов Bryum, Ceratodon, Drepanocladus, Racomitrium, Schistidium, Hygrohypnella, которые существуют в виде так называемых “ледниковых мышек” – подвижных шарообразных колоний и подушек мха [4, 48, 66, 96, 107, 177]. Такие подушки мха описаны на ледниках в Южной Америке, Исландии, Шпицбергене и особенно широко на Аляске, где на леднике могут одновременно находиться сотни куртинок мха [107]. В Центральной Африке на языках отступающих высокогорных ледников распространены агрегаты протонемы (предростков) и геммы (выводковых телец), т.е. начальных фаз развития моховидных, например таксона Ceratodon purpureus [234].
В зависимости от расположения ледника в его супрагляциальную зону попадает разнообразный свежий растительный материал (рис. 1), богатый углеродом и азотом в виде листьев, хвои, травы, а также многочисленные пыльцевые зерна [54], которые могут переноситься по воздуху на большие расстояния.
Рис. 1.
Свежее ОВ супрагляциальной зоны: a – “выпас” коллембол на грануле криоконита (Гарабаши, Кавказ); b – труп насекомого внутри гранулы криоконита, фото среза гранулы (Левый Актру, Алтай); c – лист нотофагуса, втаявший в лед (Перито-Морено, Аргентина); d – труп божьей коровки (Мижирги, Кавказ); e – труп насекомого втаял в лед, сверху покрыт биопленкой водорослей Chlamydomonas nivalis и Ancylonema nordenskioeldii (Левый Актру, Алтай); f – пыльцевые зерна в криоконите, фото со сканирующего электронного микроскопа, режим вторичных электронов (Левый Актру, Алтай); g – биопленка водорослей Chlamydomonas nivalis в фирне (Мижирги, Кавказ); h – многокомпонентная органо-минеральная пленка в мениске стакана протаивания, состоит из водорослей Chlamydomonas nivalis и Ancylonema nordenskioeldii, а также тонкой фракции мелкозема (Левый Актру, Алтай); i – органо-минеральный седимент на дне стакана протаивания (криоконит), покрытый биопленкой водорослей Chlamydomonas nivalis и труп насекомого (Левый Актру, Алтай); j – подушка мха на ледяном пьедестале, мох как термоизолятор (Бертель, Шпицберген); k – росток мха в стакане протаивания, мох как ускоритель таяния (Бертель, Шпицберген); l – насекомое на грануле криоконита в стакане протаивания (Левый Актру, Алтай). Масштабная линейка на a–e, g, h, l – 1 мм, на i–k – 10 мм, на f – 5 мкм.

Ввиду обилия водной фазы криокониты содержат много простейших, в первую очередь, инфузорий [152].
В супрагляциальной зоне обитают многочисленные беспозвоночные от коловраток до “ледяных” (“ледниковых”) червей [109, 198, 257], происходят регулярные атмосферные выпадения членистоногих [95]. Гетеротрофный блок криоконитов представлен такими беспозвоночными, как тихоходки (Tardigrada), коловратки (Rotifera), коллемболы (Collembola) и пресноводные рачки (Branchiopoda и Maxillopoda) [254]. “Ледяные” черви (Mesenchytraeus solifugus) оказывают влияние на биомассу водорослей: питаются ей [156] и привносят биогенные элементы, которые, наоборот, стимулируют увеличение водорослевой биомассы [110].
Биомасса членистоногих, особенно насекомых, богата углеродом (до 60%) и азотом (до 12%), представлена лабильными органическими соединениями, имеет низкое соотношение C : N, а в пик выпадений летом ее вклад в пул доступного C и N на леднике сопоставим со вкладом водорослей [108].
Многие крупные животные от копытных до кошачьих, а также птицы временно используют ледники и снежники в качестве укрытия, для поиска пищи, гнездования и в качестве транзитной зоны [108]. Основное ОВ, которое привносят позвоночные – это экскременты, шерсть, перья, а также пища, в том числе трупы других животных. Так же, как в почвах, на льду и снеге вблизи колоний птиц и тюленей увеличивается биопродуктивность, наблюдается обильное цветение водорослей, особенно ярко это явление выражено в Антарктике [90, 184]. Орнитогенный фактор влияет на формирование почвоподобных тел, образованных в местах скопления криоконитов [3].
Человек оказывает локальное прямое воздействие на супрагляциальную зону при туристической нагрузке [86] и научных изысканиях, а также масштабное опосредованное влияние через атмосферные выпадения поллютантов.
Мертвое ОВ ледников очень разнообразно. Наряду с ОВ микробного происхождения на ледниках присутствуют фрагменты соединений, маркирующих поступление растительного и почвенного материала; значительная часть ОВ биодоступна [199]. Состав ОВ во многом зависит от расположения, размера ледника и перигляциальной геохимии. ОВ криоконитов антарктических ледников преимущественно автохтонное, тогда как состав ОВ криоконитов в Арктике указывает на разные комбинации источников [168]. Близость лесных экосистем увеличивает количество лигнино- и таниносодержащих фрагментов, а расположение ледников на пути аэрального переноса из районов с интенсивной антропогенной нагрузкой повышает количество высококонденсированных ароматических соединений [90].
В супрагляциальной зоне ожидаемо содержится много водорастворимого органического вещества (ВОВ) [199]. Его концентрация во льду и снеге покровных ледников ниже, чем на поверхности горных ледников, так как последние ближе к наземным источникам С и лучше прогреваются, обеспечивая высокую доступность талой воды и повышая эффективность первичной продукции [185].
Аллохтонное ВОВ в основном состоит из химически устойчивых фрагментов гумино-, лигнино- и таниноподобных соединений и высококонденсированного ароматического С [67, 80, 215]. Автохтонное ВОВ имеет преимущественно липидный и белковый состав за счет развития водорослей и фотосинтезирующих микроорганизмов [36, 157]. Гетеротрофные бактериальные сообщества участвуют как в минерализации, так и в преобразовании лабильных форм ОВ с остаточным накоплением более устойчивых продуктов, например алициклических соединений с карбоксильными группами [36, 98, 157].
По данным 1H ЯМР-спектроскопии, в щелочных экстрактах из криоконита ледников Антарктиды преобладают сигналы от белков и пептидов микробного происхождения, содержатся фосфолипидные жирные кислоты и отсутствуют биомаркеры растений за исключением мхов [168]. В изолированных районах Восточной Антарктиды [40] только во фракции ВОВ, выделенной из снега, идентифицировано более 2300 химических соединений. В них доминировали алифатические фрагменты, но в структуре десятков соединений присутствовали также конденсированные ароматические соединения. ВОВ состояло из лигниноподобных соединений, белков, жиров, ненасыщенных углеводородов, углеводов и дубильных компонентов. Часть лигниноподобных соединений, по-видимому, была связана с ОВ наземного происхождения, а часть – с алициклическими соединениями, богатыми карбоксильными группами. Около 90% идентифицированных соединений (как с ароматическими, так и алифатическими фрагментами) обладало высокой биодоступностью для гетеротрофов и трансформировались микробными сообществами в краткосрочных лабораторных экспериментах посредством параллельных процессов деградации и синтеза [40]. Значительные количества легкодоступного ВОВ с высокой долей алифатических и пептидных фрагментов (например, высвобождаемые ледниками Шпицбергена и Гренландии) при поступлении в перигляциальные ландшафты могут ускорять гетеротрофную деградацию ОВ [120], т.е. приводить к прайминг-эффекту [133].
На ледниках, находящихся в окружении экосистем с доминированием сосудистых растений и развитым почвенным покровом, в составе ОВ все равно могут преобладать соединения микробного происхождения в сочетании с материалом лишайников и мхов, а не сосудистых растений. Низкие уровни молекулярных биомаркеров сосудистых растений на ледниках Аляски, указывают на то, что вклад таких источников ОВ, как леса или торфяники незначителен [215]. По данным 1H и 13С ЯМР-спектроскопии [247], ОВ криоконита с ледника Атабаска (Канадские Скалистые горы) содержало большое количество жирных кислот, н-алканолов, н-алканов, восковых эфиров и стеринов, отсутствовал лигнин, а соотношение алкильных/O-алкильных фрагментов в твердофазных 13C-ЯМР спектрах отличалось от состава ОВ окружающих почв. Анализ фосфолипидных жирных кислот подтвердил значительный вклад микроорганизмов в ОВ криоконита этого ледника, в первую очередь бактерий, а также микроскопических эукариот [209]. Однако в современном и древнем льду из керна с купола Ветреный на острове Греэм-Белл архипелага Земля Франца-Иосифа [89], несмотря на отдаленное морское положение, были идентифицированы тысячи соединений, включая гуминовые (точнее гуминоподобные) вещества (по данным масс-спектрометрии ионного циклотронного резонанса с Фурье-преобразованием – FT/ICR MS). Гуминовые вещества составляли значительную часть выделенного органического материала и указывали на его поступление из наземных экосистем.
Пирогенное ОВ. Весь спектр продуктов неполного сгорания современной биомассы и ископаемого ОВ присутствует на ледниках и схож с тем пирогенным ОВ, что выделяют в почве, включая сажу, уголь, элементный, черный и бурый С, литогенный кероген и микрокристаллический графит. Пирогенное ОВ в ледниковых кернах (черный С, левоглюкозан и др.) является важным носителем информации о хронологии пожаров и сжигании ископаемого топлива [138, 188, 258]. Классификация континуума продуктов горения крайне несовершенна, категории пирогенного ОВ пересекаются, однако во всех из них доминируют полициклические ароматические углеводороды, которые могут быть отдельно выделены с ледников [30, 140]. Среди пирогенного ОВ особенно реактивными считаются частицы черного С [1, 173, 174], даже их небольшие количества (~10–100 ppb) существенно меняют радиационный баланс поверхности ледников, поглощая солнечное излучение в широком диапазоне длин волн и снижая альбедо на 1–5% [92]. Бурый С (продукт низкотемпературного горения древесной или травянистой биомассы, бытовых отходов или ископаемого топлива, аэрозоль из дисперсного ОВ, микроорганизмов) способен поглощать свет преимущественно в диапазоне коротких волн. В буром С выделяются две крупные группы: гумусоподобных веществ (humic-like substances – HULIS), которые сходны с ОВ почв и водных сред и повсеместно присутствуют в атмосфере [87], и смолистых соединений (tar material) [60, 246]. Последние образуются при сжигании биомассы в условиях медленного горения [60] и имеют форму сферических наночастиц (30–500 нм), которые морфологически отличаются от сажи. Считается, что бурый С более химически лабилен, чем черный С. Он переносится на большие расстояния, оседает на поверхности снега и льда, где вовлекается микроорганизмами в свой жизненный цикл и затем повторно попадает в атмосферу в виде CO2 [246, 248].
Можно говорить о комплексном и разнонаправленном влиянии С-содержащих компонентов супрагляциальной зоны на радиационный баланс ледников. С одной стороны, накопление черного С в более светлом криоконите, в котором доминирует бурый С, может усиливать абляционный эффект, с другой стороны – инкапсуляция частиц черного С внутри гранул криоконита может приводить к уменьшению светопоглощения. Эта сложная взаимосвязь пока количественно не оценена [64].
Другие виды антропогенного ОВ. Совсем недавно стали появляться данные о присутствие частиц микропластика на ледниках Исландии, Альп, Тибетского нагорья, Анд и других регионов [34, 208, 260, 261] в количествах сопоставимых с загрязнением микропластиком морского льда, прибрежных отложений и некоторых почв. Окрашенные частицы микропластика среди других органических седиментов на леднике также снижают альбедо, и могут влиять на содержание и скорости трансформации ОВ, а их поверхности, будучи химически и механически протравленными в ходе транспорта и седиментации, сорбируют другие органические загрязнители и служат новыми нишами для микроорганизмов [261]. Пестициды – это еще одни компоненты ОВ, которые накапливаются на льду [81]. Эксперименты in situ показали, что помимо фото- и химической деградации, некоторые из них, например фосфорорганический инсектицид хлорпирифос, могут подвергаться биоразложению на горных ледниках [81].
Радиоуглеродный возраст ОВ. Так же, как в почве, ОВ на поверхности ледника состоит из нескольких пулов различного радиоуглеродного возраста. Несмотря на наличие в супрагляциальной зоне большого числа первичных продуцентов, радиоуглеродный возраст, определенный по общему органическому C, часто оказывается древним. Например, возраст криоконита на ледниках Шпицбергена варьировал в пределах 8200–3700 радиоуглеродных лет (BP) [13], в центральной части Норвегии превышал 5000 BP [94], на Аляске менялся в пределах 15 000–1000 BP [150], а в Антарктиде на Земле Королевы Мод – 11 000–4600 BP [145]. Это также верно для фракции ВОВ, например, в криоконите и поверхностном льду ледников Аляски и Вайоминга [215] с возрастом 7800–2640 BP. Отступающие ледники Европейских Альп выделяют биогеохимически разнообразное ВОВ, часть которого состоит из древнего С возрастом 8500–600 BP [199].
Источниками предварительно состарившегося С на поверхности ледников могут быть аэрозоли, образующиеся при сжигании топлива [215], жидкие осадки с 14С возрастом растворенного С до 8500 лет [181], аэрально-привнесенный почвенный материал голоценового возраста, а также С почв и осадков, сформировавшихся до последней трансгрессии ледников и захороненный в теле ледников [112, 118]. Примесь 1% “мертвого” по 14С активности геологического С (керогена, графита, каменного угля, продуктов сжигания ископаемого топлива и др.) дает ошибку около 80 радиоуглеродных лет. Даже существенная примесь “мертвого” С на уровне 50% приводит к удревнению, равному одному периоду полураспада 14С (~5700 лет), и не может полностью объяснять раннеголоценовый возраст супрагляциального материала [13].
Смесь древнего (14С-активного и “мертвого”) и современного С дает целый спектр разных радиоуглеродных возрастов, в зависимости от относительной доли этих компонентов. Поэтому “кажущийся” радиоуглеродный возраст в несколько тысяч лет по общему органическому С говорит лишь о том, что материал действительно содержит некоторое количество древнего углерода [94]. Для аппроксимации “истинного” возраста, необходимо построение модели, учитывающей вклад разновозрастных пулов ОВ. Например, если предположить, что супрагляциальное ВОВ происходит исключительно из пулов “мертвого” и современного C, то образец возрастом 2640 BP будет содержать 72% современного и 28% ископаемого C, а образец возрастом 7800 BP – 38 и 62% С соответственно [215].
Кроме того, 14С возраст неодинаков в разных фракциях супрагляциального материала. Разделение ОВ криоконита по денситометрическим фракциям [13] позволило уточнить, что свежее ОВ (<100 лет) ледника Альдегонда на о. З. Шпицберген содержалось в наиболее легкой фракции свободного ОВ (FPOM), а древнее ОВ (11 120 ± 40 BP и 8850 ± 30 BP) – в тяжелых фракциях (HF1 и HF2), связанных с органо-минеральными комплексами и адсорбцией на минеральной матрице.
Древний C не обязательно означает инертный. Гетеротрофы, как микроорганизмы, так и беспозвоночные, быстро поселяются на только что освободившихся от ледника поверхностях, и активно потребляют древний ледниковый С, опережая “классическую” стадию поселения первичных продуцентов, и тем самым переворачивая с ног на голову само понятие “первичной сукцессии” [47, 93]. Более того, биодоступность древнего ледникового С может положительно коррелировать с увеличением его радиоуглеродного возраста [105]. Таким образом, древний С, вытаявший с поверхности или тела ледника при его отступании, имеет большой потенциал для включения в современный круговорот и пищевые цепи. Это обусловливает своеобразный ледниковый резервуарный эффект [80], когда радиоуглеродный возраст современных беспозвоночных, живущих в предполье ледника и питающихся древним С, который прошел через несколько звеньев пищевой цепи, превышает 1000 лет [93].
Наиболее интересными представляются варианты, когда сочетаются высокая степень биогенности наледникового материала, обильная микробная биомасса и древний возраст ОВ. Например, в криоконите ледника Альдегонда на о. З. Шпицберген содержалось 4.6% органического С (δ13C = –25.83‰) и 0.2% N, при этом его возраст составлял 7540 ± 25 BP [13]. В данном случае структура микробного сообщества могла быть смещена в сторону гетеротрофного блока, который потребляет и рециркулирует древний С наряду с современным, синтезированным микробными фотоавтотрофами.
С другой стороны, при 14С датировании фракции фосфолипидных жирных кислот (PLFA) микробного происхождения было показано, что микроорганизмы криоконита могут потреблять С, который недавно находился в равновесии с атмосферой и был ассимилирован первичными продуцентами, и при этом практически не использовать древний С [150]. На ледниках юга Аляски выявлено по крайней мере два слабо пересекающихся пула С, которые различаются по составу стабильных изотопов: (1) малый пул С супрагляциального микробного сообщества и (2) большой пул преимущественно неактивного С геологического происхождения [150].
Таким образом, при разнообразных источниках и составе ОВ интерпретация измеренной 14С активности супрагляциального материала не менее сложна, чем для ОВ почв. Кроме того, в супрагляциальных системах, как и в других неравновесных системах, пулы органического C имеют разные скорости обновления.
РАЗНООБРАЗИЕ И ГЕНЕЗИС СУПРАГЛЯЦИАЛЬНЫХ ОРГАНО-МИНЕРАЛЬНЫХ ОБРАЗОВАНИЙ С ПОЗИЦИИ ПОЧВОВЕДЕНИЯ
Органические компоненты на ледниках редко существуют изолировано и тесно взаимодействуют с минеральным веществом, что приводит к формированию супрагляциальных органо-минеральных систем. Рассмотрим основные их типы в различных обстановках на ледниках и мелкоземисто-обломочных субстратах с подстиланием льдом.
Критическая зона ледника. Поверхность ледника в зоне абляции никогда не бывает чистой (рис. 2a, 2b). Фотический слой на ледниках в большинстве случаев имеет мощность в пределах 2 м, хотя коротковолновая часть солнечного спектра может проникать и до 10 м. В отличие от глубокого льда с ограниченной миграцией компонентов, включая клетки микроорганизмов [178], супрагляциальный фотический слой в условиях абляции обладает динамичным поровым пространством и проницаем для живого и мертвого ОВ, а также минеральных частиц, которые мигрируют с талыми водами, как вертикально, так и латерально [65, 115]. При этом в нескольких верхних метрах на поверхности ледника возникает вертикальная неоднородность (рис. 2e, 2f). Появляются горизонты, отличающиеся по кристаллической структуре, форме и объему пор, насыщенности талыми водами [115], а также концентрации органо-минеральных компонентов, которые могут иметь высокую скорость обновления на поверхности (быстрый пул) или оставаться в приповерхностном слое в течение n × 10 лет (медленный пул). По аналогии с плотными породами такую зону зарубежные коллеги назвали корой выветривания – weathering crust [115, 155]. В теплые периоды особенно с безоблачными днями общая пористость приповерхностного льда прогрессивно увеличивается, а кора выветривания расширяется по вертикали на большую глубину. По-русски применительно именно к ледникам термин “кора выветривания” вероятно звучит слишком радикально, что не отменяет сути феномена супрагляциальной органо-минеральной системы с трансформированными и генетически взаимосвязанными горизонтами. На наш взгляд, такой слой может также рассматриваться в качестве супрагляциальной составляющей критической зоны, которая пока отсутствует в концепции критической зоны в том числе для холодных регионов (“critical zone” – [171]). Верхняя часть такой критической зоны представлена корой таяния. Это общепринятый в гляциологии термин для обозначения верхнего сильноразрыхленного и высокопористого слоя льда толщиной в пределах первых десятков сантиметров, возникающего в области абляции ледника под действием солнечной радиации [17].
Рис. 2.
Критическая зона ледника и ледовые почвы: a – грязная супрагляциальная зона на языке ледника Левый Актру, Алтай; b – аналогичная зона на языке ледника Бертель, Шпицберген; c – крупный стакан протаивания с криоконитом и d – поры-трубки и мелкие стаканы в коре таяния с начальными стадиями формирования гранул криоконита на леднике Бертель, Шпицберген; e – профиль критической зоны (коры выветривания) на леднике Гарабаши, Кавказ; f – проникновение криоконита между кристаллами льда в верхней части критической зоны (Гарабаши, Кавказ); g – зеленый снег с цианобактериами и эукариотическими микроводорослями под криоконитом на леднике Мижирги, Кавказ; h – розовый лед с водорослями Chlamydomonas nivalis на леднике Левый Актру, Алтай; i – ледовая почва на горизонтальном участке ледника на о. Галиндез, Антарктика [24].

Развитие дренажа между кристаллами льда, а также между стаканами протаивания (рис. 2c) и порами-трубками (рис. 2d) обеспечивает механизм, с помощью которого насыщенная растворенными веществами вода распределяется по поверхности и внутри ледника, и ее поток превращается в развитую гидрологическую сеть, в том числе в глубокую – внутреннюю дренажную систему ледника [17]. Установлено, что микробные клетки, органические и неорганические вещества проникают в кору таяния и глубже в кору выветривания/критическую зону ледника и переносятся по ним талой водой [116]. В зависимости от размера пор возникает селективность по переносу и высаждению твердых частиц разных размерных фракций и связанных с ними микроорганизмов [65]. Миграция микроорганизмов через кору выветривания ледника меняет условия их освещения и доступа к питательным веществам, тем самым влияя на структуру и функции экосистемы [65]. Суточные и сезонные изменения проницаемости коры выветривания контролируют накопление и перемещение клеток и питательных веществ. Структура и функции микробного сообщества могут зависеть от глубины: горизонт вблизи поверхности льда связан с высокой интенсивностью фотосинтеза и необходимостью фотопротекторной пигментации, а более глубокий горизонт содержит организмы с лучшей адаптацией к слабой освещенности и способностью к чистой гетеротрофии [65]. Исследования инфузорий в стаканах протаивания с криоконитом выявили четкую стратификацию видов в зависимости от глубины погружения в талую воду. Таким образом, неоднородность биотических и абиотических условий влияет на микробные сообщества в микромасштабе [114].
В 2012 г. Таширев с соавт. [24] впервые описали процесс почвообразования непосредственно на леднике. Органо-минеральные тела (рис. 2i) в супрагляциальной зоне о. Галиндез (З. Антарктика) были вертикально стратифицированы на поверхностный органогенный горизонт с альго-бактериальными сообществами и нижележащий горизонт с гумифицированным ОВ, имеющим водопрочную структуру. Неполная деструкция биомассы, по-видимому, определялась дефицитом источников азота, фосфора и серы, необходимым микроорганизмам для разложения ОВ альго-бактериальных матов до конечных продуктов. Образования отличались от криоконита (рис. 2c, 2d), а также от таких широко распространенных, но эфемерных явлений, как зеленый или розовый снег (рис. 2g, 2h), связанных с развитием водорослевых сообществ, и были определены как “ледовые почвы”. Ледовая почва по Таширеву [24] представляла собой плотное органогенное образование на горизонтальных участках льда, имеющая в своем составе горизонт из гумифицированной гетеротрофными микроорганизмами некромассы альго-бактериальных криоценозов. Помимо присутствия биоты и возникновения эфемерных горизонтов функционирования, различающихся по температурному, влажностному режиму и составу ОВ (характерно для зеленого/розового льда и снега), важным свойством ледовой почвы являлось накопление in situ остаточных твердофазных продуктов функционирования (по Таргульяну [23]), причем в значительных количествах, которые могли быть диагностированы макроморфологически по темному “гумусовому” горизонту (рис. 2i).
Микропрофили под растительными сообществами. На поверхности ледников возможно образование почв с микропрофилем под моховыми сообществами. Это связано с явлением так называемых “ледниковых мышек” [4], а именно метастабильных шарообразных колоний мхов (рис. 3a–3c), то закрепляющихся на поверхности ледника, то передвигающихся по ней наподобие растений перекати-поле аридных регионов [107].
Рис. 3.
Моховые сообщества на ледниках: a – ледник Виркишекюдль, Исландия (фото Darrel Swift); b – ледник Рут, Аляска, США (фото Tim Bartholomaus); c–i – почвенный микропрофиль под моховым сообществом на леднике Бертель, о. Западный Шпицберген (далее все фото Н. Мергелова): c – подушка мха на льду – “ледниковая мышка” (вид сверх-у); d – вертикальный панорамный срез через горизонты AO и W; e – полный микропрофиль; f, g, h, i – вертикальные срезы по каждому горизонту в отдельности. Все микроморфологические изображения – проходящий свет, II николи.
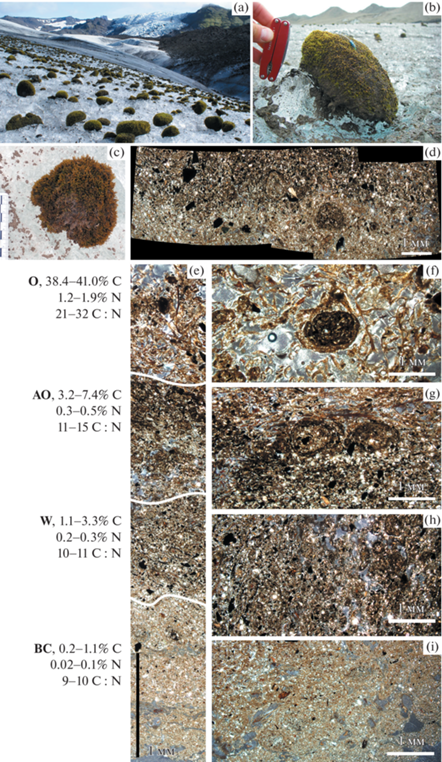
На рис. 3d–3i приведен микропрофиль слаборазвитой почвы под шарообразной колонией мха, прикрепленной к органо-минеральному материалу криоконита в небольшой лунке непосредственно на поверхности ледника Бертель (о. Западный Шпицберген). Микропрофиль достигал 4–6 см и состоял из 4 микрогоризонтов, включая аналоги таких классических почвенных горизонтов, как подстилочно-торфяный (О), грубогумусовый (AO), гумусово-слаборазвитый (W) и минеральный (BС). Между ризоидами мха повсеместно присутствовал гранулированный органо-минеральный материал криоконита. По-видимому, моховые сообщества используют биогенные элементы (C, N, P), которыми обогащен криоконит. В горизонте O содержалось 38.4–41.0% Сорг, 1.2–1.9% Nобщ, соотношение C : N варьировало в диапазоне 21–32; в горизонте AO – 3.2–7.4% С, 0.3–0.5% N, C : N 11–15; в горизонте W – 1.1–3.3% С, 0.2–0.3% N, C : N 10–11; в горизонте BC – 0.2–1.1% С, 0.02–0.1% N, C : N 9–10. В горизонте BC, который моховые сообщества используют как основной субстрат для прикрепления и получения питательных элементов, преобладали кварц, полевые шпаты, кальцит, арагонит и частицы ископаемого угля (неопубликованные авторские данные по рамановской спектроскопии), что отражает состав окружающих ледник пород и указывает на преобладание локального материала. Морфология, стратиграфия и органо-аккумулятивный тип распределения ОВ указывают на формирование или сохранение подо мхом на поверхности ледника почвенного микропрофиля.
Хотя явление “ледниковых мышек” многократно описано ботаниками и гляциологами [4, 48, 96, 107], признаки первичного почвообразования и почвенные микропрофили в данных объектах мы отмечаем впервые. Считается, что это эфемерные образования, а в их перемещении по леднику существенную роль играют ротационные механизмы (перекатывание и перевертывание подушек мха), а также скольжение. Однако обнаруженные почвенные микропрофили явно указывают на продолжительные периоды стабильности в существовании данных объектов. Такие профили не могли сформироваться в течение одного сезона, длительность почвенных процессов дифференциации должна была достигать хотя бы нескольких лет. Выделим две гипотезы образования почвенных микропрофилей: (1) перигляциальное происхождение – подушка мха с прикрепленным дифференцированным на горизонты субстратом была занесена из окружающих ледник ландшафтов и (2) супрагляциальное происхождение – микропрофиль сформировался непосредственно на леднике при закреплении мха на минеральном субстрате, например, на обломочном материале абляционной морены; споры или протонема мха были занесены ветром или птицами. Участие гранул криоконита во всех горизонтах профиля “ледниковой мышки” и тесная интеграция гранул с ризоидами мха и минеральной массой дополнительно указывают на длительное совместное образование именно в супрагляциальной обстановке.
Ботанические исследования [48] показали, что моховые подушки появляются на ледниках Шпицбергена не только в результате привноса из окружающих ландшафтов, но и в ходе развития растения из протонемы in situ, в частности, ветвления мхов на скоплениях богатого биогенными элементами криоконита. Каждая подушка мха представляет собой небольшую экосистему на льду, проходящую несколько сукцессионных стадий и являющуюся местообитанием для других организмов [48]. На ледниках Аляски после достижения зрелого размера подушки мха сохранялись в течение как минимум 6 лет [107], что при ассоциации с мелкоземом достаточно для запуска почвенных процессов и образования микропрофиля. Верхний временной предел существования одной подушки мха на леднике пока неизвестен.
Криоконит. Важнейший продукт органо-минеральных взаимодействий на леднике – это криоконит. Он представляет собой тонкодисперсный, часто структурированный и темноцветный органо-минеральный материал на поверхности ледников и многолетних снежников. Криоконит – это сложноорганизованная система из минерального мелкозема и ОВ ближних и дальних источников, микроорганизмов и мезофауны, продуктов их трансформации и в настоящее время – многочисленных антропогенных поллютантов.
Со времени, по-видимому, первого описания криоконита в XIX в. в Гренландии [163, 164] были задокументированы его биогенная зернистость, а также влияние на таяние ледников. В русскоязычной литературе термин криоконит использовал, например, Ферсман: “…в приполярных областях выявляются скопления космической пыли (криоконита), которые в других частях земли смешиваются с земной пылью и не улавливаются. Хотя до сих пор криоконит остается загадочным и малоизученным образованием, тем не менее его значение в общей геохимической истории Земли нельзя отрицать” [25].
Глазовская по материалам микроскопических исследований криоконита, правда, не называя его так, писала, что “…каждая структурная крупинка представляет собой сплетение многоклеточных нитей сине-зеленых водорослей, удерживающих внутри клубочка частицы минеральной пыли” [7]. Сейчас общепринято мнение, что зернистая или гранулярная структура криоконитов связана с нитчатыми цианобактериями и их внеклеточными полимерами, а такой продукт гетеротрофной трансформации ОВ in situ, как гумифицированное ОВ, новообразованное в гранулах криоконита, поглощает больше солнечного света, чем окружающий лед и компоненты криоконита по отдельности [216, 217].
Образование гранулированного криоконита – это чрезвычайно распространенный процесс, выявленный на ледниках обоих полушарий. Ранее было доступно несколько подробных описаний его морфологии и структуры [116, 137, 216, 217, 227, 255]. В 2022 г. крупное обобщение [187] выявило различия в окраске, морфологии и геохимии криоконита с 33 ледников мира. Важно, что криоконит был представлен как бесструктурным, так и хорошо агрегированным материалом, а встречаемость гранул возрастала с увеличением содержания органического С (от 1 до 38% С для 33 ледников).
Классификация криоконита. Предлагаем разделять инситный и переотложенный криоконит (рис. 4). Инситный криоконит накапливается и приобретает структуру в коре таяния ледника в мелких порах-трубках и более крупных округлых формах – стаканах протаивания, а также в их скоплениях и ассоциациях [43, 61, 84, 85, 136, 216, 217]. Такие ниши обеспечивают относительно стабильные условия для образования:
Рис. 4.
Формы криоконита на ледниках: a – агрегаты правильной округлой формы; b – агрегаты округлой формы c неровными краями; c – рыхлые агрегаты, покрытые нитчатыми цианобактериями; d – скопления агрегатов различных форм и размеров; e – вторичные ассоциации первичных агрегатов размером более 1 см (мегагранулы); f – сохранившаяся гранулярная структура в свежем скоплении переотложенного криоконита; g – скопление переотложенного криоконита; h – поля дисперсного криоконита; i, j – струйчатые формы переотложенного криоконита; k – переотложенный криоконит – коническая форма с ледяным ядром. Изображения на a–f, j, k – ледник Бертель, Шпицберген; g, h – Гарабаши, Кавказ; i – Левый Актру, Алтай. Масштабная линейка на a–f – 5 мм.

I. первичных (элементарных) аггрегатов, формирующихся при участии нитчатых цианобактерий (по [187] с изменениями), которые разделены на:
• агрегаты правильной округлой формы – микрогранулы (рис. 4a);
• агрегаты округлой формы c неровными краями (рис. 4b);
• слабооформленные рыхлые агрегаты, покрытые нитчатыми цианобактериями (рис. 4c);
• скопления агрегатов различных форм и размеров (рис. 4d);
II. сложных агрегатов округлой формы с концентрическими слоями, стратификацией между зоной первичной продукции и преобразования органического вещества ОВ; окислительно-восстановительными микропрофилями по направлению центр-периферия – мезогранулы (например, рис. 5b, 5d, 5h);
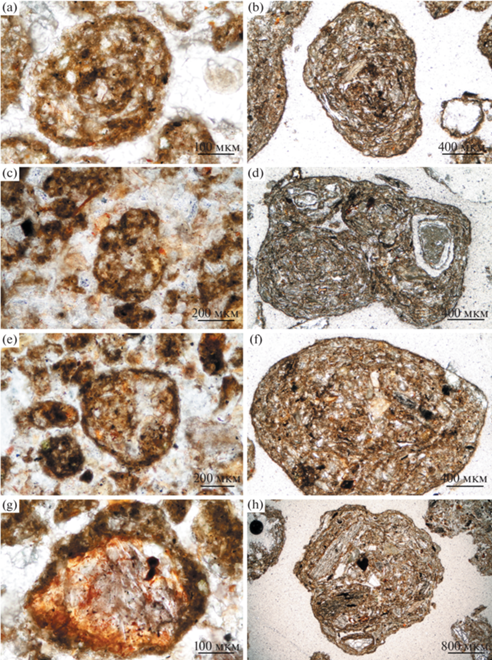
Рис. 5.
Четыре типа внутреннего строения гранул криоконита: a, b – гранулы с концентрическими слоями; c, d – гранулы с несколькими субгранулами внутри; e, f – гранулы без специфической внутренней структуры; g, h – гранулы с одним или несколькими зародышевыми зернами минералов. Все гранулы пропитаны бурым ОВ, содержание которого повышено в стенках гранул. Изображения получены в проходящем свете, II николи; левая колонка – гранулы с ледника Гарабаши (Кавказ), правая колонка – с ледника Левый Актру (Алтай).
III. вторичных ассоциаций первичных агрегатов общим размером 1 см и более – мегагранулы (рис. 4e).
По особенностям внутреннего строения гранулы могут быть разделены ([221] с изменениями) на (рис. 5):
− гранулы с концентрическими слоями (тип 1);
− гранулы с несколькими субгранулами внутри (тип 2);
− гранулы без специфической внутренней структуры (тип 3);
− гранулы с одной или несколькими крупными минеральными частицами внутри – зародышевыми зернами (тип 4).
Вероятны разные сочетания таких морфотипов гранул.
В нестабильных условиях супрагляциальной зоны криоконит проходит через многократное перемещение в рамках суточных, сезонных и годичных циклов, формирующих новые аккумуляции переотложенного криоконита (рис. 4f–4k) в виде:
− свежих скоплений с сохранившейся гранулярной структурой, часто в результате вытаивания с минимальным перемещением (рис. 4f);
− полей дисперсного криоконита (рис. 4h):
• с хорошо сохранившейся структурой (вытаивание с минимальным перемещением);
• с нарушенной структурой;
• без признаков гранулярной структуры и другой биогенной агрегации (по [187] с изменениями):
• тонкодисперсный материал с преобладанием частиц <0.5 мм;
• материал с частицами >0.5 мм.
− струйчатых аккумуляций вдоль потоков талых вод (рис. 4i, 4j);
− крупных аккумулятивных конических форм с ледяным ядром (рис. 4k), экранированным слоем криоконита мощностью в несколько сантиметров [162]. В гляциологии схожие формы называют “муравьиными кучами”, однако это понятие шире, так как экранирующий солнечную радиацию слой часто образован не криоконитом, а мореной различного типа, а также отложениями проблемного генезиса [16, 19]. Некоторые исследователи относят такие формы к особым супрагляциальным камам [210, 211].
Морфотип гранул криоконита зависит от характера ледника (ледник вершин, склонов, долин, покровный и др.), сезона, конкретного года, положения на леднике. Например, в верхней части зоны абляции гранулы могут отсутствовать, а ближе к языку ледника быть крупными и более темными, в пределах сезона они могут дезинтегрироваться из-за дождя, активного таяния, гравитационного перемещения, а также катабатических ветров [187]. Существует зависимость размера гранул и содержания в них C и N – чем гранула крупнее, тем больше в ней ОВ [235]. Предполагается, что размер гранул криоконита, который коррелирует с содержанием ОВ и цветом, оказывает решающее влияние на скорость абляции [135, 252].
Окраска криоконита зависит от минералогического и гранулометрического составов, частиц черного С, но в большей степени от содержания и структуры инситного ОВ, степени его преобразования и взаимодействия с минеральными компонентами. Эти принципы формирования окраски, похожи на те, что реализуются в почве. Чем выше содержание ОВ в криоконите, тем вероятнее образование гранул и темнее их цвет. Влияние ОВ на окраску криоконита было показано в простом эксперименте по прокаливанию при T = 550°С, после чего даже самые темные гранулы становились существенно светлее по всему объему агрегата [187]. Следовательно, именно пропитка дисперсным ОВ вносила существенный вклад в потемнение гранул. В случае антарктического криоконита в стабильных стаканах протаивания, примерно 35% поверхности гранул покрыты микробной биопленкой, из них диффузное пропитывание внеклеточными полимерами составляло ~19% [203]. Темный цвет ОВ может быть объяснен синтезом микробных пигментов, например, меланина [190], остаточным накоплением высокополимеризованных соединений, образовавшихся при бактериальном разложении ОВ [137, 216], а также сорбцией аллохтонных частиц черного С. Равномерное потемнение невозможно за счет частиц пирогенного С, который дает сильное, но точечное изменение окраски, однако легко объясняется первичной продукцией ОВ и его последующей трансформацией in situ, при которых задействовано водорастворимое ОВ, а также ОВ, тесно связанное с минеральной матрицей, включая глинистые минералы.
Таким образом, помимо минералогического состава, например исходного присутствия в гранулах меланократовых минералов (пироксенов, амфиболов, биотита и др.) и эолового поступления частиц черного С, синтез ОВ микроорганизмами и последующая его трансформация in situ служат важными дополнительными факторами биоиндуцированного снижения альбедо на ледниках [216, 217, 220, 222].
Механизмы образования гранул в криоконите до конца не раскрыты. С одной стороны, это опутывание минеральных частиц нитчатыми цианобактериями и формирование клубочков – по сути микробных матов квазисферической формы [7, 216, 217]. Отмечается роль адсорбции и адгезии с участием глинистых минералов в составе эоловой пыли, а также связывающая роль внеклеточных полимеров (полисахаридного матрикса), выделяемых цианобактериями и другими микроорганизмами [136, 137, 203]. Внеклеточные полимеры склеивают органические и неорганические компоненты в агрегаты правильной или неправильной округлой формы [136, 137, 221, 235]. Полисахаридный матрикс микробного генезиса обладает большой удельной поверхностью, на которой происходит адсорбция частиц за счет электростатических сил. Кроме того, матрикс имеет в своем составе гидрофильные и гидрофобные биополимеры. Последние играют важную роль в устойчивости гранул. По данным рамановской спектроскопии метильные функциональные группы (–СН3) приурочены именно к стенкам гранул, что свидетельствует о гидрофобных свойствах их поверхности, необходимым для стабилизации микроэкосистемы гранулы в талой воде (неопубликованные данные авторов). Микроагрегаты с высоким содержанием полисахаридов могут улавливать взвешенные твердые частицы из потоков талой воды в супрагляциальной зоне ледника.
Микробноиндуцированное формирование округлых агрегатов (гранул) происходит в широком диапазоне условий при разнообразии в составе микроорганизмов и их метаболизме. Недавно было показано [167], что гранулы криоконита и так называемые оксигенные фотогранулы, формирующиеся в системах очистки сточных вод [33], схожи и представляют собой богатые ОВ квазисферические микробные агрегаты. Как могут образовываться гранулы со значительным сходством в столь разных средах – осадках сточных вод и в талой воде на леднике? Дело в том, что в обоих случаях действует универсальный механизм: нитчатые цианобактерии, образующие маты в широком диапазоне условий, обволакивают минеральные частицы и формируют округлые агрегаты. Эта сеть нитевидных организмов и внеклеточных полимеров стабилизирует среду обитания, соединяясь с другими микроорганизмами и минеральными частицами. Среди условий образования микробноиндуцированных квазисферических агрегатов называются присутствие в микробном сообществе представителей порядка осциллаториевых (прежде всего, родов цианобактерий Oscillatoria и Phormidium), выделяющих обильные внеклеточные полимеры и способные к скользящему движению по твердой поверхности [207] по направлению к свету и от него – фототаксису. Движение цианобактерий вместе с внеклеточными полимерами усиливает связывание минеральных частиц, а также привлекает другие микроорганизмы, которые используют метаболиты цианобактерий (полимеры и кислород), способствуя дальнейшей агрегации криоконита [136, 216, 217].
Для оформления гранул могут быть важны как гидростатические условия в стаканах протаивания, способствующие росту квазисферических микробных матов, так и наличие небольшого гидродинамического сдвига (при медленном токе воды через стаканы протаивания и их слиянии), который приводит к дальнейшему оформлению гранул [167]. На наш взгляд, существует перекос в интерпретации генезиса гранул криоконита в сторону их исключительной биогенности, тогда как роль формирующего механического воздействия изучена слабо. Действительно бентосные цианобактерии растут в стабильной среде, например, на пологих горных и покровных ледниках с ограниченным движением поверхностных вод, что благоприятно для образования гранул [187]. Однако эти условия не являются строго обязательными для грануляции криоконита. В условиях интенсивной абляции на ледниках Шпицбергена гранулы также образуются ([256] и собственные наблюдения авторов). Более того, криокониты горных ледников на больших высотах часто лучше гранулированы, чем криокониты более пологих и низкорасположенных ледников Арктики и Антарктики. Например, микроагрегатный состав мелкозема с ледников Тянь-Шаня [8] выявил преобладание мезогранул размером 1–3 мм. Вероятно, криокониты на ледниках с более крутой поверхностью, испытывающие гидродинамическое воздействие, могут в ряде случаев объяснить лучшую грануляцию [167].
Анализ микроскопического строения гранул криоконита полярных ледников Бертель и Альдегонда (Шпицберген) и горного ледника Гарабаши (Кавказ) выявил, что их внутренняя часть состоит преимущественно из более крупных минеральных частиц размером в десятки микрон, в то время как периферия сложена плотно упакованными более мелкими частицами размером <10 мкм (неопубликованные данные авторов). В стенке гранулы плоские минеральные частицы часто ориентированы субпараллельно внешней поверхности гранулы, тогда как внутри гранулы частицы ориентированы случайным образом. Внутри стенки минеральные частицы образуют концентрические слои и, как правило, скреплены полимерным матриксом. Наличие биополимеров и специфическая ориентация минеральных частиц в стенке гранулы указывают на совместное действие как биологических, так и механических факторов оструктуривания материала криоконита.
Предполагается, что концентрические слои отражают стадийность процесса образования гранул, своего рода “годичные кольца”, которые образуются со скоростью ~0.2 мм/год [221]. Это является перспективным, но пока неразработанным направлением для установления возраста гранул. Следует также учитывать, что приращение цианобактериальной массы и внеклеточного полимерного матрикса, на который “садятся” минеральные частицы, может происходить несколько раз в течение сезона.
Морфология криоконита сходна с ооидами в перигляциальных почвах. Почвоведы [202, 237] указывали на роль криотурбаций и солифлюкции (гелифлюкции) в формировании округлых гранулированных структур в криосолях. В целом при палеогеографических реконструкциях ооиды интерпретируются как индикаторы перигляциальных и/или мерзлотных обстановок [236]. С одной стороны, по аналогии с почвами в формировании гранул криоконита могут играть процессы гелифлюкции (движения по мерзлой поверхности), с другой стороны, часть ооидов в перигляциальных почвах могут быть гранулами, сохранившимися при переотложении криоконита.
Стратификация в криоконитовых стаканах и гранулах. По данным [176] уже через 1 ч после осаждения органо-минерального материала в криоконитовом стакане формируется анаэробный слой мощностью несколько миллиметров. На вертикальную неоднородность концентрации кислорода влияет структура осадка, например, размер гранул криоконита, толщина слоя талой воды, а также расход кислорода в ходе гетеротрофного дыхания. Возникающий кислородный профиль в свою очередь меняет структуру микробного сообщества, и в бескислородных микронишах повышается активность анаэробов [176].
Схожее зонирование окислительно-восстановительных условий или редокс-стратификация возникает внутри крупных гранул криоконита [197]. Измерения концентрации O2, ${\text{NH}}_{4}^{ + }$, ${\text{NO}}_{2}^{ - }$ и ${\text{NO}}_{3}^{ - }$ при помощи микросенсоров выявили, что на поверхности гранул, колонизированной цианобактериями, преобладают аэробные условия, идут фотосинтез и денитрификация. В центральной части гранул могут сформироваться анаэробные условия, происходит микробное разложение ОВ, нитрификация и денитрификация. Таким образом, гранулы криоконита обусловливают неоднородность микропроцессов и образования твердофазных продуктов функционирования. В случае распространенности криоконитового материала это влияет на круговорот углерода и азота в супрагляциальной зоне в целом. При обилии в криоконите зерен кварца и проникновении света через них первичная продукция может происходить не только на поверхности криоконитовых гранул, но и в их внутренней части [99]; предполагается существование своеобразных эндокриоконитных первичных продуцентов. Элементарные микрогранулы могут не иметь зонирования по окислительно-восстановительным условиям.
Таким образом, основная структурная единица криоконита, гранула, является самостоятельной сложноорганизованной многопорядковой микроэкосистемой одновременно обособленной в водной супрагляциальной обстановке, но также и проницаемой для потоков вещества, благодаря развитой системе пор. Физические и химические механизмы стабилизации органо-минеральных агрегатов криоконита сходны с принципами агрегации в сложных коллоидных системах, например, почвах: (1) окклюзия/инкапсуляция, (2) образование гранул/ооидов, (3) адсорбция на активных минеральных поверхностях, например, глинистых минералах, (4) гидрофильно-гидрофобные взаимодействия.
Влияние криоконита на ближнюю перигляциальную зону. Криокониты являются важным источником микроорганизмов для первичных сукцессий на отложениях ледника сразу после его отступания [119], а значит и для инициации процессов почвообразования. Однако роль криоконита в этих процессах оценена качественно, но пока не количественно. По сути, криоконит является “биореактором” супрагляциальной системы, в котором происходит взаимодействие на разных уровнях организации вещества: от молекулярного до ландшафтного. Через криоконит идет наиболее активный поток биогенных веществ, в стаканах протаивания поддерживаются квазиравновесные температурные и фотические условия, что важно для развития микробных сообществ.
Ближняя перигляциальная зона находится в области переотложения органических, минеральных и органо-минеральных фаций супрагляциальной зоны. В районе конечно-моренных отложений и боковой морены локально формируются аккумуляции переотложенного криоконитового материала (чаще в виде линз и седиментов в лужах), на которых в стабильных условиях могут образовываться первичные почвы. Благодаря высокому содержанию биогенных элементов и тонкодисперсности, криоконитовый материал обладает более благоприятными условиями для почвообразования по сравнению с материалом морены [13]. Поэтому процессы первичного почвообразования на переотложенном криоконите идут на порядок быстрее, чем на окружающих обломочных субстратах, а почвы наследуют свойства криоконита: гранулированность, слоистость, повышенное содержание C, N, P.
Почвы на мелкоземисто-обломочном материале на ледниках и мертвом льду. В среднем ~4.4% площади ледников (без Антарктиды и Гренландии) покрыто мелкоземисто-обломочным материалом (supraglacial debris-cover), доля которого существенно возрастает для высокогорных ледников [195]. В данном случае не рассматриваем каменные и земляные глетчеры [10], хотя у них тоже могут быть почвы и почвенные покровы, а их разновидности, например, “каменные глетчеры с ледяным ядром” (ice-cored rock glaciers) [5], вероятно, генетически близки к перекрытым мореной блокам мертвого льда, а также погребенным и бронированным ледникам (debris-covered glaciers).
При преобладании специфических органо-минеральных образований на поверхности ледников, в некоторых случаях встречаются объекты, которые классифицируются как почвы, а некоторые полностью отвечают всем критериям почв, причем не только слаборазвитых.
Почвы на кварц-силикатных отложениях с близким подстиланием ледников. Распространенным случаем является феномен появления сосудистых растений и формирования почв на абляционной морене и коллювиальных отложениях, накапливающихся на поверхности ледника [82]. При этом ледник остается способным к движению.
Почвы в данных случаях остаются относительно слаборазвитыми, но уже классифицируемыми как почвы. На рис. 6 показаны примеры формирования почв в долине Онг в Трансантарктических ледниковых горах в сухих континентальных областях Антарктиды, где сосудистые растения отсутствуют по биоклиматическим причинам [46], которые классифицированы по Таксономии почв США как Glacic Haplorthels при близком подстилании ледника и Glacic Anhyorthels при его залегании глубже 50 см, а также мелкие почвы на леднике Милаж в итальянских Альпах [63] и на леднике Гарабаши на Кавказе (собственные данные), которые можно классифицировать как Leptosols по системе WRB [117] и сильнощебнистые пелоземы по КиДПР [15]. Во всех почвах заметна стратификация на горизонты, а подстилание массивного льда ледников Антарктиды оказывается на глубине 10–60 см. В случае континентальной Антарктиды материал ледника из-за очень низких температур не столько подвергается таянию и размыву, как в ледниках высокогорий и полярных областей северного полушария, сколько эоловому выносу мелкозема и формированию каменной мостовой и сублимации ледникового тела, которая ослабляется по мере увеличения мощности кварц-силикатного чехла над ним [194]. Содержание органического С колеблется от сотых до первых процентов. Значения рН от сильнощелочных при близком подстилании ледников до слабощелочных при увеличении глубины подстилания и возраста поверхности, а содержание солей при этом увеличивается, что связано с их эоловым привносом [194].
Почвы на мелкоземе над глыбами мертвого льда. Именно в этом случае могут формироваться полнопрофильные почвы, хотя и не всегда. Встречаемость этого варианта пока не очень ясна. Понятно, что феномен накопления субстрата над глыбами мертвого, т.е. неподвижного льда может возникать только в тех случаях, когда разность высот от зоны аккумуляции до зоны абляции весьма существенна и составляет не одну тысячу метров. В этих случаях языки ледников “уезжают” далеко вниз по долинам, как правило, в лесной пояс. В таких уже относительно теплых условиях ледники начинают таять и теряют подвижность. Кроме этого, должны выполняться условия возможности поверхностного накопления мелкозема на поверхности ледника, помимо абляционной морены, например, вулканической тефры. Такие случаи отмечены для ледников Анд в Чили на границе с Аргентиной на 4° S [180], ледников Аляски [209] и на леднике Клутлан на северо-западе Канады [193] (рис. 7).
Рис. 7.
Почвы с подстиланием слабоподвижных ледников и глыб мертвого (не движущегося) льда: a – ледник Каса Панг, Чили, деревья нотофагуса растут на слаборазвитых каменистых почвах [180]; b и c – глыбы мертвого льда около ледников Аляски под полноценным лесом из ситкинской ели: b – ледник Фэруэтер (фото Tom Bean), данных по почвам нет, c – ледник Куштака, почва – инициальный подзол, подстилаемый чистым льдом на глубине 90 см [209]; d–g – почвы на тефре, аккумулированной на морене с ледяным ядром ледника Клутлан, залегающим на глубине около 2 м, провинция Юкон, Канада [193]: d – общий вид – формы ледового карста с “пьяным лесом” и выходами тефры на поверхность на крутых склонах, e – разреженный напочвенный покров и биокорка на поверхности крутых склонов, f – Dystric Brunic Arenosol (Gelic), g – Dystric Cambisol (Arenic, Gelic, Humic).
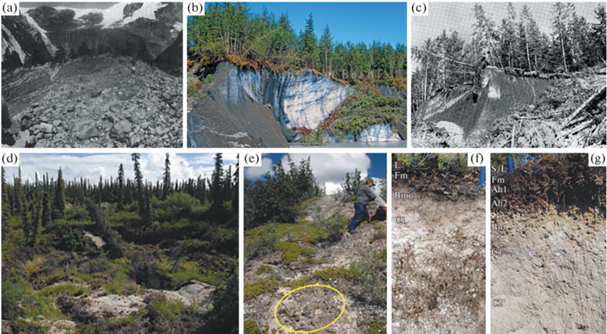
В Андах наблюдается переходный случай – часть ледника Каса Панг на склонах горы Тронадор находится в движущемся состоянии, а часть – под моренными грядами в почти неподвижном состоянии, что дало возможность здесь сформироваться лесным сообществам с преобладанием нотофагуса (Nothofagus dombeyi) с кустарниковым подлеском и с папоротниками, мхами и лишайниками в напочвенном покрове. Мощность надледникового материала на грядах составляет 3–4 м. Почвы кислые (рН 4.6 до 4.7), содержание N от 0.01 до 0.02%, а Сорг от 0.1 до 0.2%, что свидетельствует о слабом развитии почв. Содержание Ca невелико, но, в целом, содержание биофильных элементов Ca, K и P выше, чем в образцах материала на леднике. Авторы это связывают с почвообразованием.
Над ледниками Аляски, вероятно, в некоторых локациях, где на маломощных рыхлых каменистых субстратах, близко подстилаемых ледником, произрастают настоящие сомкнутые леса из ситкинской ели (Picea sitchensis) с подлеском из ольхи и моховым покровом. Описана почва под таким лесом на леднике Куштака (60°25′ N, 144°05′ W) [209]. Горизонты 13 см – лесная подстилка, 3 см осветленный серый горизонт (по автору А2), 15 см – темно-бурый горизонт (B), до 90 см – очень гравелистый опесчаненный легкий суглинок серого цвета (горизонт С), подстилается плотным чистым льдом. Автор определяет данную почву как инициальный (неполноразвитый) подзол и указывает, что подобные почвы имеют место и около ледников Герберт и Мендехолл. По современной классификации WRB эту почву, вероятно (так как нет химических характеристик), можно классифицировать как Protospodic Glacic Cryosol.
Наконец, наиболее развитые почвы описаны на тефре, аккумулированной на морене с ледяным ядром ледника Клутлан (провинция Юкон, Канада), залегающим на глубине около 2 м, (61°36′ N, 140°39′ W) [193]. Исследования проводились на высоте 1050 м. Здесь отчетливо выражен ледовый карст – неравномерное протаивание ледяного ядра приводит к формированию неоднородного почвенно-растительного покрова (рис. 7d, 7e). На наиболее стабильных участках формируются леса из ели белой (Picea glauca) с примесью березы, на нарушенных участках в растительном покрове преобладает кустарник из ольхи, а на совсем сильно нарушенных пятнах имеет место биологическая корка из лишайников. Соответственно, наиболее развитые почвы авторы отнесли к Брунисолям по канадской классификации. Полноценные химические данные позволили классифицировать их по WRB как Dystric Cambisols (Arenic, Gelic, Humic) (рис. 7g). Менее развитые почвы (Регосоли по канадской системе) классифицированы как Dystric Brunic Arenosols (Gelic) (рис. 7f). На крутых склонах ледово-карстовых форм тефра и другие минеральные частицы удерживаются вместе органическим детритом и лишайниками с образованием поверхностной почвенной корки. Авторы никак не классифицируют эти образования, но по WRB они соответствуют почвам Protic Arenosols (Gelic?). Интересно, что несмотря на существенную роль тефры почвы не соответствуют критериям andic и vitric.
ТИПИЗАЦИЯ СУПРАГЛЯЦИАЛЬНЫХ ОРГАНО-МИНЕРАЛЬНЫХ ОБРАЗОВАНИЙ С ПОЗИЦИИ ПОЧВОВЕДЕНИЯ
Экспонированные поверхности льда и снега. Предпочвы. Как же можно рассматривать тонкие, не всегда организованные в микрогоризонты или структурные агрегаты, органические, органо-минеральные и минеральные гетерогенные дисперсные системы на поверхности ледников с позиции теоретического почвоведения? Напрямую такие объекты ни в общей теории педогенеза [22], ни в концепции экстремального почвообразования [11, 12] не рассматривались, так как для почвоподобных тел (солоидов) требуется хоть какая-то организация материала. Ближе всего они относятся к такому элементу почвенных систем как “предпочвы” [22], т.е. недифференцированные in situ на уровне твердой фазы тела, в которых может наблюдаться зонирование по температурному, влажностному режиму и функционированию микробных сообществ. Однако в концепции почвенных систем предполагается, что с течением времени предпочвы станут почвами. С такими системами, как зеленый/розовый снег и лед, биопленки и органо-минеральные заполнения между кристаллами льда в коре таяния ледника это происходит не всегда. В зоне аккумуляции ледника они будут перекрыты снежным покровом и перейдут в тело ледника в виде несвязанных прослоев, а в зоне абляции или (1) будут смыты талыми водами, перенесены ниже по склону и попадут в твердый сток гидрологической сети или (2) будут организованы в криокониты, которые являются уже существенно иными супрагляциальными образованиями. Предлагается для подобных органо-минеральных систем ввести понятие супрагляциальных предпочв, среди которых есть как эфемерные разности, так и те, что разовьются в почвоподобные тела и почвы (рис. 8a).
Рис. 8.
Типизация супрагляциальных органо-минеральных образований с позиции почвоведения: a – предпочвы и почвоподобные тела во льду и снеге; b – ледовые почвы с остаточным накоплением твердофазных продуктов in situ и макроскопическими горизонтами; c – метастабильные почвы с микропрофилем О(AO)–W–С под мхами; d – метастабильные почвоподобные тела на криоконите с микропрофилями внутри крупных гранул (d1) и микропрофилями на переотложенном криоконите (d2); e – почвы на мелкоземисто-обломочном материале на ледниках; f – почвы на погребенном/мертвом льду.
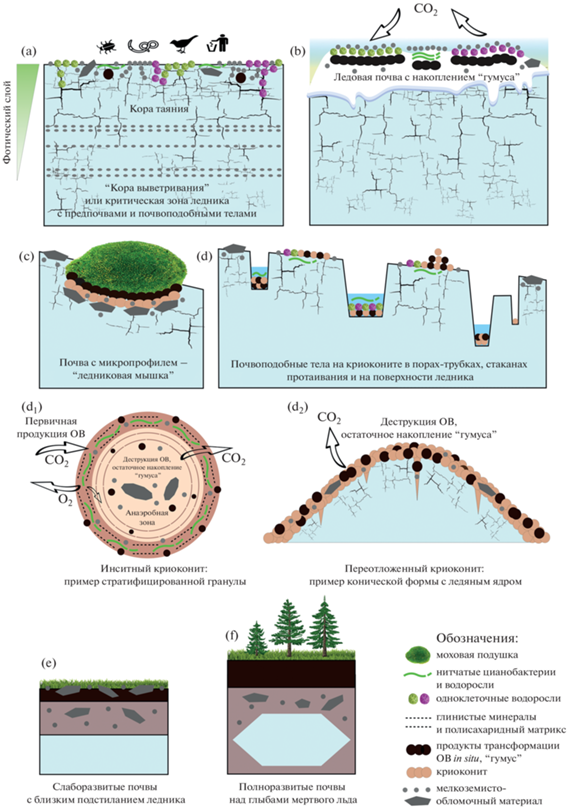
Почвы и почвоподобные тела. Ранее гляциологов интересовали свойства примесей для определения альбедо и провенанса – источника привноса частиц. Инситные изменения, например, смена зеленого и красного цвета поверхностных водорослей на бурый и темно-серый в результате фотохимической и гетеротрофной трансформации ОВ практически не изучались. До Таширева [24] слово “почвы” применительно к объектам на открытой поверхности ледников упоминалось лишь при анализе источника светопоглощающих примесей, а термин “почвоподобные тела” (soil-like bodies) только недавно стали применять к скоплениям криоконита [1, 28–30, 151, 175].
Наиболее очевидными образованиями, которые можно отнести к почвам без перекрытия льда чехлом мелкоземисто-щебнистых отложений, являются органо-минеральные системы с длительным остаточным накоплением гумифицированного ОВ на горизонтальных и наклонных участках ледников или снежных покровов (рис. 8b). Они описаны на острове Галиндез в Западной Антарктике как ледовые почвы [24] и, вероятно, распространены шире в Субантарктике на невысоких островах, где ледники имеют слабонаклонную поверхность, а климат относительно теплый и влажный (по сравнению с континентальной Антарктикой) и предполагает относительную термостатированность условий, способствующую развитию мощных альго-бактериальных покровов на снеге и льду. Ледовые почвы образуются в “гравитационных” ловушках, связанных с локальным протаиванием льда и снега или механическим барьером в виде крупнообломочного материала и выходов плотных пород, которые препятствуют удалению продуктов трансформации ОВ. Их яркой особенностью является стратификация на макроскопические горизонты первичной продукции ОВ (органогенные) и на горизонты с гетеротрофной трансформацией ОВ (гумусовые).
К почвам также следует отнести органо-минеральные системы под моховыми сообществами на леднике с небольшим участием минерального материала (рис. 8c). В данном случае образуются микропрофили, которые могут состоять из подстилочно-торфяного (О), грубогумусового (AO), гумусово-слаборазвитого (W) и минеральных горизонтов с участием криоконита и небольших количеств неоструктуренного мелкозема или мелкообломочного материала. Это метастабильные образования, которые то закрепляются, то перемещаются по поверхности ледника, но могут существовать и развиваться на нем в течение как минимум нескольких лет. По-видимому, наиболее широко такие почвы с микропрофилем распространены на ледниках Аляски, Шпицбергена, Исландии, Гренландии, Южной Америки и островов в Субантарктике.
Метастабильные почвоподобные тела на криоконите (рис. 8d) могут образовываться как внутри гранул (рис. 8d1) с концентрическими микрогоризонтами первичной продукции на поверхности и гетеротрофной трансформации ОВ внутри гранул, так и на скоплениях переотложенного криоконита (рис. 8d2), на которых преимущественно идет деградация ОВ и временное накопление продуктов гумификации. Структура крупных гранул наиболее сложна: наружный горизонт поддерживается за счет тесного взаимодействия глинистых минералов, нитчатых цианобактерий и других микроскопических фотоавтотрофов с обильным полимерным матриксом, а во внутреннем микрогоризонте поддерживаются преимущественно анаэробные условия и накапливаются остаточные твердофазные продукты трансформации ОВ. Наличие многочисленных минеральных поверхностей (глинистые минералы, выветрелые зерна первичных минералов) создает дополнительные условия для стабилизации ОВ. Метастабильные почвоподобные тела на криоконите, по-видимому, могут присутствовать в очень широком диапазоне условий на всех типах ледников за исключением самых удаленных внутренних участков ледяных щитов. Время существования таких почвоподобных тел зависит от локальных условий и варьирует от недель до десятков лет, например, в запечатанных ледяной крышкой стаканах с криоконитом на ледниках Антарктиды.
Поверхности с перекрытием льда чехлом мелкоземисто-обломочного материала. В условиях более мощной и более стабильной полиминеральной матрицы почвообразование проходит стадию предпочвы и почвоподобного тела, и уже через годы и десятки лет появляются почвы со слаборазвитым (рис. 8e), а затем и полноразвитым профилем (рис. 8f). Здесь возможен широкий спектр вариантов от петроземов, щебнистых пелоземов и псаммоземов, инициальных подзолов и камбисолей до более развитых почв в зависимости от глубины залегания льда, стабильности субстрата и длительности почвообразования. Супрагляциальные почвы на мелкоземисто-обломочном материале на ледниках и мертвом льду описаны в Антарктиде, Андах и Альпах, на Кавказе, Аляске и в Канаде, и, вероятно, имеют очень широкое распространение, а их классификационное разнообразие выявлено далеко неполностью.
Проведя типизацию супрагляциальных органо-минеральных образований, идентифицировали в них следующие процессы, характерные для почв и почвоподобных тел (табл. 1): аккумуляция ОВ, его фотохимическая и гетеротрофная трансформация, стабилизация ОВ на минеральных поверхностях, образование темноцветного “гумифицированного” ОВ, накопление остаточных твердофазных продуктов функционирования in situ, агрегация мелкозема и его биогенное оструктуривание, биохимическое выветривание минеральных частиц, и в итоге – формирование микро- и макропрофилей почв и почвоподобных тел на льду или на силикатных отложениях над льдом.
Таблица 1.
Процессы в супрагляциальных органо-минеральных образованиях, характерные для почв и почвоподобных тел
| Органо-минеральные образования | Процесс | Ссылка | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Аккумуляция ОВ | Фотохимическая трансформация ОВ | Гетеротрофная трансформация ОВ | Образование темноцветного “гумифицированного” ОВ | Стабилизация ОВ на минеральных поверхностях | Накопление остаточных твердофазных продуктов функционирования in situ | Агрегация мелкозема, биогенное структурообразование | Биохимическое выветривание | Формирование микропрофилей | Формирование макропрофилей | ||
| Во льду и снеге на леднике | |||||||||||
| Зеленый, розовый, “арбузный” лед/снег | ++ | ++ | + | ? | + | ++ | ? | ? | - | - | [39, 40, 90, 108, 192, 201] |
| Ледовые почвы | ++ | ++ | ++ | +++ | + | +++ | ++ | + | + | ++ | [24, 39, 40, 192] |
| Слоистые седименты в критической зоне ледника / годичные слои/ мелкозем между кристаллами льда | + | + | + | ? | ? | ? | ? | + | ? | ? | Данные авторов |
| Под растениями на леднике | |||||||||||
| Почвы с микропрофилем под моховыми сообществами – “ледниковые мышки” | ++ | ? | + | + | ++ | ++ | ++ | ++ | +++ | – | [4, 48, 107, 110, 177], данные авторов |
| Инситный криоконит на леднике | |||||||||||
| Элементарные агрегаты (микрогранулы) | ++ | ++ | + | ++ | ++ | ++ | ++ | + | + | – | [137, 187, 192, 216, 217, 221, 255] |
| Сложные агрегаты (мезогранулы) | +++ | ++ | ++ | +++ | ++ | +++ | +++ | ++ | ++ | – | |
| Переотложенный криоконит на леднике | |||||||||||
| Поля дисперсного криоконита и струйчатые аккумуляции вдоль ручьев / конические формы с ледяным ядром | + | +++ | +++ | +++ | ++ | ++ | + | + | + | – | Данные авторов, [128, 162] |
| На мелкоземисто-обломочном материале на ледниках и мертвом льду | |||||||||||
| Почвы на кварц-силикатных отложениях с близким подстиланием ледников | + | н/п | ++ | + | ++ | ++ | + | ++ | н/п | + | [46, 63, 82, 83, 194] |
| Почвы на мелкоземисто-обломочном материале на ледниках и мертвом льду | ++ | н/п | ++ | + | ++ | ++ | ++ | ++ | н/п | +++ | [180, 193, 209] |
Степень выраженности процесса: слабо (+), средне (++), сильно (+++), неизвестно (?), неприменимо (н/п). Под микропрофилями подразумевается последовательность почвенных горизонтов О, АO и W под моховыми сообществами на леднике или окислительно-восстановительная стратификации внутри биогенных агрегатов (гранул) и их скоплениях на леднике.
ОБМЕН ПАРНИКОВЫХ ГАЗОВ НА ПОВЕРХНОСТИ ЛЕДНИКОВ И В ПЕРИГЛЯЦИАЛЬНОМ ЛАНДШАФТЕ
Можно выделить четыре потенциально значимых компонента, влияющих на круговорот биогенных парниковых газов (ПГ) (CO2, СН4 и N2O) в структуре ледникового биома: (1) поверхность ледников с активной супрагляциальной системой, (2) перигляциальные отложения, (3) талые воды и (4) подледные отложения. Это отражает уникальную особенность ледникового биома по сравнению с остальными: в современном “тающем” мире он является автотрофно-гетеротрофной экосистемой и донором биогенных элементов, накопленных как за прошлые ледниковые эпохи, так и в современной супрагляциальной зоне. Различные компоненты супрагляциальной зоны и перигляциального ландшафта могут быть как нетто-стоками, так и источниками ПГ (рис. 9).
Рис. 9.
Ледниковый биом и потоки парниковых газов (ПГ) в супрагляциальной и ближней перигляциальной зоне.
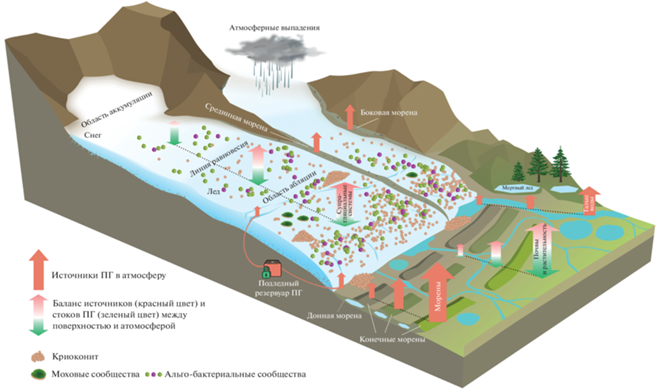
Поверхность ледников. Как показывают расчеты на основе немногочисленных опубликованных данных [100], ледники за сезон абляции (обычно эта оценка принимается за годовое дыхание) с учетом криоконита в стаканах протаивания и дисперсного криоконита в процессе микробного дыхания выделяют 12–14 кг С км2. Это суммарно дает лишь 0.22 млн т С в год, что нельзя считать существенным вкладом в биосферное дыхание. Только российские тундры в конце XX в. выделяли в процессе дыхания 474 млн т С в год, что на три порядка больше [252]. Если учесть первичную продукцию на ледниках, то оказывается, что это преимущественно нетто-автотрофные системы, где первичная продукция преобладает над валовым дыханием [34, 64, 100], хотя встречаются и нетто-гетеротрофные криоконитовые экосистемы [99]. Однако натурных оценок собственно NEP (net ecosystem production = валовая первичная продукция – дыхание экосистемы) на ледниках, и по сей день, очень мало. Согласно обзору [64], внутри криоконитовых стаканов валовое дыхание менялось от 1.86 до 42.1, а нетто-баланс от –2.03 (С-источник) до +14.6 (С-сток) мкг C–CO2/(г сут). По данным того же обзора, из 27 найденных в литературе публикаций по первичной продуктивности, экосистемному дыханию или нетто-балансу супрагляциального материала, лишь 10 содержали оценки последнего показателя, охватывая период с 2007 по 2015. Авторы [212, 213] провели единственное в своем роде масштабное обследование вдоль 70 км трансекты поперек края Гренландского ледяного щита, надежно показав, что экосистемы криоконита в период таяния поглощают углерод, т.е. являются преимущественно автотрофными. Основными факторами нетто-баланса являются влияние талых вод и крутизны склона – дальше от края к центру ледника склон становится более пологим, что способствует увеличению количества и тонкослойному распределению массы внутри стаканов протаивания с криоконитом; в них дольше задерживаются биогенные элементы и выше первичная продукция. Но ближе к зоне таяния, где идет мощный смыв талыми водами, вещества криоконитов и микробиоты становится меньше, и нетто-баланс вновь снижается. Эта зона таяния постепенно движется вверх в ходе теплого сезона. Отсюда следует, что нельзя просто экстраполировать данные с малых (горно-долинных) ледников на мощные покровные.
Вскипание криоконита в результате пузырькового транспорта отмечено как важный путь поступления СО2 и метана в приземный слой воздуха из тающего льда, что может скачкообразно и синхронно повышать фоновую концентрацию CH4 на 20 ppb, а CO2 на 5 ppm, за интервалы времени порядка 10–15 мин [259]. В результате до 79% поступающего метана из талых ледниковых вод обязано именно пузырьковому транспорту [262].
Первая (и пока единственная) попытка оценить глобальный вклад поверхности ледников в биосферный нетто-баланс СО2 была предпринята авторами [36]. Экстраполируя имеющиеся данные, и исходя из преобладания автотрофии в стаканах протаивания с криоконитом, авторы рассчитали глобальную оценку стока в –0.064 млн т С в год для поверхности ледников вне Антарктики. Вновь сравнивая с приведенной выше оценкой для российской тундровой зоны (сток – 11.6 млн т С в год), можно видеть насколько первая величина незначительна. Однако средние удельные площадные оценки продукции и дыхания криоконитов на 3–4 порядка выше, чем на общей поверхности ледников, и сопоставимы с эмиссией из арктических озер. Используя эти данные, общий вклад криоконита в нетто-обмен СО2 между поверхностью ледников и атмосферой можно минимально оценить, как 77%.
Такие экстраполяции относятся исключительно к сезонам таяния льда, при этом наиболее длительный, зимний период фактически игнорируется, что может значительно занижать оценки экосистемного дыхания и переоценивать нетто-сток СО2 за год. Кроме того, почти все данные касаются покровных ледников Гренландии, Антарктики, а также ледников Шпицбергена. Сейчас активные исследования по обмену ПГ стали проводиться на Тибетском плато – крупнейшем ледниковом массиве на суше после Антарктики и Гренландии [259]. Российская территория Евразии остается в этом отношении практически неохваченной. Исключение составляют недавние работы в перигляциальной зоне крупнейшего покровного ледника России на архипелаге Новая Земля [20] и первые оценки баланса парниковых газов на ледниках Кавказа и Алтая [14].
Однако значение ледников как биосферных агентов определяется не существующей “прижизненной” величиной баланса СО2 на их поверхности (что во многом зависит от присутствия криоконита), а консервирующими свойствами массы льдов, а также их высоким альбедо. Поэтому баланс СО2 и других биогенных ПГ гораздо важнее изучать на месте сошедшего ледника, а также в процессе его таяния.
Кроме изучения перигляциальных потоков ПГ в локальном масштабе, крайне скромно представлены исследования, охватывающие ледниковый ландшафт в целом, включая вклад абиотических процессов. Одной из пионерных здесь является работа [239] по Западному Тянь-Шаню, которая показала, что весь ледниковый бассейн за сезон таяния оказался небольшим, но значимым стоком СО2 величиной –0.05 над чистым льдом, и –0.07 мкмоль/(м2 с) над молодыми моренами с ледяным основанием. Важно, что основным механизмом в данном случае является абиотическое поглощение СО2 талой водой с образованием угольной кислоты. Таким образом, “химический насос”, выкачивающий СО2 из атмосферы, заменил собой насос биологический, действующий через фотосинтез. При этом были отмечены сильные колебания стока СО2 на часовом шаге измерений, что связывается со скачкообразным высвобождением газов из пузырьков, содержащихся в таящем льду [189]. В принципе, такой механизм должен приводить к увеличению эмиссии в атмосферу ПГ, таких как СО2, что противоречит результатам, демонстрирующим его преимущественное поглощение. Однако воздух в пузырьках, образующихся при таянии, содержит концентрацию СО2 меньшую, чем в современной атмосфере, т.е. ~295 ррm, что характерно для дотехнологической эпохи. Видимо выделение этих дополнительных молекул успевает компенсироваться их химическим связыванием в ландшафтном и сезонном масштабах. При этом 75% С растаявших ледников выносится в растворенном виде в океан, в форме ${\text{НСО}}_{3}^{ - }$, и только 25% в газообразном виде сразу поступает в атмосферу [189].
Этот факт указывает на активное направление, которое сформировалось за последнее десятилетие в отношении эмиссии СО2 и метана из талых ледниковых вод, перигляциальных образований и подледных отложений, что включает в исследовательскую орбиту ледниковый ландшафт в целом, и граничащие с ледниками наземные и водные биомы. Наиболее подробно в этом отношении исследован обмен Гренландского ледника [35, 56, 134, 189], и начато подробное изучение Тибетского плато [259]. Все реки и озера выделяют респираторный СО2 в атмосферу, но в ледниковых реках и озерах становится важен вклад химического выветривания с участием карбонатов и силикатов, в ходе которого СО2 переходит в форму гидрокарбонат-аниона ${\text{НСО}}_{3}^{ - }$, что может считаться стоком углерода, если оперировать характерными временами жизни гидрокарбонат-аниона в водах суши (102–103 лет) или в океане (105 лет) [189]. Множественный анализ водных проб показывает, что при удалении от Гренландского щита к океану содержание растворенного СО2 постепенно уменьшается, а ${\text{НСО}}_{3}^{ - }$ увеличивается, затем, по мере установления равновесия с атмосферой (примерно, через 20 км от ледника), содержание С в этих формах перестает меняться. Согласно предварительным оценкам, на 50% это СО2, содержащийся в поверхностных водах, стекающих с ледника, а остальное количество выделяется из-под самого ледяного щита. Вода из-под Гренландского ледника поступает очень насыщенная СО2, но при этом этот газ выделяется в атмосферу в очень небольших количествах – порядка 0.11 млн т С в год. Тем не менее, модели показывают, что при наиболее напряженных климатических сценариях, по достижении в ходе таяния нижних слоев льда, насыщенных СО2, к 2100 г. эмиссия может достигнуть 100–170 млн т С(СО2) в год [189], что сравнимо, например, с современной эмиссией от лесных пожаров в России.
Концентрации СО2 и метана в талых ледниковых водах могут на порядки отличаться между участками Гренландского ледника, а сами воды могут быть как перенасыщенными, так и недонасыщенными этими газами [165]. В частности, существенные различия в величине подледниковых источников СО2, при относительном равенстве скоростей атмосферных стоков, приводят к тому, что местные талые воды являются либо нетто-источниками, либо поглотителями атмосферного CO2. Эти результаты подчеркивают ранее не признанную степень неоднородности в динамике парниковых газов под ледяным щитом. При этом ледниковые воды всегда остаются источниками метана [259]. В целом метан, содержащийся в талых ледниковых водах Земли, представляет собой потенциальный источник в атмосферу около 1 млн т в год при его запасах под ледниками в форме газогидратов до 480 млрд т С–СН4, а общее поступление углерода во всех формах с абляцией составляет не менее 6 млн т С в год [238].
Сейчас появились новейшие крупномасштабные исследования, оценивающие баланс C-содержащих ПГ во всех основных компонентах ледникового ландшафта Тибетского плато, включая супрагляциальные поверхности, криоконитовые стаканы, перигляциальные отложения и перигляциальные реки и озера [259], где показано значительно усиление эмиссии метана и СО2 из моренных отложений и талых ледниковых вод в результате потепления климата. Распространение застойного увлажнения, озер и болот на Тибетском плато в результате прогрессирующего отступания ледников увеличило эмиссию СН4 до 0.96 млн т в год в 2000-е гг., что по независимым оценкам, превысило скорость его поглощения на той же площади (0.74 млн т в год) [242]. При том, что стаканы протаивания с криоконитом в этом ландшафте могут быть как нетто-стоками, так и источниками СО2, несомненно то, что они выступают нетто-источниками метана [259].
Среди трех наиболее важных биогенных ПГ, меньше всего исследований в супрагляциальной и перигляциальной зонах посвящено балансу N2O, что объясняется недостаточной величиной его потоков для инструментальной оценки. Значимые выбросы N2O фиксируются только на моренных отложениях при появлении на них растительности [226] или под влиянием гуано в колониях птиц [240]. При этом важными факторами баланса между продукцией и поглощением газа являются рН и степень аэрации почв, также возможен существенный вклад абиогенной продукции закиси азота.
Продолжающееся потепление климата диктует необходимость объединения в прогностических моделях биосферной динамики всех компонентов ледникового биома (супрагляциальных поверхностей, субгляциальных отложений, морен и талых вод), и учета значимых положительных обратных связей. При этом важно включение в биосферные модели биотической составляющей баланса ПГ ледников. Существует отдельная нехватка данных по газообмену ПГ покровных и горных ледников, расположенных на территории России, все еще представляющей в этом отношении “белое пятно”. Необходимость таких исследований связана с тем, что показатели продукции, поглощения и транспорта ПГ могут существенно зависеть от местных особенностей строения ледников, климата, антропогенного влияния, и предшествующей геологической истории.
ЗАКЛЮЧЕНИЕ
Супрагляциальные органо-минеральные образования обладают наиболее разнообразным составом и самой высокой скоростью биологического круговорота среди всех компонентов ледникового биома. В период абляции на леднике нет недостатка в жидкой воде, органических и минеральных примесях, взаимодействие которых приводит к появлению органо-минеральных тел с новыми свойствами, меняющими структуру сообщества организмов, химический состав вещества и спектральные характеристики поверхности ледника, так как не сделал бы ни один из компонентов по отдельности (эмерджентность системы). Анализ разнообразия, строения и процессов формирования супрагляциальных органо-минеральных образований показал, что некоторые из них имеют признаки почв и почвоподобных тел. Важнейшими из таких признаков являются накопление ОВ, его стабилизация на минеральных поверхностях и в агрегатах, трансформация ОВ с остаточным накоплением темноцветных твердофазных продуктов in situ. Среди супрагляциальных образований предлагается выделять предпочвы, в том числе эфемерные, и почвоподобные тела во льду и снеге, метастабильные почвоподобные тела на криоконите и почвы с микропрофилями под моховыми сообществами на льду, а также относительно стабильные почвы с макропрофилями на мелкоземисто-обломочных отложениях с подстиланием ледников и мертвого льда. Легкодоступное ОВ, полученное в супрагляциальном “реакторе”, в том числе в его ячейках – почвах и почвоподобных телах, оказывает существенное влияние на перигляциальную зону, приводя к резервуарному и прайминг эффектам и воздействуя на все звенья трофической цепи, а также на формирование инициального почвенного покрова после отступания ледников.
Супрагляциальная зона ледников представляет собой автотрофно-гетеротрофную экосистему. Ее органо-минеральные компоненты, включая почвы и почвоподобные тела, при разных условиях являются как нетто-стоками, так и источниками парниковых газов. Однако в современном “тающем” мире биосферное значение ледников определяется не только “прижизненной” величиной баланса парниковых газов на их поверхности, но и консервирующими свойствами массы льдов, а также их альбедо. Поэтому баланс парниковых газов необходимо изучать во всех компонентах ледникового ландшафта, в том числе на месте сошедших ледников и в процессе их таяния.
Список литературы
Абакумов Е.В., Темботов Р.Х. Влияние светопоглощающих частиц на дегляциацию ледников полярных и горных территорий // Самарская Лука: проблемы региональной и глобальной экологии. 2020. Т. 29. № 2. С. 5–11.
Абакумов Е.В., Темботов Р.Х. Биохимические свойства криоконитов ледников Центрального Кавказа // Самарская Лука: проблемы региональной и глобальной экологии. 2021. Т. 30. № 3. С. 38–46.
Абакумов Е.В., Жиянски М., Чиграй С.Н., Поляков В.И. Роль птиц в формировании органо-минеральных криоконитов на ледниках Субантарктики // Русский орнитологический журн. 2020. № 29(1957). С. 3540–3544.
Белкина О.А., Мавлюдов Б.Р. Мхи на ледниках Шпицбергена // Ботанический журн. 2011. № 96(5). С. 582–596.
Галанин А.А. Каменные глетчеры-особый тип современного горного оледенения северо-востока Азии // Вестник Дальневосточного отделения Российской академии наук. 2005. № 5. С. 59–70.
Глазовская М.А. Эоловые мелкоземистые накопления на ледниках хребта Терскей Ала-Тау // Тр. Института географии АН СССР. 1952. Вып. 49. С. 55–69.
Глазовская М.А. Эоловые отложения на ледниках Тянь-Шаня // Природа. 1954. № 2. С. 90–92.
Глазовская М.А. Субаэральные покровные пылеватые суглинки и почвы в высокогорьях Внутреннего Тянь-Шаня // Многоликая география. Развитие идей Иннокентия Петровича Герасимова (к 100-летию со дня рождения). 2005. С. 132–163.
Геннадиев А.Н. Изучение почвообразования методом хронорядов (на примере почв Приэльбрусья) // Почвоведение. 1978. № 12. С. 33–43.
Горбунов А., Горбунова И. География каменных глетчеров мира. М.: Товарищество научных изданий КМК, 2010.
Горячкин C.В., Мергелов Н.С., Таргульян В.О. Генезис и география почв экстремальных условий: элементы теории и методические подходы // Почвоведение. 2019. № 1. С. 5–19.
Горячкин С.В. География экстремальных почв и почвоподобных систем // Вестник РАН. 2022. № 92(6). С. 564–571.
Зазовская Э.П., Мергелов Н.С., Шишков В.А., Долгих А.В., Добрянский А.С., Лебедева М.П., Турчинская С.М., Горячкин С.В. Криокониты как факторы развития почв в условиях быстрого отступания ледника Альдегонда, Западный Шпицберген // Почвоведение. 2022. № 3. С. 281–295. https://doi.org/10.31857/S0032180X22030157
Карелин Д.В., Кутузов С.С., Горячкин С.В., Зазовская Э.П., Котляков В.М. Российские горные ледники в “тающем” мире: первые оценки баланса парниковых газов на Кавказе и Алтае // Доклады РАН. Науки о Земле. 2022. Т. 504(1). С. 104–109.
Классификация и диагностика почв России. Смоленск: Ойкумена, 2004. 342 с.
Котляков В.М., Рототаева О.В., Носенко Г.А., Десинов Л.В., Осокин Н.И., Чернов Р.А. Кармадонская катастрофа: что случилось и чего ждать дальше. М.: Издательский дом “Кодекс”, 2014. 184 с.
Мавлюдов Б.Р. Дренажные системы ледников. М.: Изд-во Ин-та географии РАН. 2006.
Миндлин С.З., Петрова М.А. О происхождении и распространении устойчивости к антибиотикам: результаты изучения древних бактерий из многолетнемерзлых отложений // Молекулярная генетика, микробиология и вирусология. 2017. 35(4). С. 123–132.
Москалевский М.Ю. О роли криогенного фактора при формировании донно-моренных отложений (в условиях покровного оледенения Северной Земли) // Проблемы криолитологии. 1978. Вып. VIII. С. 178–183.
Никитин Д.А., Лысак Л.В., Бадмадашиев Д.В., Холод С.С., Мергелов Н.С., Долгих А.В., Горячкин С.В. Биологическая активность почв в условиях покровного оледенения в северной части архипелага Новая Земля // Почвоведение. 2021. №10. С. 1207–1230. https://doi.org/10.31857/S0032180X21100087
Носенко Г.А., Никитин С.А., Хромова, Т.Е. Изменение площади и объема ледников Горного Алтая (Россия) с середины ХХ в. по данным космических съемок // Лед и снег. 2015. № 54(2). С. 5–13.
Таргульян В.О. Теория педогенеза и эволюции почв. М.: Издательство ГЕОС. 2019. 297 с.
Таргульян B.О. Элементарные почвообразовательные процессы // Почвоведение. 2005. № 12. С. 1413–1422.
Таширев А.Б., Таширева А.А., Березкина А.Е. Роль криоценозов в формировании почв на ледниках Западной Антарктики // Доповiдi Національної академії наук України. 2012. № 4. С. 155–161.
Ферсман А.Е. Геохимия и минералогия полярных областей // Доклады АН СССР. 1938. Т. 19. № 8.
Чумаков Н.М. Оледенения Земли История, стратиграфическое значение и роль в биосфере // Тр. Геологического ин-та. 2015. № 611.
Шишков В.А., Зазовская Э.П., Лебедева М.П., Мергелов Н.С., Долгих А.В. Особенности микростроения почв, развитых на криоконитах в экстремальных условиях зоны отступания ледника Альдегонда (Западный Шпицберген) // Морфология почв от макро- до субмикроуровня. 2016. С. 359–363.
Abakumov E., Nizamutdinov T., Polyakov V. Analysis of the polydispersity of soil-like bodies in glacier environments by the laser light scattering (diffraction) method // Biol. Comm. 2021. V. 66(3). P. 198–209. https://doi.org/10.21638/ spbu03.2021.302
Abakumov E., Tembotov R., Kushnov I., Polyakov V. Micromorphology of cryoconite on Garabashi and Skhelda glaciers and soils of Baksan Gorge, Mt. Elbrus, Central Caucasus // Polish Polar Res. 2021. V. 43(1). P. 1–20. https://doi.org/10.24425/ppr.2021.138590
Abakumov E., Kushnov I., Nizamutdinov T., Tembotov R. Cryoconites as biogeochemical markers of anthropogenic impact in high mountain regions: analysis of polyaromatic pollutants in soil-like bodies // One Ecosystem. 2022. V. 7. P. 1–26. https://doi.org/10.3897/oneeco.7.e78028
Abakumov E., Gangapshev A., Gezhaev A., Tembotov R. Radionuclide activity in cryoconite from glaciers of the Central Caucasus, Russia // Solid Earth Sci. 2022. V. 7(4). P. 268–275. https://doi.org/10.1016/j.sesci.2022.08.001
Abbot D.S., Pierrehumbert R.T. Mudball: Surface dust and snowball Earth deglaciation // J. Geophys. Res.: Atmospheres. 2010. V. 115(D3). P. 1–11. https://doi.org/10.1029/2009JD012007
Abouhend A.S., Milferstedt K., Hamelin J., Ansari A.A., Butler C., Carbajal-González B.I., Park C. Growth progression of oxygenic photogranules and its impact on bioactivity for aeration-free wastewater treatment // Environ. Sci. Technol. 2019. V. 54(1). P. 486-496. https://doi.org/10.1021/acs.est.9b04745
Ambrosini R., Azzoni R.S., Pittino F., Diolaiuti G., Franzetti A., Parolini M. First evidence of microplastic contamination in the supraglacial debris of an alpine glacier // Environ. Poll. 2019. V. 253. P. 297–301. https://doi.org/10.1016/j.envpol.2019.07.005
Andrew L.C. Greenland’s subglacial methane released // Nature. 2019. V. 565(7737). P. 31–32. https://doi.org/10.1038%2Fd41586-018-07762-7
Anesio A.M., Hodson A.J., Fritz A., Psenner R., Sattler B. High microbial activity on glaciers: importance to the global carbon cycle // Global Change Biol. 2009. V. 15(4). P. 955–960. https://doi.org/10.1111/j.1365-2486.2008.01758.x
Anesio A.M., Laybourn-Parry J. Glaciers and ice sheets as a biome // Trends Ecology Evolution. 2012. V. 27(4). P. 219–225. https://doi.org/10.1016/j.tree.2011.09.012
Anesio A.M., Mindl B., Laybourn-Parry J., Hodson A.J., Sattler B. Viral dynamics in cryoconite on a high Arctic glacier (Svalbard) // J. Geophy. Res. 2007. V. 112(G4). P. 1–10. https://doi.org/10.1029/2006JG000350
Antony R., Grannas A.M., Willoughby A.S., Sleighter R.L., Thamban M., Hatcher P.G. Origin and sources of dissolved organic matter in snow on the East Antarctic ice sheet // Environ. Sci. Technol. 2014. V. 48(11). P. 6151–6159. https://doi.org/10.1021/es405246a
Antony R., Willoughby A.S., Grannas A.M., Catanzano V., Sleighter R.L., Thamban M., Hatcher P.G., Nair S. Molecular insights on dissolved organic matter transformation by supraglacial microbial communities // Environ. Sci. Technol. 2017. V. 51(8). P. 4328–4337. https://doi.org/10.1021/acs.est.6b05780
Baccolo G., Di Mauro B., Massabò D., Clemenza M., Nastasi M., Delmonte B., Prata M., Prati P., Previtali E., Maggi V. Cryoconite as a temporary sink for anthropogenic species stored in glaciers // Sci. Rep. 2017. V. 7(1). P. 1–11. https://doi.org/10.1038/s41598-017-10220-5
Baccolo G., Łokas E., Gaca P., Massabò D., Ambrosini R., Azzoni R.S., Clason C., Di Mauro B., Franzetti A., Nastasi M., Prata M., Prati P., Previtali E., Delmonte B., Maggi V. Cryoconite: an efficient accumulator of radioactive fallout in glacial environments // The Cryosphere. 2020. V. 14(2). P. 657–672. https://doi.org/10.5194/tc-14-657-2020
Bagshaw E.A., Tranter M., Fountain A.G., Welch K.A., Basagic H., Lyons W.B. Biogeochemical evolution of cryoconite holes on Canada Glacier, Taylor Valley, Antarctica // J. Geophys. Res. 2007. V. 112(G4). P. 1–8. https://doi.org/10.1029/2007JG000442
Bagshaw E.A., Tranter M., Fountain A.G., Welch K., Basagic H.J., Lyons W.B. Do cryoconite holes have the potential to be significant sources of C, N and P to downstream depauperate ecosystems of Taylor Valley, Antarctica? // Arct. Antarct. Alp. Res. 2013. V. 45(4). P. 1–15. https://doi.org/10.1657/1938-4246-45.4.440
Bagshaw E.A., Tranter M., Wadham J.L., Fountain A.G., Dubnick A., Fitzsimons S. Processes controlling carbon cycling in Antarctic glacier surface ecosystems // Geochem. Perspect. Lett. 2016. V. 2(1). P. 44–54. https://doi.org/10.7185/geochemlet.1605
Balks M.R., López-Martínez J., Goryachkin S., Mergelov N.S., Schaefer C.E.G.R., Simas F.N.B., Almond P.C., Claridge G.G.C., McLeod M., Scarrow J. Windows on Antarctic Soil-Landscape relations across selected regions of Antarctica // Geological Society London Special Publications. 2013. V. 381 (1). P. 397–410. https://doi.org/10.1144/SP381.9
Bardgett R.D., Richter A., Bol R., Garnett M.H., Bäumler R., Xu X., Lopez-Capel E., Manning D., Hobbs P., Hartley I., Wanek W. Heterotrophic microbial communities use ancient carbon following glacial retreat // Biology letters. 2007. V. 3(5). P. 487–490. https://doi.org/10.1098%2Frsbl.2007.0242
Belkina O.A., Vilnet A.A. Some aspects of the moss population development on the Svalbard glaciers // Czech Polar Rep. 2015. V. 5(2). P. 160–175. https://doi.org/10.5817/CPR2015-2-14
Bellas C.M., Anesio A.M., Telling J. et al. (2013) Viral impacts on bacterial communities in Arctic cryoconite // Environ. Res. Lett. V. 8. P. 045021.
Benn D.I., Le Hir G., Bao H., Donnadieu Y., Dumas C., Fleming E.J., et al Orbitally forced ice sheet fluctuations during the Marinoan Snowball Earth glaciation // Nat. Geosci. 2015. V. 8(9). P. 704–707.
Beraldi-Campesi H. Early life on land and the first terrestrial ecosystems // Ecol. Process. 2013. V. 2(1). P. 1–17. https://doi.org/10.1186/2192-1709-2-1
Blank C.E., Sanchez-Baracaldo P. Timing of morphological and ecological innovations in the cyanobacteria–a key to understanding the rise in atmospheric oxygen // Geobiology. 2010. V. 8(1). P. 1–23. https://doi.org/10.1111/j.1472-4669.2009.00220.x
Bond T., Doherty S., Fahey D., Forster P., Berntsen T., DeAngelo B.J., Flanner M., Ghan S., Kaercher B., Koch D. et al. Bounding the role of black carbon in the climate system: A scientific assessment // J. Geophys. Res. Atmos. 2013. V. 118. P. 5380–5552. https://doi.org/10.1002/jgrd.50171
Bourgeois J.C., Gajewski K., Koerner R.M. Spatial patterns of pollen deposition in arctic snow // J. Geophys. Res. Atmos. 2001. V. 106(D6). P. 5255–5265. https://doi.org/10.1029/2000JD900708
Buda J., Łokas E., Pietryka M., Richter D., Magowski W., Iakovenko N.S., Porazinska D.L., Budzik T., Grabiec M., Grzesiak J., Klimaszyk P., Gaca P., Zawierucha K. Biotope and biocenosis of cryoconite hole ecosystems on Ecology Glacier in the maritime Antarctic // Sci. Total Environ. 2020. V. 724. P. 138112. https://doi.org/10.1016/j.scitotenv.2020.138112
Burns R., Wynn P.M., Barker P., McNamara N., Oakley S., Ostle N., Stott A.W., Tuffen H., Zhou Z., Tweed F.S., Chesler A. Stuart M. Direct isotopic evidence of biogenic methane production and efflux from beneath a temperate glacier // Sci. Rep. 2018. V. 8(17118). P. 1–8. https://doi.org/10.1038/s41598-018-35253-2
Butterfield N.J. Early evolution of the Eukaryota // Palaeontology. 2015. V. 58(1). P. 5–17. https://doi.org/10.1111/pala.12139
Cameron K.A., Hodson A.J., Osborn A.M. Structure and diversity of bacterial, eukaryotic and archaeal communities in glacial cryoconite holes from the Arctic and the Antarctic // FEMS Microbiol. Ecol. 2012. V. 82(2). P. 254–267. https://doi.org/10.1111/j.1574-6941.2011.01277.x
Casey K.A., Kääb A., Benn D.I. Geochemical characterization of supraglacial debris via in situ and optical remote sensing methods: a case study in Khumbu Himalaya, Nepal // Cryosphere. 2012. V. 6(1). P. 85–100. https://doi.org/10.5194/tc-6-85-2012
Chakrabarty R.K., Moosmüller H., Chen L.W., Lewis K., Arnott W.P., Mazzoleni C., Dubey M.K., Wold C.E., Hao W.M., Kreidenweis S.M. Brown carbon in tar balls from smoldering biomass combustion // Atmos. Chem. Phys. 2010. V. 10(13). P. 6363–6370. https://doi.org/10.5194/acp-10-6363-2010
Christner B.C., Kvitko B.H., Reeve J.N. Molecular identification of bacteria and eukarya inhabiting an Antarctic cryoconite hole // Extremophiles. 2003. V. 7(3). P. 177–183. https://doi.org/10.1007/s00792-002-0309-0
Cohen P.A., Macdonald F.A. The Proterozoic record of eukaryotes // Paleobiology. 2015. V. 41(4). P. 610–632. https://doi.org/10.1017/pab.2015.25
Collier E., Nicholson L.I., Brock B.W., Maussion F., Essery R., Bush A.B.G. Representing moisture fluxes and phase changes in glacier debris cover using a reservoir approach // The Cryosphere. 2014. V. 8(4). P. 1429–1444. https://doi.org/10.5194/tc-8-1429-2014
Cook J., Edwards A., Takeuchi N., Irvine-Fynn T. Cryoconite: the dark biological secret of the cryosphere // Prog. Phys. Geogr. 2016. V. 40(1). P. 66–111. https://doi.org/10.1177/0309133315616574
Cook J.M., Hodson A.J., Irvine-Fynn T.D. Supraglacial weathering crust dynamics inferred from cryoconite hole hydrology / Hydrological Processes. Epubahead of print. 2016. https://doi.org/10.1002/hyp.10602
Coulson S.J., Midgley N.G. The role of glacier mice in the invertebrate colonisation of glacial surfaces: the moss balls of the Falljökull, Iceland // Polar Biol. 2012. V. 35(11). P. 1651–1658. https://doi.org/10.1007/s00300-012-1205-4
D'Andrilli J., Foreman C.M., Sigl M., Priscu J.C., McConnell J.R. 2017. A 21 000-year record of fluorescent organic matter markers in the WAIS Divide ice core // Clim. Past V. 13(5). P. 533–544. https://doi.org/10.5194/cp-13-533-2017
Darcy J.L., Gendron E., Sommers P., Porazinska D.L., Schmidt S.K. Island biogeography of cryoconite hole bacteria in Antarctica’s Taylor Valley and around the world // Front. Ecol. Evol. 2018. V. 6(180). https://doi.org/10.3389/fevo.2018.00180
Dawson J.B., Hinton R.W., Steele I.M. The composition of anorthoclase and nepheline in Mount Kenya phonolite and Kilimanjaro trachyte, and crystal–glass partitioning of elements // Can. Mineral. 2008. V. 46(6). P. 1455–1464. https://doi.org/10.3749/canmin.46.6.1455
de Menezes G.C.A., Porto B.A., Simões J.C., Rosa C.A., Rosa L.H. Fungi in snow and glacial ice of Antarctica / Fungi of Antarctica: diversity, ecology and biotechnological applications. 2019. P. 127–146. https://doi.org/10.1007/978-3-030-18367-7_6
de Menezes G.C.A., Amorim S.S., Gonçalves V.N., Godinho V.M., Simões J.C., Rosa C.A. Rosa L.H. Diversity, Distribution, and Ecology of Fungi in the Seasonal Snow of 918 Antarctica // Microorganisms. 2019. V. 7. P. 445–445. https://doi.org/10.3390/microorganisms7100445
Deuerling K.M., Lyons W.B., Welch S.A., Welch K.A. The characterization and role of aeolian deposition on water quality, McMurdo Dry Valleys, Antarctica // Aeolian Res. 2014. V. 13. P. 7–17. https://doi.org/10.1016/j.aeolia.2014.01.002
Di Mauro B., Baccolo G., Garzonio R., Giardino C., Massabò D., Piazzalunga A., Rossini M., Colombo R. Impact of impurities and cryoconite on the optical properties of the Morteratsch Glacier (Swiss Alps) // Cryosphere. 2017. V. 11. P. 2393–2409. https://doi.org/10.5194/tc-11-2393-2017
Di Mauro B., Fava F., Ferrero L., Garzonio R., Baccolo G., Delmonte B., Colombo R. Mineral dust impact on snow radiative properties in the European Alps combining ground, UAV, and satellite observations // J. Geophys. Res. 2015. V. 120. P. 6080–6097. https://doi.org/10.1002/2015JD023287
Di Mauro B., Garzonio R., Rossini M., Filippa G., Pogliotti P., Galvagno M., Morra di Cella U., Migliavacca M., Baccolo G., Clemenza M., Delmonte B., Maggi, Dumont V.M., Tuzet F., Lafaysse M., Morin S., Cremonese E., Colombo R. Saharan dust events in the European Alps: Role in snowmelt and geochemical characterization // Cryosphere. 2019. V. 13. P. 1147–1165. https://doi.org/10.5194/tc-13-1147-2019
Edwards A., Anesio A.M., Rassner S.M., Sattler B., Hubbard B., Perkins W.T., Young M. Possible interactions between bacterial diversity, microbial activity and supraglacial hydrology of cryoconite holes in Svalbard // ISME J. 2011. V. 51(1). P. 150–160. https://doi.org/10.1038/ismej.2010.100
Edwards A., Pachebat J.A., Swain M. et al. A metagenomic snapshot of taxonomic and functional diversity in an alpine glacier cryoconite ecosystem // Environ. Res. Lett. 2013. V. 8. P. 035003. https://doi.org/10.1088/1748-9326/8/3/035003
Edwards M.B. Late Precambrian glacial loessites from north Norway and Svalbard // J. Sediment. Res. 1979. V. 49(1). P. 85–91. https://doi.org/10.1306/212F76C6-2B24-11D7-8648000102C1865D
Evans D.A. Stratigraphic, geochronological, and paleomagnetic constraints upon the Neoproterozoic climatic paradox // Am. J. Sci. 2000. V. 300(5). P. 347–433. https://doi.org/10.2475/ajs.300.5.347
Fellman J.B., Hood E., Raymond P.A., Hudson J., Bozeman M., Arimitsu M. Evidence for the assimilation of ancient glacier organic carbon in a proglacial stream food web // Limnol. Oceanogr. 2015. V. 60(4). P. 1118–1128. https://doi.org/10.1002/lno.10088
Ferrario C., Pittino F., Tagliaferri I., Gandolfi I., Bestetti G., Azzoni R.S., Diolaiuti G., Franzetti A., Ambrosini R., Villa S. Bacteria contribute to pesticide degradation in cryoconite holes in an Alpine glacier // Environ. Pollut. 2017. V. 230. P. 919–926. https://doi.org/10.1016/j.envpol.2017.07.039
Fickert T., Friend D., Grüninger F., Molnia B., Richter M. Did Debris-Covered Glaciers Serve as Pleistocene Refugia for Plants? A New Hypothesis Derived from Observations of Recent Plant Growth on Glacier Surfaces // Arct. Antarct. Alp. Res. 2007. V. 39(2). P. 245–257. https://doi.org/10.1657/1523-0430(2007)39[245:DDGSAP]2.0.CO;2
Fickert T., Friend D., Molnia B., Grüninger F., Richter M. Vegetation ecology of debris-covered glaciers (DCGs)–site conditions, vegetation patterns and implications for DCGs serving as quaternary cold-and warm-stage plant refugia // Diversity. 2022. V. 14(2). P. 114. https://doi.org/10.3390/d14020114
Fountain A.G., Tranter M., Nylen T.H., Lewis K.J., Mueller D.R. Evolution of cryoconite holes and their contribution to meltwater runoff from glaciers in the McMurdo Dry Valleys, Antarctica // J. Glaciol. 2004. V. 50(168). P. 323–335.
Fountain A.G., Nylen T.H., Tranter M., Bagshaw E., Temporal variations in physical and chemical features of cryoconite holes on Canada Glacier, McMurdo Dry Valleys, Antarctica // J. Geophys. Res. Biogeosci. 2008. V. 113(G1). https://doi.org/10.1029/2007JG000430
Goodwin K., Loso M.G., Braun M. Glacial transport of human waste and survival of fecal bacteria on Mt. McKinley’s Kahiltna Glacier, Denali National Park, Alaska // Arct. Antarct. Alp. Res. 2012. V. 44(4). P. 432–445. https://doi.org/10.1657/1938-4246-44.4.432
Graber E.R., Rudich Y. Atmospheric HULIS: How humic-like are they? A comprehensive and critical review // Atmos. Chem. Phys. 2006. V. 6(3). P. 729–753. https://doi.org/10.5194/acp-6-729-2006
Graham L.E., Cook M.E., Wilcox L.W., Graham J., Taylor W., Wellman C.H., Lewis L. Resistance of filamentous chlorophycean, ulvophycean, and xanthophycean algae to acetolysis: testing Proterozoic and Paleozoic microfossil attributions // Int. J. Plant Sci. 2013. V. 174(6). P. 947–957. https://doi.org/10.1086/670591
Grannas A.M., Hockaday W.C., Hatcher P.G., Thompson L.G., Mosley-Thompson E. New revelations on the nature of organic matter in ice cores // J. Geophys. Res. Atmos. 2006. V. 111(D4). https://doi.org/10.1029/2005JD006251
Gray A., Krolikowski M., Fretwell P., Convey P., Peck L.S., Mendelova M., Smith A.G., Davey M.P. Remote sensing reveals Antarctic green snow algae as important terrestrial carbon sink // Nat. Commun. 2020. V. 11(1). P. 2527. https://doi.org/10.1038/s41467-020-16018-w
Guo B., Liu Y., Liu K., Shi Q., He C., Cai R., Jiao N. Different dissolved organic matter composition between central and southern glaciers on the Tibetan Plateau // Ecol. Indic. 2022. V. 139. P. 108888. https://doi.org/10.1016/j.ecolind.2022.108888
Hadley O.L., Kirchstetter T.W. Black-carbon reduction of snow albedo // Nat. Clim. Change 2012. V. 2(6). P. 437–440. https://doi.org/10.1038/nclimate1433
Hågvar S., Ohlson M. Ancient carbon from a melting glacier gives high 14C age in living pioneer invertebrates // Sci. Rep. 2013. V. 3(1). P. 1–4. https://doi.org/10.1038/srep02820
Hågvar S., Ohlson M., Brittain J.E. A melting glacier feeds aquatic and terrestrial invertebrates with ancient carbon and supports early succession // Arct. Antarct. Alp. Res. 2016. V. 48(3). P. 551–562. https://doi.org/10.1657/AAAR0016-027
Hansen R.R., Hansen O.L.P., Bowden J.J., Normand S., Bay C., Sørensen J.G., Høye T.T. High spatial variation in terrestrial arthropod species diversity and composition near the Greenland ice cap // Polar Biol. 2016. V. 39. P. 2263–2272. https://doi.org/10.1007/s00300-016-1893-2
Heusser C.J. Polsters of the moss Drepanocladus berggrenii on Gilkey Glacier, Alaska // Bulletin of the Torrey Botanical Club. 1972. P. 34–36. https://doi.org/10.2307/2484240
Hodson A. Understanding the dynamics of black carbon and associated contaminants in glacial systems // Wiley Interdiscip. Rev. Water. 2014. V. 1(2). P. 141–149. https://doi.org/10.1002/wat2.1016
Hodson A., Anesio A.M., Tranter M., Tranter M., Fountain A., Osborn M., Priscu J., Laybourn-Parry J., Sattler B. Glacial ecosystems // Ecol. Monogr. 2008. V. 78(1). P. 41–67. https://doi.org/10.1890/07-0187.1
Hodson A., Cameron K., Bøggild C., Irvine-Fynn T., Langford H., Pearce D., Banwar S. The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier: Longyearbreen, Svalbard // J. Glaciol. 2010. V. 56(196). P. 349–362. https://doi.org/10.3189/002214310791968403
Hodson A.J., Anesio A.M., Ng F., Watson R., Quirk J., Irvine-Fynn T., Dye A., Clark C., McCloy P., Kohler J. A glacier respires: quantifying the distribution and respiration CO2 flux of cryoconite across Arctic supraglacial ecosystem // J. Geophys. Res. 2007. V. 112(G4). P. G04S36. https://doi.org/10.1029/2007JG000452
Hoffman P.F. Cryoconite pans on Snowball Earth: supraglacial oases for Cryogenian eukaryotes? // Geobiology. 2016. V. 14(6). P. 531–542. https://doi.org/10.1111/gbi.12191
Hoffman P.F., Li Z.X. A palaeogeographic context for Neoproterozoic glaciation // Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009. V. 277(3–4). P. 158–172. https://doi.org/10.1016/j.palaeo.2009.03.013
Homann M., Sansjofre P., Van Zuilen M., Heubeck C., Gong J., Killingsworth B., Foster I.S., Airo A., Van Kranendonk M.J., Lalonde S.V. Microbial life and biogeochemical cycling on land 3,220 million years ago // Nat. Geosci. 2018. V. 11(9). P. 665. https://doi.org/10.1038/s41561-018-0190-9
Hood E., Battin T.J., Fellman J., O’neel S., Spencer R.G. Storage and release of organic carbon from glaciers and ice sheets // Nat. Geosci. 2015. V. 8(2). P. 91–96. https://doi.org/10.1038/ngeo2331
Hood E., Fellman J., Spencer R.G., Hernes P.J., Edwards R., D’Amore D., Scott D. Glaciers as a source of ancient and labile organic matter to the marine environment // Nature. 2009. V. 462(7276). P. 1044–1047. https://doi.org/10.1038/nature08580
Horneck G. The microbial world and the case for Mars // Planet. and Space Sci. 2000. V. 48(11). P. 1053–1063. https://doi.org/10.1016/S0032-0633(00)00079-9
Hotaling S., Bartholomaus T.C., Gilbert S.L. Rolling stones gather moss: movement and longevity of moss balls on an Alaskan glacier // Polar Biol. 2020. V. 43. P. 735–744. https://doi.org/10.1007/s00300-020-02675-6
Hotaling S., Lutz S., Dial R.J., Anesio A.M., Benning L.G., Fountain A.G., Kelley J.L., McCutcheon J., McKenzie S.S., Hamilton T.L. Biological albedo reduction on ice sheets, glaciers, and snowfields // EarthSci. Rev. 2021. V. 220. P. 103728. https://doi.org/10.1016/j.earscirev.2021.103728
Hotaling S., Shain D.H., Lang S.A., Bagley R.K., Tronstad L.M., Weisrock D.W., Kelley J.L. Long-distance dispersal, ice sheet dynamics and mountaintop isolation underlie the genetic structure of glacier ice worms // Proc. R. Soc. 2019. V. 286(1905). P. 20190983.
Hotaling S., Wimberger P.H., Kelley J.L., Watts H. Macroinvertebrates on glaciers: a key resource for terrestrial food webs? // Ecology. 2020. V. 101(4). P. 1–3. https://doi.org/10.1002/ecy.2947
Huang J., Kang S., Ma M., Guo J., Cong Z., Dong Z., Yin R., Xu J., Tripathee L., Ram K., Wang F. Accumulation of atmospheric mercury in glacier cryoconite over Western China // Environ. Sci. Technol. 2019. V. 53(12). P. 6632–6639. https://doi.org/10.1021/acs.est.8b06575
Humlum O., Elberling B., Hormes A., Fjordheim K., Hansen O.H., Heinemeier J. Late-Holocene glacier growth in Svalbard, documented by subglacial relict vegetation and living soil microbes // The Holocene. 2005. V. 15(3). P. 396–407. https://doi.org/10.1191/0959683605hl817rp
Hyde W.T., Crowley T.J., Baum S.K., Peltier W.R. Neoproterozoic ‘snowball Earth’ simulations with a coupled climate/ice-sheet model // Nature. 2000. V. 405(6785). P. 425–429. https://doi.org/10.1038/35013005
Irvine-Fynn T.D.L., Edwards A., Newton S., Langford H., Rassner S.M., Telling J., Anesio A.M., Hodson A.J. Microbial cell budgets of an Arctic glacier surface quantified using flow cytometry // Environ. Microbiol. 2012. V. 14(11). P. 2998–3012. https://doi.org/10.1111/j.1462-2920.2012.02876.x
Irvine-Fynn T.D., Edwards A. A frozen asset: the potential of flow cytometry in constraining the glacial biome // Cytometry part A. 2014. V. 85(1). P. 3–7.
Irvine-Fynn T.D., Bridge J.W., Hodson A.J. In situ quantification of supraglacial cryoconite morphodynamics using time-lapse imaging: an example from Svalbard // J. Glaciol. 2011. V. 57(204). P. 651–657. https://doi.org/10.3189/002214311797409695
IUSS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps / World Soil Resources Reports. 2015. V. 106. FAO, Rome.
Kabala C., Zarat J. Recent, relic and buried soils in the forefield of Werenskiold Glacier, SW Spitsbergen // Pol. Polar Res. 2009. V. 30(2). P. 161–178.
Kaczmarek Ł., Jakubowska N., Celewicz-Gołdyn S., Zawierucha K. The microorganisms of cryoconite holes (algae, Archaea, bacteria, cyanobacteria, fungi, and Protista): a review // Polar Rec. 2016. V. 52(2). P. 176–203. https://doi.org/10.1017/S0032247415000637
Kastovska K., Elster J., Stibal M., Santruckova H. Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (High Arctic) // Microb. Ecol. 2005. V. 50. P. 396–407. https://doi.org/10.1007/s00248-005-0246-4
Kellerman A.M., Vonk J., McColaugh S., Podgorski D.C., van Winden E., Hawkings J.R., Johnston S.E., Humayun M., Spencer R.G. Molecular signatures of glacial dissolved organic matter from Svalbard and Greenland // Global Biogeoch. Cycles. 2021. V. 35(3). P. e2020GB006709. https://doi.org/10.1029/2020GB006709
Khan A.L., Dierssen H.M., Scambos T.A., Höfer J., Cordero R.R. Spectral characterization, radiative forcing and pigment content of coastal Antarctic snow algae: Approaches to spectrally discriminate red and green communities and their impact on snowmelt // Cryosphere. 2021. V. 15. P. 133–148. https://doi.org/10.5194/tc-15-133-2021
Khromova T., Nosenko G., Nikitin S., Muraviev A., Popova V., Chernova L., Kidyaeva V. Changes in the mountain glaciers of continental Russia during the twentieth to twenty-first centuries // Reg. Environ. Change 2019. V. 19. P. 1229–1247. https://doi.org/10.1007/s10113-018-1446-z
Kirschvink J.L. Late Proterozoic low-latitude global glaciation / The snowball Earth. 1992. P. 51–52.
Knoll A.H. The early evolution of eukaryotes: a geological perspective // Science. 1992. V. 256(5057). P. 622–627. https://doi.org/10.1126/science.1585174
Knoll A.H. Paleobiological perspectives on early eukaryotic evolution // Cold Spring Harb. Perspect. Biol. 2014. V. 6(1). P. a016121. https://doi.org/10.1101/cshperspect.a016121
Kushnov I., Abakumov E., Tembotov R., Nizamutdinov T. Migration of organic carbon and trace elements in the system glacier-soil in the Central Caucasus alpine environment // J. Mt. Sci. 2022. V. 19(12). P. 3458–3474. https://doi.org/10.1007/s11629-022-7589-x
Kushnov I., Abakumov E., Tembotov R., Polyakov V. Geochemistry of cryoconite and soils in the Central Caucasus region and its environmental implications // J. Mt. Sci. 2021. V. 18(12). P. 3109–3124. https://doi.org/10.1007/s11629-021-6945-6
Kutuzov S., Lavrentiev I., Smirnov A., Nosenko G., Petrakov D. Volume changes of Elbrus glaciers from 1997 to 2017 // Front. Earth Sci. 2019. P. 153. https://doi.org/10.3389/feart.2019.00153
Kutuzov S., Legrand M., Preunkert S., Ginot P., Mikhalenko V., Shukurov K., Poliukhov A., Toropov P. History of desert dust deposition recorded in the Elbrus ice core // Atmos. Chem. Phys. 2019. P. 1–26. https://doi.org/10.5194/acp-2019-411
Kutuzov S., Shahgedanova M., Krupskaya V., Goryachkin S. Optical, Geochemical and Mineralogical Characteristics of Light-Absorbing Impurities Deposited on Djankuat Glacier in the Caucasus Mountains // Water. 2021. V. 13. P. 2993. https://doi.org/10.3390/w13212993
Kutuzov S., Shahgedanova M., Mikhalenko V., Ginot P., Lavrentiev I., Kemp S. High-resolution provenance of desert dust deposited on Mt. Elbrus, Caucasus in 2009–2012 using snow pit and firn core records // Cryosphere. 2013. V. 7. P. 1481–1498. https://doi.org/10.5194/tc-7-1481-2013
Kuzyakov Y. Priming effects: interactions between living and dead organic matter // Soil Biol. Biochem. 2010. V. 42(9). P. 1363–1371. https://doi.org/10.1016/j.soilbio.2010.04.003
Lamarche-Gagnon G., Wadham J.L., Lollar B.S., Arndt S., Fietzek P., Beaton A.D., Tedstone A.J., Telling J., Bagshaw E.A., Hawkings J.R., Kohler T.J., Zarsky J.D., Mowlem M.C., Anesio A.M., Stibal G.M., Lamarche-Gagnon J.L., Wadham B.S., Lollar et al. Greenland melt drives continuous export of methane from the ice-sheet bed // Nature. 2019. V. 565. P. 73–77. https://doi.org/10.1038/s41586-018-0800-0
Langford H., Hodson A., Banwart S. Using FTIR spectroscopy to characterise the soil mineralogy and geochemistry of cryoconite from Aldegondabreen glacier, Svalbard // Appl. Geochem. 2011. V. 26. P. S206–S209. https://doi.org/10.1016/j.apgeochem.2011.03.105
Langford H., Hodson A., Banwart S., Bøggild C. The microstructure and biogeochemistry of Arctic cryoconite granules // Ann. Glaciol. 2010. V. 51(56). P. 87–94. https://doi.org/10.3189/172756411795932083
Langford H.J., Irvine-Fynn T.D.L., Edwards A., Banwart S.A., Hodson A.J. A spatial investigation of the environmental controls over cryoconite aggregation on Longyearbreen glacier, Svalbard // Biogeosciences. 2014. V. 11(19). P. 5365–5380. https://doi.org/10.5194/bg-11-5365-2014
Legrand M., McConnell J., Fischer H., Wolff E.W., Preunkert S., Arienzo M., Chellman N., Leuenberger D., Maselli O., Place P., Sigl M., Schüpbach S., Flannigan M. Boreal fire records in Northern Hemisphere ice cores: a review // Clim. Past. 2016. V. 12(10). P. 2033–2059. https://doi.org/10.5194/cp-12-2033-2016
Li Z.X., Evans D.A.D., Halverson G.P. Neoproterozoic glaciations in a revised global palaeogeography from the breakup of Rodinia to the assembly of Gondwanaland // Sediment. Geol. 2013. V. 294. P. 219–232. https://doi.org/10.1016/j.sedgeo.2013.05.016
Li Q., Kang S., Wang N., Li Y., Li X., Dong Z., Chen P. Composition and sources of polycyclic aromatic hydrocarbons in cryoconites of the Tibetan Plateau glaciers // Sci. Total Environ. 2017. V. 574. P. 991–999. https://doi.org/10.1016/j.scitotenv.2016.09.159
Li X., Ding Y., Xu J., He X., Han T., Kang S., Wu Q., Mika S., Yu Z., Li Q. Importance of mountain glaciers as a source of dissolved organic carbon // J. Geophys. Res. Earth Surf. 2018. V. 123(9). P. 2123–2134. https://doi.org/10.1029/2017JF004333
Li Y., Kang S., Chen J., Hu Z., Wang K., Paudyal R., Liu J., Wang X., Qin X., Sillanpää M. Black carbon in a glacier and snow cover on the northeastern Tibetan Plateau: Concentrations, radiative forcing and potential source from local topsoil // Sci. Total Environ. 2019. V. 686. P. 1030–1038. https://doi.org/10.1016/j.scitotenv.2019.05.469
Łokas E., Zaborska A., Kolicka M., Różycki M., Zawierucha K. Accumulation of atmospheric radionuclides and heavy metals in cryoconite holes on an Arctic glacier // Chemosphere. 2016. V. 160. P. 162–172. https://doi.org/10.1016/j.chemosphere.2016.06.051
Łokas E., Zawierucha K., Cwanek A., Szufa K., Gaca P., Mietelski J.W., Tomankiewicz E. The sources of high airborne radioactivity in cryoconite holes from the Caucasus (Georgia) // Sci. Rep. 2018. V. 8(1). P. 10802. https://doi.org/10.1038/s41598-018-29076-4
Lutz S., Ziolkowski L.A., Benning L.G. The Biodiversity and Geochemistry of Cryoconite Holes in Queen Maud Land, East Antarctica // Microorganisms. 2019. V. 7(6). P. 1–16. https://doi.org/10.3390/microorganisms7060160
Lutz S., Anesio A.M., Edwards A., Benning L.G. Linking microbial diversity and functionality of arctic glacial surface habitats // Environ. Microbiol. 2017. V. 19(2). P. 551–565. https://doi.org/10.1111/1462-2920.13494
Makowska N., Zawierucha K., Nadobna P., Piątek-Bajan K., Krajewska A., Szwedyk J., Iwasieczko P., Mokracka J., Koczura R. Occurrence of integrons and antibiotic resistance genes in cryoconite and ice of Svalbard, Greenland, and the Caucasus glaciers // Sci. Total Environ. 2020. V. 716. P. 137022.
Makowska-Zawierucha N., Mokracka J., Małecka M., Balazy P., Chełchowski M., Ignatiuk D., Zawierucha K. Quantification of class 1 integrons and characterization of the associated gene cassettes in the high Arctic–Interplay of humans and glaciers in shaping the aquatic resistome // Ecol. Indic. 2022. V. 145. P. 109633.
Margesin R., Fell J.W. Mrakiella cryoconite gen. nov., sp. Nov., a psychrophilic, anamorphoc, basidiomycetous yeast from alpine and arctic habitats // Int. J. Syst. Evol. Microbiol. 2008. V. 58. P. 2977–2982. https://doi.org/10.1099/ijs.0.2008/000836-0
McCrimmon D.O., Bizimis M., Holland A., Ziolkowski L.A. Supraglacial microbes use young carbon and not aged cryoconite carbon // Org. Geochem. 2018. V. 118. P. 63–72. https://doi.org/10.1016/j.orggeochem.2017.12.002
Mergelov N.S., Zazovskaya E.P., Goryachkin S.V. Exploring principles of aggregation between organic and mineral phases on ice: insights from cryoconite granules of two mountain glaciers / Biogenic – abiogenic interactions in natural and anthropogenic systems. VII International Symposium. Saint Petersburg: Skifia-print. 2022. P. 17–18.
Mieczan T., Tarkowska-Kukuryk M., Górniak D., Świątecki A., Zdanowski M., Adamczuk M. Vertical microzonation of ciliates in cryoconite holes in Ecology Glacier, King George Island // Pol. Polar Res. 2013. V. 2. P. 201–212.
Miroshnikov A., Flint M., Asadulin E., Aliev R., Shiryaev A., Kudikov A., Khvostikov V. Radioecological and geochemical peculiarities of cryoconite on Novaya Zemlya glaciers // Sci. Rep. 2021. V. 11(1). P. 1–15. https://doi.org/10.1038/s41598-021-02601-8
Miteva V. Bacteria in snow and glacier ice / Psychrophiles: from biodiversity to biotechnology. 2008. P. 31–50. Springer, Berlin, Heidelberg.
Müller F., Keeler C.M. Errors in short-term ablation measurements on melting ice surfaces // J. Glaciol. 1969. V. 8(52). P. 91–105.
Murakami T., Segawa T., Bodington D., Dial R., Takeuchi N., Kohshima S., Hongoh Y. Census of bacterial microbiota associated with the glacier ice worm Mesenchytraeus solifugus // FEMS Microbiol. Ecol. 2015. V. 91(3). P. fiv003. https://doi.org/10.1093/femsec/fiv003
Musilova M., Tranter M., Wadham J., Telling J., Tedstone A., Anesio A.M. 2017. Microbially driven export of labile organic carbon from the Greenland ice sheet // Nat. Geosci. V. 10(5). P. 360–365.
Naegeli K., Damm A., Huss M., Wulf H., Schaepman M., Hoelzle M. Cross-Comparison of Albedo Products for Glacier Surfaces Derived from Airborne and Satellite (Sentinel-2 and Landsat 8) Optical Data // Remote Sens. 2017. V. 9. P. 110. https://doi.org/10.3390/rs9020110
Naegeli K., Huss M., Hoelzle M. Change detection of bare-ice albedo in the Swiss Alps // Cryosphere 2019. V. 13. P. 397–412. https://doi.org/10.5194/tc-13-397-2019
Nagatsuka N., Takeuchi N., Nakano T., Kokado E., Li Z. Sr, Nd and Pb stable isotopes of surface dust on Ürümqi glacier No. 1 in western China // Ann. Glaciol. 2010. V. 51(56). P. 95–105. https://doi.org/10.3189/172756411795931895
Nansen F. The Norwegian North Polar Expedition 1893–1896: Scientific Results. Longmans, Green and Co, London. 1906.
Nizamutdinov T., Mavlyudov B., Polyakov V., Abakumov E. Sediments from cryoconite holes and dirt cones on the surface of Svalbard glaciers: main chemical and physicochemical properties // Acta Geochimica. 2023. V. 42(2). P. 346–359. https://doi.org/10.1007/s11631-022-00586-3
Nordenskiöld A.E. Account of an expedition to Greenland in the year 1870 // Geol. Mag. 1870. V. 9(98). P. 355–368.
Nordenskiöld A.E. Cryoconite found 1870, july 19th–25th, on the inland ice, east of Auleitsivik Fjord, Disco Bay Greenland // Geol. Mag., Decade. 1875. V. 2(2). P. 157–162.
Pain A.J., Jonathan B. Martin, Ellen E. Martin, Rennermalm Å.K., Shaily Rahman. Heterogeneous CO2 and CH4 content of glacial meltwater from the Greenland Ice Sheet and implications for subglacial carbon processes // The Cryosphere. 2021. V. 15. P. 1627–1644. https://doi.org/10.5194/tc-15-1627-2021
Painter T.H., Barrett A.P., Landry C.C., Neff J., Cassidy M.P., Lawrence C., McBride K.E., Farmer G.L. Impact of dis-turbed desert soils on duration of mountain snow cover // Geophys. Res. Lett. 2007. V. 34. P. L12502. https://doi.org/10.1029/2007GL030284
Park C., Takeuchi N. Unmasking photogranulation in decreasing glacial albedo and net autotrophic wastewater treatment // Environ. Microbiol. 2021. V. 23(11). P. 6391–6404. https://doi.org/10.1111/1462-2920.15780
Pautler B.G., Dubnick A., Sharp M.J., Simpson A.J., Simpson M.J. Comparison of cryoconite organic matter composition from Arctic and Antarctic glaciers at the molecular-level // Geochim. Cosmochim. Acta. 2013. V. 104. P. 1–18. https://doi.org/10.1016/j.gca.2012.11.029
Perini L., Gostinčar C., Anesio A.M., Williamson C., Tranter M., Gunde-Cimerman N. Darkening of the Greenland ice sheet: Fungal abundance and diversity are associated 1246 with algal bloom // Front. Microbiol. 2019. V. 10. P. 557. https://doi.org/10.3389/fmicb.2019.00557
Pey J., Revuelto J., Moreno N., Alonso-González E., Bartolomé M., Reyes J., Gascoin S., López-Moreno J.I. Snow impurities in the central Pyrenees: from their geochemical and mineralogical composition towards their impacts on snow Albedo // Atmosphere. 2020. V. 11. P. 937. https://doi.org/10.3390/atmos11090937
Pi K., Bieroza M., Brouchkov A., Chen W., Dufour L.J., Gongalsky K.B., … Van Cappellen P. The cold region critical zone in transition: Responses to climate warming and land use change // Annu. Rev. Environ. Resour. 2021. V. 46. P. 111–134. https://doi.org/10.1146/annurev-environ-012220-125703
Pittino F., Maglio M., Gandolfi I., Azzoni R.S., Diolaiuti G., Ambrosini R., Franzetti A. Bacterial communities of cryoconite holes of a temperate alpine glacier show both seasonal trends and year-to-year variability // Ann. Glaciol. 2018. V. 59(77). P. 1–9. https://doi.org/10.1017/aog.2018.16
Polyakov V., Abakumov E., Mavlyudov B. Black Carbon as a Source of Trace Elements and Nutrients in Ice Sheet of King George Island, Antarctica // Geosciences. 2020. V. 10(11). P. 465. https://doi.org/10.3390/geosciences10110465
Polyakov V.I., Abakumov E.V., Tembotov R.Kh. Black carbon as a factor in deglaciation in polar and mountain ecosystems: A Review // Bulletin of Tomsk State University. Biology. 2020. V. 52. P. 6–33. (in Russian)
Polyakov V., Zazovskaya E., Abakumov E. Molecular composition of humic substances isolated from selected soils and cryconite of the Grønfjorden area, Spitsbergen // Pol. Polar Res. 2019. V. 40(2). P. 105–120. https://doi.org/10.24425/ppr.2019.128369
Poniecka E.A., Bagshaw E.A., Tranter M., Sass H., Williamson C.J., Anesio A.M., Team B.A.B. Rapid development of anoxic niches in supraglacial ecosystems // Arct. Antarct. Alp. Res. 2018. V. 50(1). P. S100015. https://doi.org/10.1080/15230430.2017.1420859
Porter P.R., Evans A.J., Hodson A.J., Lowe A.T., Crabtree M.D. Sediment–moss interactions on a temperate glacier: Falljökull, Iceland // Ann. Glaciol. 2008. V. 48. P. 25–31. https://doi.org/10.3189/172756408784700734
Price P.B. Microbial life in glacial ice and implications for a cold origin of life // FEMS Microbiol. Ecol. 2007. V. 59(2). P. 217–231. https://doi.org/10.1111/j.1574-6941.2006.00234.x
Procházková L., Leya T., Křížková H., Nedbalová L. Sanguina nivaloides and Sanguina aurantia gen. et spp. nov. (Chlorophyta): the taxonomy, phylogeny, biogeography and ecology of two newly recognised algae causing red and orange snow // FEMS Microbiol. Ecol. 2019. V. 95(6). P. fiz064. https://doi.org/10.1093/femsec/fiz064
Rabassa J., Rubulis S., Suarez J. Moraine in-transit as parent material for soil development and the growth of Valdivian rain forest on moving ice: Casa Pangue glacier, mount Tronador (lat. 41010'5), Chile // Ann. Glaciol. 1981. V. 2. P. 97–102. https://doi.org/10.3189/172756481794352342
Raymond P.A. The composition and transport of organic carbon in rainfall: Insights from the natural (13C and 14C) isotopes of carbon // Geophys. Res. Lett. 2005. V. 32. P. L14402. https://doi.org/10.1029/2005GL022879
Remias D., Lütz-Meindl U., Lütz C. Photosynthesis, pigments and ultrastructure of the alpine snow alga Chlamydomonas nivalis // Eur. J. Phycol. 2005. V. 40(3). P. 259–268. https://doi.org/10.1080/09670260500202148
Remias D., Schwaiger S., Aigner S., Leya T., Stuppner H., Lütz C. Characterization of an UV-and VIS-absorbing, purpurogallin-derived secondary pigment new to algae and highly abundant in M esotaenium berggrenii (Z ygnematophyceae, Chlorophyta), an extremophyte living on glaciers // FEMS Microbiol. Ecol. 2012. V. 79(3). P. 638–648. https://doi.org/10.1111/j.1574-6941.2011.01245.x
Remias D., Wastian H., Lütz C., Leya T. Insights into the biology and phylogeny of Chloromonas polyptera (Chlorophyta), an alga causing orange snow in Maritime Antarctica // Antarct. Sci. 2013. V. 25. P. 648–656. https://doi.org/10.1017/S0954102013000060
Ren Z., Martyniuk N., Oleksy I.A., Swain A., Hotaling S. Ecological stoichiometry of the mountain cryosphere // Front. Ecol. Evol. 2019. V. 7. P. 360. https://doi.org/10.3389/fevo.2019.00360
Rooney A.D., Strauss J.V., Brandon A.D., Macdonald F.A. A Cryogenian chronology: Two long-lasting synchronous Neoproterozoic glaciations // Geology. 2015. V. 43(5). P. 459–462. https://doi.org/10.1130/G36511.1
Rozwalak P., Podkowa P., Buda J., Niedzielski P., Kawecki S., Ambrosini R., … Zawierucha K. Cryoconite–From minerals and organic matter to bioengineered sediments on glacier’s surfaces // Sci. Total Environ. 2022. V. 807. P. 150874. https://doi.org/10.1016/j.scitotenv.2021.150874
Rubino M., D’Onofrio A., Seki O., Bendle J.A. Ice-core records of biomass burning // Anthr. Rev. 2016. V. 3(2). P. 140–162. https://doi.org/10.1177/2053019615605117
Ryu J.S., Jacobson A.D. CO2 evasion from the Greenland Ice Sheet: a new carbon–climate feedback // Chemical Geol. 2012. V. 320–321. P. 80–95. https://doi.org/10.1016/j.chemgeo.2012.05.024
Sajjad W., Din G., Rafiq M., Iqbal A., Khan S., Zada S., Ali B., Kang S. Pigment production by cold-adapted bacteria and fungi: colorful tale of cryosphere with wide range applications // Extremophiles. 2020. V. 24. P. 447–473. https://doi.org/10.1007/s00792-020-01180-2
Samui G., Antony R., Thamban M. Chemical characteristics of hydrologically distinct cryoconite holes in coastal Antarctica // Ann. Glaciol. 2018. V. 59(77). P. 69–76. https://doi.org/10.1017/aog.2018.30
Samui G., Antony R., Thamban M. Fate of dissolved organic carbon in Antarctic Surface Environments during Summer // J. Geophys. Res. Biogeosci 2020. V. 125(12). P. e2020JG005958.
Sanborn P. Soil formation on supraglacial tephra deposits, Klutlan Glacier, Yukon Territory // Can. J. Soil Sci. 2010. V. 90. P. 611–618. https://cdnsciencepub.com/doi/10.4141/cjss10042
Scarrow J.W., Balks M.R., Almond P.C. Three soil chronosequences in recessional glacial deposits near the polar plateau, in the Central Transantarctic Mountains, Antarctica // Antarct. Sci. 2014. V. 26(5). P. 573–583. https://doi.org/10.1017/S0954102014000078
Scherler D., Wulf H., Gorelick N. Global assessment of supraglacial debris-cover extents. Geophys. Res. Lett. 2018. V. 45(21). P. 11–798. https://doi.org/10.1029/2018GL080158
Schulze-Makuch D., Grinspoon D.H. Biologically enhanced energy and carbon cycling on Titan? // Astrobiology. 2005. V. 5(4). P. 560–567. https://doi.org/10.1089/ast.2005.5.560
Segawa T., Takeuchi N., Mori H., Rathnayake R.M., Li Z., Akiyoshi A., Satoh H., Ishii S. Redox stratification within cryoconite granules influences the nitrogen cycle on glaciers // FEMS Microbiol. Ecol. 2020. V. 96(11). P. fiaa199. https://doi.org/10.1093/femsec/fiaa199
Shain D.H., Halldórsdóttir K., Pálsson F., Aðalgeirsdóttir G., Gunnarsson A., Jónsson Þ., et al. Colonization of maritime glacier ice by bdelloid Rotifera //Mol. Phylogenet. Evol. 2016. V. 98. P. 280–287. https://doi.org/10.1016/j.ympev.2016.02.020
Singer G.A., Fasching C., Wilhelm L., Niggemann J., Steier P., Dittmar T., Battin T.J. Biogeochemically diverse organic matter in Alpine glaciers and its downstream fate // Nat. Geosci. 2012. V. 5(10). P. 710–714. https://doi.org/10.1038/ngeo1581
Singh P., Singh S.M. Characterisation of yeasts and filamentous fungi isolated from cryoconite holes of Svalbard, Arctic // Polar Biol. 2012. V. 35. P. 575–583. https://doi.org/10.1007/s00300-011-1103-1
Smirnova M., Miamin U., Kohler A., Valentovich L., Akhremchuk A., Sidarenka A., Dolgikh A., Shapaval V. Isolation and characterization of fast-growing green snow bacteria from coastal East Antarctica // Microbiology Open. 2021. V. 10(1). P. e1152. https://doi.org/10.1002/mbo3.1152
Smith C.A.S., Fox C.A., Hargrave A.E. Development of soil structure in some turbic cryosols in the Canadian low Arctic // Can. J. Soil Sci.1991. V. 71(1). P. 11–29. https://doi.org/10.4141/cjss91-002
Smith H.J., Schmit A., Foster R., Littman S., Kuypers M.M., Foreman C.M. Biofilms on glacial surfaces: hotspots for biological activity // Biofilms and Microbiomes. 2016. V. 2(1). P. 1–4. https://doi.org/10.1038/npjbiofilms.2016.8
Sodemann H., Palmer A.S., Schwierz C., Schwikowski M., Wernli H. The transport history of two Saharan dust events archived in an Alpine ice core // Atmos. Chem. Phys. Discuss. 2006. V. 6. P. 667–688. https://doi.org/10.5194/acp-6-667-2006
Sommers P., Darcy J.L., Porazinska D.L., Gendron E., Fountain A.G., Zamora F., et al. Comparison of microbial communities in the sediments and water columns of frozen cryoconite holes in the McMurdo Dry Valleys, Antarctica // Front. Microbiol. 2019. V. 10. P. 65. https://doi.org/10.3389/fmicb.2019.00065
Sommers P., Fontenele R.S., Kringen T., Kraberger S., Porazinska D.L., Darcy J.L., Vincent K., Cawley K.M., Solon A.J., Vimercati L., Varsani J.R. A. Single-stranded DNA viruses in antarctic cryoconite holes // Viruses. 2019. V. 11(11). P. 1022. https://doi.org/10.3390/v11111022
Stal L.J. Cyanobacterial mats and stromatolites / Ecology of Cyanobacteria II: Their Diversity in Space and Time, B.A. Whitton. 2012. P. 65–125. Netherlands: Springer.
Stefánsson H., Peternell M., Konrad-Schmolke M., Hannesdóttir H., Ásbjörnsson E.J., Sturkell E. Microplastics in glaciers: first results from the Vatnajökull ice cap // Sustainability. 2021. V. 13(8). P. 4183. https://doi.org/10.3390/su13084183
Stephens F.R. A forest ecosystem on a glacier in Alaska / Arctic. 1969. V. 22. P. 441–444.
Stibal M., Lawson E.C., Lis G.P. et al. Organic matter content and quality in supraglacial debris across the ablation zone of the Greenland ice sheet // Ann. Glaciol. 2010. V. 51(56). P. 1–8. https://doi.org/10.3189/172756411795931958
Stibal M., Sabacka M., Kastova K. Microbial communities on glacier surfaces in Svalbard: impact of physical and chemical properties on abundance and structure of cyanobacteria and algae // Microb. Ecol. 2006. V. 52(4). P. 644–654. https://doi.org/10.1007/s00248-006-9083-3
Stibal M., Jon T., Cook J., Mak K.M., Hodson A., Anesio A.M. Environmental Controls on Microbial Abundance and Activity on the Greenland Ice Sheet: A Multivariate Analysis Approach // Microb. Ecol. 2012. V. 63. P. 74–84. https://doi.org/10.1007/s00248-011-9935-3
Stibal M., Šabacká M., Žárský J. Biological processes on glacier and ice sheet surfaces // Nat Geosci. 2012. V. 5(11). P. 771–774. https://doi.org/10.1038/ngeo1611
Stibal M., Box J.E., Cameron K.A., Langen P.L., Yallop M.L., Mottram R.H., Khan A.L., Molotch N.P., Chrismas N.A.M., Quaglia F.C., Remias D., Smeets C.J.P.P., van den Broeke M.R., Ryan J.C., Hubbard A., Tranter M., van As D., Ahlstrøm A.P. Algae Drive Enhanced Darkening of Bare Ice on the Greenland Ice Sheet // Geophys. Res. Lett. 2017. V. 44(11). P. 463–471. https://doi.org/10.1002/2017GL075958
Stubbins A., Hood E., Raymond P.A., Aiken G.R., Sleighter R.L., Hernes P.J., Butman D., Hatcher P.G., Striegl R.G., Schuster P., Abdulla H.A.N., Vermilyea A.W., Scott D.T., Spencer R.G. Anthropogenic aerosols as a source of ancient dissolved organic matter in glaciers // Nat. Geosci. 2012. V. 5(3). P. 198–201. https://doi.org/10.1038/ngeo1403Spencer
Takeuchi N., Kohshima S., Seko K. Structure, formation, darkening process of albedo reducing material (cryoconite) on a Himalayan glacier: a granular algal mat growing on the glacier // Arct. Antarct. Alp. 2001. V. 33. P. 115–122. https://doi.org/10.1080/15230430.2001.12003413
Takeuchi N., Kohshima S., Goto-Azuma K., Koerner R.M. Biological characteristics of dark colored material (cryoconite) on Canadian Arctic glaciers (Devon and Penny ice caps) // Memoirs of National Institute of Polar Research. Special issue. 2001. V. 54. P. 495–505.
Takeuchi N. Optical characteristics of cryoconite (surface dust) on glaciers: the relationship between light absorbency and the property of organic matter contained in the cryoconite // Ann. Glaciol. 2002. V. 34. P. 409–414.
Takeuchi N. Surface albedo and characteristics of cryoconiteonan Alaska glacier (Gulkana Glacier in the Alaska Range) // Bulletin of Glaciological Research. 2002. V. 19. P. 63–70.
Takeuchi N., Nagatsuka N., Uetake J., Spatial variations in impurities (cryoconite) on glaciers in northwest Greenland // Bulletin of Glaciological Research. 2014. V. 32. P. 85–94. https://doi.org/10.5331/bgr.32.85
Takeuchi N., Nishiyama H., Li Z. Structure and formation process of cryoconite granules on Urumqi glacier No.1, Tien Shan, China // Ann. Glaciol. 2010. V. 51(56). P. 9–14. https://doi.org/10.3189/172756411795932010
Takeuchi N., Li Z. Characteristics of surface dust on Urumqi Glacier No.1 in the Tien Shan Mountains, China // Arct. Antarct. Alp. 2008. V. 40(4). P. 744–750. https://doi.org/10.1657/1523-0430(07-094)
Takeuchi N. Temporal and spatial variations in spectral reflectance and characteristics of surface dust on Gulkana Glacier, Alaska Range // J. Glaciol. 2009. V. 55. P. 701–709, https://doi.org/10.3189/002214309789470914
Takeuchi N., Fujisawa Y., Kadota T., Tanaka S., Miyairi M., Shirakawa T., Kusaka R., Fedorov A.N., Konstantinov P., Ohata T. The effect of impurities on the surface melt of a glacier in the Suntar-Khayata mountain range, Russian Siberia // Front. Earth Sci. 2015. V. 3. P. 82. https://doi.org/10.3389/feart.2015.00082
Takeuchi N., Uetake J., Fujita K., Aizen V.B., Nikitin S.D. A snow algal community on Akkem glacier in the Russian Altai mountains // Ann. Glaciol. 2006. V. 43. P. 378–384. https://doi.org/10.3189/172756406781812113
Tanaka S., Takeuchi N., Miyairi M., Fujisawa Y., Kadota T., Shirakawa T., Kusaka R., Takahashi S., Enomoto H., Ohata T., Yabuki H., Konya K., Konstantinov A.F. Snow algal communities on glaciers in the Suntar-Khayata Mountain Range in eastern Siberia, Russia // Polar Sci. 2016. V. 10(3). P. 227–238. https://doi.org/10.1016/j.polar.2016.03.004
Tedesco M., Foreman C.M., Anton J. et al. Comparative analysis of morphological, mineralogical and spectral properties of cryoconite in Jakobshavn Isbrae, Greenland, and Canada Glacier, Antarctica // Ann. Glaciol. 2013. V. 54(63). P. 147–157. https://doi.org/10.3189/2013AoG63A417
Thevenon F., Chiaradia M., Adatte T., Hueglin C., Poté J. Characterization of Modern and Fossil Mineral Dust Transported to High Altitude in the Western Alps: Saharan Sources and Transport Patterns // Adv. Meteorol. 2012. P. 674385. https://doi.org/10.1155/2012/67438
Thomazini A., Mendonça E.S., Teixeira D.B., Almeida I.C.C., La Scala N., Jr., Canellas L.P., Spokas K.A., Milori D.M.B.P., Turbay C.V.G., Fernandes R.B.A., Schaefer C.E.G.R. CO2 and N2O emissions in a soil chronosequence at a glacier retreat zone in Maritime Antarctica // Sci. Total Environ. V. 521–522. 2015. P. 336–345.
Tielidze L.G., Wheate R.D. The greater Caucasus glacier inventory (Russia, Georgia and Azerbaijan) // The Cryosphere. 2018. V. 12(1). P. 81–94. https://doi.org/10.5194/tc-12-81-2018
Tranter M., Bagshaw E.A., Fountain A.G., Foreman C.M. The biogeochemistry and hydrology of McMurdo Dry Valley glaciers: Is there life on Martian ice now? // Life in Antarctic Deserts and Other Cold, Dry Environments / Astrobiological Analogues / Ed. Doran P.T. Cambridge: Cambridge University Press. 2010. P. 195–220.
Tranter M., Fountain A.G., Fritsen C.H., Lyons B.W., Priscu J.C., Statham P.J., Welch K.A. Extreme hydrochemical conditions in natural microcosms entombed within Antarctic ice // Hydrol. Process. 2004. V. 18(2). P. 379–387. https://doi.org/10.1002/hyp.5217
Trindade R.I., Macouin M. Palaeolatitude of glacial deposits and palaeogeography of Neoproterozoic ice ages // Comptes Rendus Geoscience. 2007. V. 339(3–4). P. 200–211. https://doi.org/10.1016/j.crte.2007.02.006
Uetake J., Tanaka S., Hara K., Tanabe Y., Samyn D., Motoyama H., Imura S., Kohshima S. Novel biogenic aggregation of moss gemmae on a disappearing African glacier // PLoS One. 2014. V. 9(11). P. e112510. https://doi.org/10.1371/journal.pone.0112510
Uetake J., Tanaka S., Segawa T., Takeuchi N., Nagatsuka N., Motoyama H., Aoki T. Microbial community variation in cryoconite granules on Qaanaaq Glacier, NW Greenland // FEMS Microbiol. Ecol. 2016. V. 92(9). P. fiw127. https://doi.org/10.1093/femsec/fiw127
Van Vliet-Lanoë B. Frost and soils: implications for paleosols, paleoclimates and stratigraphy // Catena. 1998. V. 34(1–2). P. 157–183. https://doi.org/10.1016/S0341-8162(98)00087-3
Van Vliet-Lanoë B. Frost effects in soils // Soils and Quaternary Landscape Evolution / Ed. Boardman J. Wiley, Chichester. 1985. P. 117–158.
Wadham J.L., Hawkings J.R., Tarasov L. Gregoire L.J., Spencer R.G.M., Gutjahr M., Ridgwell A., Kohfeld K.E. Ice sheets matter for the global carbon cycle // Nat. Commun. 2019. V. 10. P. 3567. https://doi.org/10.1038/s41467-019-11394-4
Wang J., Haidong H., Shiqiang Z. Carbon dioxide flux in the ablation area of Koxkar glacier, western Tien Shan, China // Ann. Glaciol. 2014. V. 55(66). https://doi.org/10.3189/2014AoG66A060
Wang P., D’Imperioc L., Biersmad E.M., Rannikuc R., Xuc W., Tiana Q., Ambusc P., Elberlingc B. Combined effects of glacial retreat and penguin activity on soil greenhouse gas fluxes on South Georgia, sub-Antarctica // Sci. Total Environ. 2019. V. 718. P. 135255. https://doi.org/10.1016/j.scitotenv.2019.135255
Watanabe Y., Martini J.E., Ohmoto H. Geochemical evidence for terrestrial ecosystems 2.6 billion years ago // Nature. 2000. V. 408(6812). P. 574. https://doi.org/10.1038/35046052
Wei D., Wang X. Recent climatic changes and wetland expansion turned Tibet into a net CH4 source // Clim. Change. 2017. V. 144. P. 657–670. https://doi.org/10.1007/s10584-017-2069-y
Weisleitner K., Perras A.K., Unterberger S.H., Moissl-Eichinger C., Andersen D.T., Sattler B. Cryoconite hole location in East-Antarctic Untersee Oasis shapes physical and biological diversity // Front. Microbiol. 2020. V. 11. P. 1165. https://doi.org/10.3389/fmicb.2020.01165
Wiscombe J.W., Warren S.G. A Model for the Spectral Albedo of Snow. II: Snow Containing Atmospheric Aerosols // J. Atmos. Sci. 1980. V. 37. P. 2734–2745. https://doi.org/10.1175/1520-0469(1980)037<2734:AMFTSA>2.0.CO;2
Wouters B., Gardner A.S., Moholdt G. Global glacier mass loss during the GRACE satellite mission (2002–2016) //Front. Earth Sci. 2019. V. 7. P. 96. https://doi.org/10.3389/feart.2019.00096
Wu G.M., Cong Z.Y., Kang S.C., Kawamura K., Fu P.Q., Zhang Y.L., Wan X., Gao S.-P., Liu B. Brown carbon in the cryosphere: Current knowledge and perspective // Adv. Clim. Change Res. 2016. V. 7(1–2). P. 82–89. https://doi.org/10.1016/j.accre.2016.06.002
Xu Y., Simpson A.J., Eyles N., Simpson M.J. Sources and molecular composition of cryoconite organic matter from the Athabasca Glacier, Canadian Rocky Mountains // Org. Geochem. 2010. V. 41. P. 177–186. https://doi.org/10.1016/j.orggeochem.2009.10.010
Yan J., Wang X., Gong P., Wang C., Cong Z. Review of brown carbon aerosols: Recent progress and perspectives // Sci. Total Environ. 2018. V. 634. P. 1475–1485. https://doi.org/10.1016/j.scitotenv.2018.04.083
Young G.M., Brunn V.V., Gold D.J., Minter W.E.L. Earth’s oldest reported glaciation: physical and chemical evidence from the Archean Mozaan Group (∼2.9 Ga) of South Africa // J. Geol. 1998. V. 106(5). P. 523–538. https://doi.org/10.1086/516039
Yue X., Li Z., Zhao J., Fan J., Takeuchi N., Wang L. Variation in Albedo and Its Relationship with Surface Dust at Urumqi Glacier No. 1 in Tien Shan, China // Front. Earth Sci. 2020. V. 8. P. 110. https://doi.org/10.3389/feart.2020.00110
Zamolodchikov D.G., Karelin D.V. An empirical model of carbon fluxes in Russian tundra // Glob. Chang. Biol. 2001. V. 7(2). P. 147–161. https://doi.org/10.1046/j.1365-2486.2001.00380.x
Zarsky J.D., Stibal M., Hodson A., Sattler B., Schostag M., Hansen L.H., Jacobsen C.S., Psenner R. Large cryoconite aggregates on a Svalbard glacier support a diverse microbial community including ammonia-oxidising archaea // Environ. Res. Lett. 2013. V. 8. P. 035044. https://doi.org/10.1088/1748-9326/8/3/035044
Žárský J., Žárský V., Hanáček M., Žárský V. Cryogenian glacial habitats as a plant terrestrialisation cradle–the origin of the anydrophytes and Zygnematophyceae split // Front. Plant Sci. 2022. V. 12. P. 735020. https://doi.org/10.3389/fpls.2021.735020
Zawierucha K., Coulson S., Michalcyzk M., Current knowledge of the Tardigrada of Svalbard with the first records of water bears from Nordaustlandet (High Arctic) // Polar Res. 2013. V. 32. P. 20886. https://doi.org/10.3402/polar.v32i0.20
Zawierucha K., Baccolo G., Di Mauro B., Nawrot A., Szczuciński W., Kalińska E. Micromorphological features of mineral matter from cryoconite holes on Arctic (Svalbard) and alpine (the Alps, the Caucasus) glaciers // Polar Sci. 2019. V. 22. P. 100482. https://doi.org/10.1016/j.polar.2019.100482
Zawierucha K., Buda J., Nawro A. Extreme weather event results in the removal of invertebrates from cryoconite holes on an Arctic valley glacier (Longyearbreen, Svalbard) // Ecol. Res. 2019. V. 34(3). P. 370–379. https://doi.org/10.1111/1440-1703.1276
Zawierucha K., Stec D., Lachowska-Cierlik D., Takeuchi N., Li Z., Michalczyk Ł. High mitochondrial diversity in a new water bear species (Tardigrada: Eutardigrada) from mountain glaciers in central Asia, with the erection of a new genus Cryoconicus // Annales Zoologici. 2018. V. 68(1). P. 179–201. https://doi.org/10.3161/00034541ANZ2018.68.1.007
Zennaro P., Kehrwald N., McConnell J.R., Schüpbach S., Maselli O.J., Marlon J., Vallelonga P., Leuenberger D., Zangrando R., Spolaor A., Borrotti M., Barbaro E., Gambaro A., Barbante C. Fire in ice: two millennia of boreal forest fire history from the Greenland NEEM ice core // Clim. Past. 2014. V. 10(5). P. 1905–1924. https://doi.org/10.5194/cp-10-1905-2014
Zhang Y., Kang S., Wei D., Luo X., Wang Z., Gao T. Sink or source? Methane and carbon dioxide emissions from cryoconite holes, subglacial sediments, and proglacial river runoff during intensive glacier melting on the Tibetan Plateau // Fundam. Res. 2021. V. 1(3). P. 232–239. https://doi.org/10.1016/j.fmre.2021.04.005
Zhang Y., Gao T., Kang S., Allen S., Luo X., Allen D. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics // Sci. Total Environ. 2021. V. 758. P. 143634. https://doi.org/10.1016/j.scitotenv.2020.143634
Zhang Y., Gao T., Kang S., Shi H., Mai L., Allen D., Allen S. Current status and future perspectives of microplastic pollution in typical cryospheric regions // Earth Sci. Rev. 2022. V. 226. P. 1–16. https://doi.org/10.1016/j.earscirev.2022.103924
Zhou Y., Zhou L., He X., Jang K.S., Yao X., Hu Y., Zhang Y., Li X., Spencer R.G.M., Brookes J.D., Jeppesen E. Variability in dissolved organic matter composition and biolability across gradients of glacial coverage and distance from glacial terminus on the Tibetan Plateau // Environ. Sci. Technol. 2019. V. 53(21). P. 12207–12217. https://doi.org/10.1021/acs.est.9b03348
Дополнительные материалы отсутствуют.



