Коллоидный журнал, 2023, T. 85, № 6, стр. 806-817
Модели стабилизации заряженных частиц поверхностно-активными веществами в неполярных средах
П. С. Поповецкий *
Институт неорганической химии им. А.В. Николаева СО РАН
630090 Новосибирск,
пр. ак. Лаврентьева, 3, Россия
* E-mail: popovetskiy@niic.nsc.ru
Поступила в редакцию 17.07.2023
После доработки 23.08.2023
Принята к публикации 23.08.2023
- EDN: HDBQJI
- DOI: 10.31857/S0023291223600621
Аннотация
Стабилизация заряженных частиц в неполярных средах является одним из наиболее сложных вопросов современной коллоидной химии. Отнесение к коллоидной химии в данном случае абсолютно справедливо: в неполярных средах заряженные частицы, как правило, имеют супрамолекулярную природу. Низкие значения диэлектрической проницаемости среды обуславливают энергетическую невыгодность существования ионов в классическом понимании. Условием нахождения заряженных частиц в неполярных средах является их стерическая стабилизация, что требует некоторого пересмотра представлений о строении двойного электрического слоя, прежде всего его диффузной части. Важность детального анализа структуры двойного электрического слоя в неполярных средах обусловлена высокой практической значимостью электрокинетических явлений в подобных системах. В данной обзорной работе рассматриваются основные модели стерической стабилизации заряженных частиц поверхностно-активными веществами различной природы в дисперсионных средах со значением диэлектрической проницаемости ниже 5. Внимание уделено не только области концентраций, соответствующих образованию мицелл обратного типа, но и концентрациям ниже критической концентрации мицеллообразования. Кроме того, рассмотрены некоторые нетипичные примеры электрокинетических явлений в органозолях.
1. ВВЕДЕНИЕ
Формирование заряженных частиц в неполярных средах относится к достаточно новой и слабо изученной области физической химии коллоидных систем. Первые работы, посвященные вопросам природы возникновения и механизмов стабилизации заряженных частиц в неполярных средах, были опубликованы в 30-х гг. XX века [1–5]. В этих работах впервые обнаружено наличие ионов в среде углеводородов. Существование заряженных частиц часто связывают с явлением электрофореза, одного из методов их обнаружения, важного для практических целей. Явление электрофореза в средах с низкой диэлектрической проницаемостью (около 2) впервые было обнаружено и описано в работах ван дер Минне и Германи [6]. Явление неводного электрофореза на тот момент уже было известно, но только для сред с диэлектрической проницаемостью около 10–15, для которых применимы методики детектирования, используемые для “водных” систем. Авторы предложили критерии того, что наблюдаемое явление перемещения частиц дисперсной фазы под действием внешнего электрического поля является электрофорезом:
1) частицы дисперсной фазы должны двигаться равномерно и прямолинейно;
2) скорость движения частиц не зависит от их положения в электрическом поле;
3) скорость частиц прямо пропорциональна напряженности электрического поля, при смене знака направление движения частиц меняется на противоположное.
Авторы называют наблюдаемое явление “истинным электрофорезом” (“real electrophoresis”, “pure electrophoresis”). Чаще по отношению к данному явлению используется термин “линейный электрофорез”. При увеличении напряженности внешнего электрического поля могут наблюдаться отклонения от линейного характера движения частиц, что отмечено самими авторами [6], и наблюдалось в ряде других работ [7, 8].
Все эти критерии основаны на классическом уравнении Гельмгольца–Смолуховского, хотя для случая неполярных сред обычно применимо уравнение Хюккеля–Онзагера. Оба этих уравнения являются частным случаем более общего уравнения Генри, описывающего связь между скоростью движения частиц дисперсной фазы и электрокинетическим (дзета) потенциалом:
где v – скорость движения частиц, k – численный коэффициент, зависящий от формы частиц и отношения их размера к толщине двойного электрического слоя (2/3 в приближении Хюккеля–Онзагера и 1 в приближении Гельмгольца–Смолуховского), ζ – электрокинетический потенциал, H – напряженность внешнего электрического поля, ε – диэлектрическая проницаемость среды, ε0 – диэлектрическая постоянная, η – вязкость среды.Из уравнения Генри также становятся понятны основные сложности, связанные с детектированием электрофореза в средах углеводородов – скорость электрофореза при прочих равных ниже примерно в 40 раз в сравнении с водой.
Но с практической точки зрения исследование данных процессов необходимо. Контроль возникновения заряженных частиц важен для нефтехимии. Электрический заряд может накапливаться в нефтяных танкерах [9], особенно при воздействии влаги и температуры [10]. В нефтехимии образование заряженных частиц – процесс крайне нежелательный. Накопление заряженных частиц может приводить к электрическим пробоям и весьма плачевным последствиям [11]. С развитием технологий электронных устройств количество работ, посвященных неводному электрофорезу, заметно выросло: это явление лежит в основе принципа работы электронной бумаги. В качестве электронных (хотя правильнее их было бы назвать электрофоретическими) чернил используются различные современные высокодисперсные частицы – углеродные материалы, в том числе наноматериалы [12, 13], наночастицы оксидов кремния или титана [14, 15], полиметилметакрилата [16] и другие [17, 18]. Неизменным остается одно – неполярная дисперсионная среда.
Сложности развития данной области были обусловлены (и, вообще говоря, обусловлены до сих пор) недостаточным пониманием того, как в принципе в столь неблагоприятной среде могут возникнуть заряженные частицы, и что позволяет им остаться заряженными, а не коалесцировать. Первая удачная попытка критического анализа механизмов возникновения и стабилизации заряженных частиц в неполярных средах была осуществлена Моррисоном в 90-е годы [19]. В обзоре Моррисона внимание уделено физическим процессам разделения зарядов – сопоставления энергии теплового и электростатического взаимодействия. Для описания таких процессов во многих работах [20, 21], в том числе и обзорных [22, 23], авторы часто используют понятие длины Бьеррума, что, по сути, аналогично рассуждениям Моррисона. Физический смысл данной величины довольно прост: это расстояние, на котором энергии теплового и электростатического взаимодействий двух заряженных частиц будут равны:
(2)
${{E}_{{{\text{el}}}}} = \frac{{{{e}^{2}}}}{{4\pi h\varepsilon {{\varepsilon }_{0}}}} = {{k}_{{\text{B}}}}T,$(3)
$h = \,\,{{\lambda }_{{\text{B}}}} = \frac{{{{{\text{e}}}^{2}}}}{{4\pi \varepsilon {{\varepsilon }_{0}}{{k}_{{\text{B}}}}T}}.$В этих формулах Eel – энергия электростатического взаимодействия, e – элементарный заряд, h – расстояние между центрами взаимодействующих ионов, ε и ε0 – диэлектрическая проницаемость среды и диэлектрическая постоянная, kB – постоянная Больцмана, λB – длина Бьеррума.
В случае разноименно заряженных частиц с элементарным зарядом, на расстояниях меньше длины Бьеррума электростатическое притяжение будет превышать тепловое взаимодействие [24]. Длина Бьеррума – удобный инструмент для моделирования процессов взаимодействия заряженных частиц между собой [25]. Для воды при стандартных условиях длина Бьеррума составляет около 0.7 нм [16]. Если рассмотреть какой-нибудь простой электролит типа NaCl, то длина Бьеррума превышает сумму вандерваальсовых радиусов натрия и хлора менее чем в два раза. Очевидно, что даже очень тонкой сольватационной оболочки будет достаточно, чтобы разделить ионы на достаточное расстояние [19]. В распространенных неполярных средах, таких как углеводороды, длина Бьеррума при аналогичных условиях составит около 28 нм [22]. Таким образом, для эффективной стабилизации ионов в углеводородах толщина их “защитной” оболочки должна составлять не менее 14 нм [19, 26]. То есть эта защитная оболочка должна представлять собой некую супрамолекулярную структуру – мицеллу. Притом мицелла в целом рассматривается как стерически стабилизированный макроион, то есть не должна являться электронейтральной системой [26]. Нескомпенсированный заряд при этом, очевидно, локализован в полярной полости мицеллы. Далее по тексту для подобных систем, в которых присутствуют заряженные мицеллы (речь, конечно же, идет о мицеллах обратного типа), будет использоваться термин “микроэмульсия”. Сложно представить себе ион, покрытый исключительно оболочкой поверхностно-активного вещества (ПАВ). Ион должен быть сольватирован, что подразумевает наличие полярной фазы. Также следует отметить, что практически всегда в коммерческих образцах ПАВ присутствует вода (очистка ПАВ от воды – процедура непростая и не всегда целесообразная) [27–29]. По мнению ряда авторов, именно полярные примеси, в основном вода, и являются своего рода центрами мицеллообразования [29, 30]. Еще в 60–70-ых годах было отмечено существенное влияние количества примесной воды на критическую концентрацию мицеллообразования (ККМ) [31]. Другими словами, если в растворе ПАВ присутствуют заряженные мицеллы, то его корректнее называть именно микроэмульсией, даже если полярную фазу в него не добавляли намеренно.
Могут возникнуть логичные вопросы: как вообще мицелла может быть заряженной и откуда берется нескомпенсированный заряд? Раз “среда не благоволит”, разве не выгоднее мицелле оставаться электронейтральной? Конечно выгоднее. Заряженные мицеллы в достаточном для детектирования количестве образуются далеко не во всех микроэмульсиях. И даже если они образуются, их доля очень невелика. Для удобства и простоты в дальнейшем те типы ПАВ, для которых наблюдается формирование стерически стабилизированных ионов в неполярных средах, в данном обзоре будут называться “заряжающими”. В англоязычной литературе используется термин “charge control agents” [32, 33], имеющий аналогичный смысл. Далее будут рассмотрены основные механизмы возникновения заряженных частиц в микроэмульсиях на основе различных типов ПАВ. Первыми будут рассмотрены анионные ПАВ, так как в их случае механизм стабилизации более простой и интуитивно понятный. Вторыми будут рассмотрены неионные ПАВ. Работы, посвященные стабилизации заряженных частиц в неполярных средах при помощи катионных ПАВ, на данный момент практически не встречаются либо вовсе отсутствуют.
Рассмотрение механизмов стабилизации заряженных частиц логично начать с краткого рассмотрения более общих и широких понятий, таких как двойной электрический слой (ДЭС) в целом и его диффузная часть в частности. Уже после рассмотрения диффузной части ДЭС будут рассмотрены те типы ПАВ, обратные мицеллы которых могут ее образовывать.
2. ДВОЙНОЙ ЭЛЕКТРИЧЕСКИЙ СЛОЙ В НЕПОЛЯРНЫХ СРЕДАХ
В соответствии с классическими представлениями, поверхностный заряд может быть обусловлен адсорбцией ионов на поверхности частиц дисперсной фазы, диссоциацией поверхностных групп, либо ориентированием на поверхности полярных молекул. Предполагается, что в неполярных средах механизмы возникновения заряда поверхности довольно близки, хотя и не до конца понятны [34]. Существенная разница в количестве зарядов на поверхности. И для одних и тех же частиц она может составлять несколько порядков, что отмечено в работе [34].
Различия проявляются и при рассмотрении диффузной части ДЭС. И начинаются они с границы скольжения, разделяющей плотную и диффузную части ДЭС. Потенциал этой границы скольжения принято называть электрокинетическим, или дзета-потенциалом. Это важный параметр, определяющий электростатическую составляющую энергии парного межчастичного взаимодействия. Анализ баланса различных составляющих энергии парного межчастичного взаимодействия позволяет предсказывать стабильность дисперсных систем, для чего используется классическая теория Дерягина–Ландау–Фервея–Овербека (ДЛФО). А стабильность – ключевая характеристика любой дисперсной системы.
Ряд теоретических работ посвящен развитию подхода Дерягина, позволяющего оценить в линейном приближении энергию взаимодействия двух заряженных частиц выпуклой формы. Формула для расчета энергии электростатического взаимодействия двух сферических частиц разного размера и с разным поверхностным потенциалом предложена в работе Хогга, Хили и Фюрстенау (Hogg, Healy, Fuerstenau) [35] и имеет следующий вид:
(4)
$\begin{gathered} {{E}_{{{\text{edl}}}}} = \frac{{\varepsilon {{\varepsilon }_{0}}{{r}_{1}}{{r}_{2}}\left( {\varphi _{1}^{2} + \varphi _{2}^{2}} \right)}}{{4({{r}_{1}} + {{r}_{2}})}} \times \\ \times \,\,\left[ {\frac{{2{{\varphi }_{1}}{{\varphi }_{2}}}}{{\left( {\varphi _{1}^{2} + \varphi _{2}^{2}} \right)}}{\text{ln}}\left( {\frac{{\left( {1 + {{{\text{e}}}^{{ - \kappa h}}}} \right)}}{{\left( {1 - {{{\text{e}}}^{{ - \kappa h}}}} \right)}}} \right) + {\text{ln}}\left( {1 - {{{\text{e}}}^{{ - 2\kappa h}}}} \right)} \right], \\ \end{gathered} $Авторы отмечают, что в случае взаимодействия одинаковых частиц (r1 = r2 = r, φ1 = φ2 = φ) формула (4) совпадает с уравнением Дерягина:
(5)
${{E}_{{{\text{edl}}}}} = \frac{{\varepsilon {{\varepsilon }_{0}}r{{\varphi }^{2}}}}{2}{\text{ln}}\left( {1 + {{{\text{e}}}^{{ - \kappa h}}}} \right).$Также авторами отмечено, что формула (4) корректна для малых значений потенциалов взаимодействующих частиц (менее 25 мВ), а толщина ДЭС мала в сравнении с размером частиц. Для случая неполярных дисперсионных сред толщина ДЭС значительно выше, нежели для полярных, что потребовало дополнительного уточнения формулы Хогга–Хили–Фюрстенау. В ряде работ [36–38] для случая κh < 1 применяется модифицированная формула следующего вида:
(6)
$\begin{gathered} {{E}_{{{\text{edl}}}}} = \pi \varepsilon {{\varepsilon }_{0}}\frac{{{{r}_{1}}{{r}_{2}}}}{{{{r}_{1}} + {{r}_{2}}}}\left[ {{{{\left( {{{\varphi }_{1}} + {{\varphi }_{2}}} \right)}}^{2}}{\text{ln(}}1 + {{{\text{e}}}^{{ - \kappa h}}}{\text{)}} + } \right. \\ + \,\,{{\left( {{{\varphi }_{1}} - {{\varphi }_{2}}} \right)}^{2}}{\text{ln}}\left. {(1 - {{{\text{e}}}^{{ - \kappa h}}})} \right]. \\ \end{gathered} $Для случая взаимодействия одинаковых частиц формула (6) также принимает более привычный вид:
(7)
${{E}_{{{\text{edl}}}}} = 2\pi \varepsilon {{\varepsilon }_{0}}r{{\varphi }^{2}}{{{\text{e}}}^{{ - \kappa h}}}.$Так как формула (6) также получена в линейном приближении, на нее также накладывается ограничение на малость поверхностных потенциалов взаимодействующих частиц. Следует отметить, что в данных уравнениях фигурирует поверхностный потенциал, а не электрокинетический. Поверхностный потенциал является статической характеристикой частицы и часто определяется на границе неподвижной части ДЭС. При этом полагается, что неподвижная часть ДЭС мала в сравнении с диффузной. Для неполярной среды это справедливо, поэтому для них часто постулируется равенство поверхностного и электрокинетического потенциалов. В данном случае определяющим является именно потенциал на границе плотного слоя ДЭС, а не на границе твердой фазы, так как частица рассматривается вместе с неподвижным слоем ДЭС. Даже в случае наличия заряда на границе твердой фазы частица может вести себя как электронейтральная (электрокинетические явления будут отсутствовать при включении внешнего электрического слоя), если заряд поверхности твердой фазы будет полностью компенсироваться неподвижным слоем ДЭС, что отмечено в работе [39]. Полная компенсация заряда твердой фазы неподвижным слоем ДЭС наблюдалась авторами при низкой концентрации ПАВ и, соответственно, невозможности стерической стабилизации заряженных частиц в диффузной части ДЭС. Но даже при достаточной концентрации ПАВ в микроэмульсии, заряженных частиц в неполярных средах намного меньше, чем в полярных. Это приводит к низким значениям электропроводности и очень медленному падению поверхностного потенциала в диффузной части ДЭС. Схематически строение ДЭС в органозолях представлено на рис. 1.
Рис. 1.
Схема строения двойного электрического слоя в коллоидной системе c неполярной дисперсионной средой.
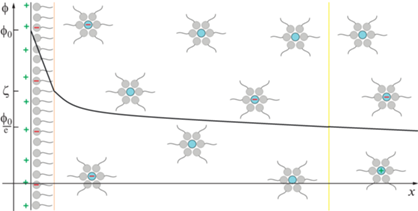
Электрокинетический потенциал является динамической характеристикой и определяется из подвижности частиц во внешнем электрическом поле. В этом заключается одно из важных отличий от поверхностного потенциала, прямые методы экспериментального определения которого отсутствуют. Однако в качестве примера можно привести работу [40]. Методом рентгеновской фотоэлектронной спектроскопии авторы показали линейную зависимость между энергией связи 2p орбитали кремния и радиусом катиона в электролите. Также подтверждением служило, что дзета-потенциал частиц оксида кремния диаметром около 9 нм, диспергированных в 50 мМ растворах хлоридов щелочных металлов, изменялся с –54 до –40 мВ при переходе от хлорида лития к хлориду цезия. В приближении нулевого радиуса катиона авторы смогли впервые определить поверхностный потенциал частиц оксида кремния. Также в работе [41] отмечено, что потенциал границы неподвижного слоя ДЭС и его толщина также могут быть определены для частиц с известной полидисперсностью. Поэтому вполне очевидно, что с точки зрения экспериментального определения дзета-потенциал является значительно более удобной величиной, нежели поверхностный потенциал. В качестве основного метода определения электрофоретической подвижности и дзета-потенциала используется электрофорез в свободной среде с различными вариантами детектирования движения, в основном визуальными или оптическими [34, 42–44], и лазерный электрофорез с детектированием движения частиц по фазовому сдвигу рассеянного света (в англоязычной литературе – phase analysis light scattering, PALS) [16, 45, 46]. В ряде работ отмечено, что такой подход позволяет добиться более высокой чувствительности, в сравнении с классическим лазерным допплеровским электрофорезом, что критически важно для неполярных сред [47, 48]. Также следует отметить работу [49], в которой авторами показано, что для определения дзета-потенциала частиц сложной геометрии (гидрофобных нанотрубок) достаточно знать их электроосмотическую подвижность.
Типичные значения электропроводности обратных микроэмульсий на основе заряжающих ПАВ лежат в районе от фСм/см до нСм/см в зависимости от концентрации и содержания воды [26, 50, 51]. Следовательно, диффузный слой становится более размытым (длина Дебая в случае неполярных сред намного выше). Существенное влияние на увеличение толщины диффузной части ДЭС оказывает и размер заряженных частиц. Типичный диаметр обратных мицелл без дополнительно введенной полярной фазы составляет от 3 до ~10 нм. В работе [52] авторами отмечен слабый и дальнодействующий характер взаимодействия мицелл ПАВ, который с увеличением концентрации меняется на более сильный и короткодействующий.
3. СТАБИЛИЗАЦИЯ ЗАРЯЖЕННЫХ ЧАСТИЦ АНИОННЫМИ ПАВ
3.1. Классический заряжающий анионный ПАВ – аэрозоль ОТ
Если рассмотреть все многообразие коммерчески доступных ПАВ, можно заметить, что в большинстве своем это водорастворимые ПАВ. Стабилизаторы обратных микроэмульсий встречаются намного реже. Пожалуй, самым популярным маслорастворимым ПАВ является аэрозоль ОТ (АОТ, реже NaAOT, бис-2-(этилгексил)сульфосукцинат натрия). Подробно свойства аэрозоля ОТ и его близких аналогов описаны в цикле из 4 работ с заголовком “What is so special about аerosol-OT” (“Что такого особенного в аэрозоле ОТ?”) [53–56]. А особенного в аэрозоле ОТ достаточно – растворим в неполярных органических растворителях (хорошо) и воде (хуже, но в достаточных количествах); популярный и во многих аспектах оптимальный стабилизатор для микроэмульсионного синтеза; может быть функционализирован различными группами. Но в данном обзоре внимание будет уделяться в первую очередь его заряжающей способности.
Коммерческий аэрозоль ОТ представляет собой натриевую соль. Однако при помощи, например, катионообменной экстракции, можно заменить натрий на другой катион с бóльшим размером или зарядом [57]. В некоторых работах встречаются Ca(AOT)2 [58], AgAOT [59], Cu(AOT)2 [60, 61] и другие соли [62, 63]. Поэтому, чтобы исключить возможное неверное трактование, в дальнейшем под АОТ будет подразумеваться остаток бис-2-(этилгексил)сульфоянтарной кислоты. А натриевая соль будет обозначаться как NaAOT.
Как правило, для придания наночастицам измеримых значений дзета-потенциала используются достаточно высокие концентрации ПАВ. Под высокой концентрацией ПАВ будет подразумеваться диапазон концентраций, которые обычно используются для микроэмульсионного способа получения высокодисперсных частиц или достаточно близкий к ним. Другими словами, такой диапазон концентраций, в котором концентрация ПАВ, находящегося в молекулярной форме, пренебрежимо мала. Диапазон концентраций ПАВ ниже или на уровне ККМ, в котором обратные мицеллы либо отсутствуют вовсе, либо концентрации ПАВ, находящихся в молекулярной и агрегированной форме, достаточно близки, практически не рассматривается. Область низких концентраций NaAOT рассмотрена в работе [64]. В качестве среды использовалась смесь гексадекана и хлороформа (диэлектрическая проницаемость от 2 до 4.8). В системах с высоким содержанием хлороформа обнаружены значения электрофоретической подвижности, соответствующие величинам дзета-потенциала до 100 мВ. Диффузионно-ориентированная ЯМР-спектроскопия показала, что при концентрации в 0.25 мМ в хлороформе NaAOT присутствует в молекулярной форме. По мнению авторов, носителями заряда в данной системе выступают сольватированные ионы АОТ– и (Na2AOT)+. В качестве подтверждения данной гипотезы показана линейная зависимость электропроводности растворов NaAOT от концентрации в диапазоне 0.1–0.5 мМ.
(8)
$2{{\left( {{\text{NaAOT}}} \right)}_{{{\text{solv}}}}} \Leftrightarrow \left( {{\text{N}}{{{\text{a}}}_{{\text{2}}}}{\text{AOT}}} \right)_{{{\text{solv}}}}^{ + } + {\text{ }}\left( {{\text{AOT}}} \right)_{{{\text{solv}}}}^{ - }$(9)
$\left[ {\left( {{\text{N}}{{{\text{a}}}_{{\text{2}}}}{\text{AOT}}} \right)_{{{\text{solv}}}}^{ + }} \right] = \left[ {\left( {{\text{AOT}}} \right)_{{{\text{solv}}}}^{ - }} \right] = {{C}_{{{\text{NaAOT}}}}}\sqrt K $Квадратные скобки означают концентрацию соответствующих ионов, K – константу реакции (8).
Хлороформ более полярный растворитель, нежели предельные углеводороды; длина Бьеррума при стандартных условиях составляет около 12 нм. С учетом размера молекулы NaAOT, толщина сольватационного слоя должна составлять не менее 5 нм, что представляется вполне достижимой величиной.
Более типичный диапазон концентраций NaAOT, используемый для исследования электрокинетических явлений в неполярных средах – десятки или сотни мМ. По мнению ряда исследователей, механизм возникновения заряженных мицелл NaAOT связан с флуктуационными обменными процессами [65]. За счет способности NaАОТ к диссоциации содержимое полярной полости фактически представляет собой раствор электролита. Сама обратная мицелла является довольно лабильным агрегатом, способным к межмицеллярному обмену (эта способность проявляется в эффекте солюбилизации и активно используется при мицеллярном синтезе наночастиц). Соответственно, за счет флуктуационных колебаний электролитного состава полярной полости часть мицелл в определенный момент времени может иметь неравное число катионов и анионов. Схематически процесс изображен на рис. 2. Измерив электропроводность раствора ПАВ, зная его концентрацию и числа агрегации мицелл, можно легко оценить число заряженных мицелл. Соответствующие расчеты для раствора NaAOT в додекане представлены в работе [52]. В диапазоне концентраций 3–200 мМ доля заряженных мицелл составляла около 1.2 × 10–5. Зависимость от концентрации отсутствовала.
Обычно NaAOT используется как заряжающая добавка для коммерческих образцов высокодисперсных частиц, например оксида кремния [45] или полиметилметакрилата [66]. Встречаются отдельные работы, в которых NaAOT использован как нанореактор, позволяющий получать заряженные наночастицы металлов [42, 44], оксида кремния [67]. Полученные значения электрофоретической подвижности довольно близки.
3.2. Цирконил 2-этилгексонат
Вторым по популярности анионным заряжающим ПАВ является цирконил 2-этилгексонат (обычно обозначается как ZrO(Oct)2). И по популярности он существенно уступает не только NaАОТ, но и всем неионным ПАВ, которые будут обсуждаться далее. Его структура довольно близка к NaАОТ, механизм действия как заряжающего агента аналогичен. В работе [45] ZrO(Oct)2 использовался для заряжения наночастиц оксида кремния наряду с NaАОТ и неионным OLOA 11000 (более детально будет обсуждаться в главе 4.1). Авторы отмечают, что характер влияния каждого из ПАВ на электрофоретическую подвижность довольно близок. При низких концентрациях наночастицы не заряжены по причине отсутствия достаточного количества мицелл для стабилизации противоионов.
4. СТАБИЛИЗАЦИЯ ЗАРЯЖЕННЫХ ЧАСТИЦ НЕИОННЫМИ ПАВ
Сам по себе термин “неионные заряжающие ПАВ” вполне способен вызвать диссонанс у читателя. Это действительно похоже на некий оксюморон – заряжение частицы подразумевает формирование ионов, а неионная природа ПАВ, напротив, подразумевает их отсутствие. Однако работы, посвященные контролю электрокинетического потенциала наночастиц в неполярных средах при помощи неионных ПАВ, также присутствуют, притом в сопоставимом объеме. Наиболее распространены два типа неионных заряжающих ПАВ – эфиры сорбитола и жирных кислот (торговая марка Span) и полиизобутиленсукцинимиды (торговая марка OLOA). Часто в литературе можно встретить аббревиатуры PIBS или PIBSI (в дальнейшем для полиизобутиленсукцинимида будет использоваться аббревиатура PIBSI, так как в публикациях встречаются не только коммерческие ПАВ под торговой маркой OLOA, но и синтезируемые авторами [68, 69]). Для данных ПАВ будет рассматриваться только область концентраций выше ККМ. В работе [65] можно обратить внимание, что PIBSI имеют даже более высокие константы диспропорционирования, в сравнении с анионным NaАОТ, в до-мицеллярной области концентраций достаточного для детектирования электрокинетических явлений содержания заряженных частиц не образуется. Хотя и в мицеллярной области концентраций их существование тоже не особо очевидно.
4.1. Полиизобутиленсукцинимиды
Первые примеры исследования влияния добавок неионных заряжающих ПАВ на стабильность коллоидных систем с неполярной дисперсионной средой были опубликованы в 80-х годах. Авторы работы [70] использовали ПАВ с торговым названием OLOA 1200 (раствор PIBSI в минеральном масле, 1 : 1 по массе) для стабилизации частиц сажи в додекане. Значения дзета-потенциала частиц достигали более –100 мВ. В этой же работе предложен механизм, как неионный PIBSI может зарядить частицы. Более поздние работы [23, 46, 71] при описании “заряжения” частиц неионными ПАВ, как правило, руководствуются именно этим механизмом, предложенным Фоуксом (F.M. Fowkes) с соавт. [70, 72, 73].
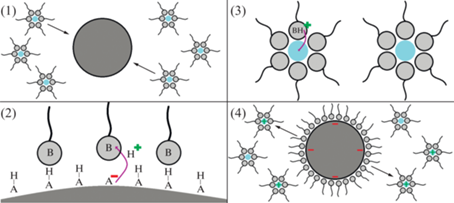
Механизм основан на кислотно-основном взаимодействии, для PIBSI обычно используют термин “основный” ПАВ [74]. По гипотезе Фоукса стадии формирования заряженных частиц в коллоидах, стабилизированных PIBSI, следующие:
1) адсорбция мицелл на частицах дисперсной фазы;
2) диссоциация функциональных групп на поверхности частиц дисперсной фазы или десорбция ионизуемых частиц с поверхности частиц дисперсной фазы;
3) трансфер носителя заряда и его стабилизация функциональными группами ПАВ в полярной полости обратной мицеллы;
4) десорбция мицелл в виде стерически стабилизированных ионов.
Схематически данный механизм проиллюстрирован на рис. 3 для случая “основного” заряжающего ПАВ типа PIBSI. Сложность данного механизма заключается в том, что эффективность заряжения частиц дисперсной фазы определяется не только свойствами самого ПАВ и наличием определенных функциональных групп, способных к стабилизации ионов, но и химией поверхности самих частиц дисперсной фазы. Поэтому наиболее популярные объекты “для заряжения” – частицы оксидов кремния или титана, природа возникновения поверхностного заряда для которых хорошо изучена. Взаимодействие кислых силанольных групп на поверхности частицы оксида кремния и основных амино-групп, входящих в состав PIBSI, обеспечивают высокие значения дзета-потенциала частиц [34, 75].
Рис. 3.
Схематическое изображение модели стабилизации заряженных частиц в микроэмульсиях “основных” неионных ПАВ, предложенная Фоуксом [70]. Стадии процесса: (1) адсорбция обратных мицелл на частице дисперсной фазы; (2) перенос носителя заряда (в данном случае иона Н+ как наиболее простого и распространенного) с локализованных на поверхности частицы дисперсной фазы функциональных групп А–Н (“Acid”), обладающих кислыми свойствами, на функциональные группы В (“Base”) молекулы ПАВ, обладающих основными свойствами; (3) стабилизация носителя заряда полярной полостью обратной мицеллы; (4) десорбция заряженных обратных мицелл с частиц дисперсной фазы.
Также следует отметить, что эффективность неионных ПАВ как заряжающих агентов как минимум не уступает ионным. В работе [76] отмечено, что для OLOA 1200 численная доля заряженных мицелл очень высока (около 0.017) и может даже превосходить таковую для случая NaAOT.
4.2. Эфиры сорбитола и жирных кислот
Наверное, почти так же часто, как и NaАОТ, в статьях, посвященных стабилизации заряженных частиц в неводных средах, можно встретить такой ПАВ, как Span 80 (сорбитан моноолеат). Он обсуждается после PIBSI не по причине меньшей популярности, а по причине более поздних работ, посвященных данному ПАВ. Так же, как и NaАОТ, Span 80 может использоваться одновременно как заряжающий агент и стабилизатор при синтезе наночастиц [77, 78]. Дзета-потенциал наночастиц серебра, стабилизированных Span 80, составлял около +30 мВ. На данный момент коммерчески доступна весьма широкая линейка эфиров сорбитола разных производителей, что и обуславливает их популярность и большое количество работ, опубликованных за последние десятилетия. Под торговой маркой Span выпускаются: лаурат (Span 20), пальминат (Span 40), стеарат (Span 60), тристеарат (Span 65), триолеат (Span 85) и другие. И все это так называемое “семейство Span” (выражение “Span family” можно встретить, например, в работах [16, 79]) является заряжающими ПАВ, в большей или меньшей степени [80]. В ряде работ эти ПАВ называют “кислотными” [71, 81] (в противоположность “основным” PIBSI). Соответственно, предполагаемый механизм действия также кислотно-основный, только в качестве кислоты выступает ПАВ, а в качестве основания – частицы дисперсной фазы.
В качестве подтверждения кислотно-основного механизма можно привести исследование группы профессора Берга. В работе [46] приводится сравнение значений дзета-потенциала функционализированных наночастиц оксида кремния при использовании в качестве заряжающих агентов Span 80 и OLOA 11000 (дисперсия PIBSI с молекулярной массой около 1.2 кДа в минеральном масле [82]). Для придания поверхности наночастиц кислотных свойств они были функционализированы 3-глицидоксипропилтриметоксисиланом, а для придания основных свойств – аминопропилтриэтоксисиланом. Оба ПАВ заряжали частицы как с “кислой”, так и с “основной” поверхностью. Но Span 80 эффективнее заряжал основные частицы (+93 против +27 мВ), а OLOA 11000 – напротив, кислые (–77 против –19 мВ). В более поздней работе [74] этой группы также отмечена важность рассмотрения химии поверхности частиц дисперсной фазы. В предыдущих работах при использовании OLOA 11000 авторами были получены только отрицательно заряженные частицы. В данной работе авторы исследовали оксиды кремния, титана, цинка, алюминия и магния. И в случае оксида магния были получены положительно заряженные частицы. Оксид магния обладает наиболее выраженными основными свойствами и, видимо, в данном случае полиимид OLOA 11000 выступает не как основание, а как кислота. При этом авторы отмечают, что предложенный механизм до конца не понятен и требует уточнения.
4.3. Нетипичные примеры стабилизации заряженных частиц
Первый пример электрокинетических явлений в органозолях, стабилизированных оксиэтилированными ПАВ, представлен в работе [83]. Этоксилаты относятся к неионным ПАВ, соответственно, не обладают способностью к диссоциации. Кислотно-основные свойства этоксилатов в литературе также не обсуждаются. Соответственно, описанные ранее механизмы стабилизации заряженных частиц для обратных микроэмульсий, стабилизированных этоксилатами, неприменимы. Авторы сделали предположение, что роль носителя заряда в таких системах отводится примесным ионам хлора. Наличие значительного количества хлора показано как качественно, реакцией с нитратом серебра, так и полуколичественно, методом синхронного термического анализа с масс-спектрометрическим детектированием уходящих газов.
5. ЗАКЛЮЧЕНИЕ
В данной обзорной работе рассмотрены основные механизмы стабилизации заряженных частиц поверхностно-активными веществами в средах с низкой диэлектрической проницаемостью, имеющие значение для важных областей науки и техники. Рассмотрены основные типы популярных заряжающих ПАВ и отмечена их довольно близкая эффективность. Для анионных ПАВ описанный в научной литературе механизм возникновения, стабилизации и транспорта заряда автору данного обзора представляется логичным и понятным, в основном по причине его простоты. В случае неионных ПАВ в качестве возможного механизма в литературе предлагается кислотно-основное взаимодействие. Хотя в ряде работ, опубликованных в авторитетных изданиях, представлены эксперименты, хорошо соответствующие данной концепции, назвать ее однозначной автор данной работы не возьмется. Сложность данной модели обусловлена тем, что электрокинетические явления в подобных системах определяются не только химией самих ПАВ, но и химией поверхности частиц дисперсной фазы. А она далеко не всегда бывает проста и понятна. Поэтому стабилизация заряженных частиц неионными ПАВ в средах с диэлектрической проницаемостью около 2 представляется на данный момент недостаточно изученной областью коллоидной химии, как с точки зрения теоретических представлений, так и с точки зрения их экспериментального подтверждения, особенно с учетом новых результатов [83] и результатов, которые до конца не понятны и требуют уточнения [74, 83].
Список литературы
Mer V.K., Downes H.C. Indicator studies of acids and bases in benzene // Chem. Rev. 1933. V. 13. № 1. P. 47–60. https://doi.org/10.1021/cr60044a004
Fuoss R.M. Properties of electrolytic solutions // Chemical Reviews. 1935. V. 17. № 1. P. 27–42. https://doi.org/10.1021/cr60056a002
Mer V.K., Downes H.C. Acidity in non-aqueous solvents. Conductimetric and electrometric titrations of acids and bases in benzene // Journal of the American Chemical Society. 1931. V. 53. № 3. P. 888–896. https://doi.org/10.1021/ja01354a009
Mer V.K., Downes H.C. Indicator studies of acids and bases in benzene // Journal of the American Chemical Society. 1933. V. 55. № 5. P. 1840–1864. https://doi.org/10.1021/ja01332a010
Strong L.E., Kraus C.A. Properties of electrolytic solutions. XLV. Conductance of some salts in benzene at higher concentrations // Journal of the American Chemical Society. 1950. V. 72. № 1. P. 166–171. https://doi.org/10.1021/ja01157a047
van der Minne J.L., Hermanie P.H.J. Electrophoresis measurements in benzene-correlation with stability. I. Development of method // Journal of Colloid Science. 1952. V. 7. № 6. P. 600–615. https://doi.org/10.1016/0095-8522(52)90042-1
Scher H., Shlesinger M.F., Bendler J.T. Time-scale invariance in transport and relaxation // Physics Today. 1991. V. 44. № 1. P. 26–34. https://doi.org/10.1063/1.881289
Beunis F., Strubbe F., Neyts K. et al. Power-law transient charge transport in a nonpolar liquid // Applied Physics Letters. 2007. V. 90. № 18. https://doi.org/10.1063/1.2734511
Matsubara Y., Matsushima S., Jones T.B. Charge accumulation in an oil tank during loading operations // Journal of Electrostatics. 1997. V. 40. P. 191–197. https://doi.org/10.1016/S0304-3886(97)00036-3
Perisse F., Vazquez J., Paillat T. et al. Gasoline electrification: Moisture and temperature influence // Journal of Electrostatics. 2005. V. 63. № 6–10. P. 481–487. https://doi.org/10.1016/j.elstat.2005.03.006
Sun K., Liu Q., Li X. Simulation test on charge density and surface potential in an oil tank during filling operation // Journal of Electrostatics. 2009. V. 67. № 2–3. P. 340–341. https://doi.org/10.1016/j.elstat.2009.01.043
Kim J., Anderson J.L., Garoff S. et al. Ionic conduction and electrode polarization in a doped nonpolar liquid // Langmuir. 2006. V. 22. № 18. P. 7942. https://doi.org/10.1021/la061883w
Patel M.N., Smith P.G., Kim J. et al. Electrophoretic mobility of concentrated carbon black dispersions in a low-permittivity solvent by optical coherence tomography // Journal of Colloid and Interface Science. 2010. V. 345. № 2. P. 194–199. https://doi.org/10.1016/j.jcis.2010.01.055
Thwala J.M., Goodwin J.W., Mills P.D. Electrokinetic studies of colloidal silica particles dispersed in non-aqueous media in the presence of a nonionic surfactant, dodecylhexaethylene glycol monoether (C12E6) // Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2009. V. 335. № 1–3. P. 33–42. https://doi.org/10.1016/j.colsurfa.2008.10.050
Novotny V. Contributions of particles to electrical conductivity of colloids // Colloids and Surfaces. 1986. V. 21. P. 219–233. https://doi.org/10.1016/0166-6622(86)80093-2
Espinosa C.E., Guo Q., Singh V. et al. Particle charging and charge screening in nonpolar dispersions with nonionic surfactants // Langmuir. 2010. V. 26. № 22. P. 16941–16948. https://doi.org/10.1021/la1033965
Zhang Z., Wang Y., Chen Q. et al. Application of high potential electrophoretic particles modified with high ionization mono ionic liquid for electrophoretic displays // Micromachines. 2022. V. 13. № 8. P. 1235. https://doi.org/10.3390/mi13081235
Gao A., Cao M., Yan J. et al. Research on electrophoretic display ink and its microencapsulation // Lecture Notes in Electrical Engineering. 2019. V. 543. P. 788–793. https://doi.org/10.1007/978-981-13-3663-8_106
Morrison I.D. Electrical charges in nonaqueous media // Colloids and Surfaces A: Physicochemical and Engineering Aspects. 1993. V. 71. № 1. P. 1–37. https://doi.org/10.1016/0927-7757(93)80026-B
Fertig D., Sarkadi Z., Valiskó M. et al. Scaling for rectification of bipolar nanopores as a function of a modified Dukhin number: The case of 1 : 1 electrolytes // Molecular Simulation. 2022. V. 48. № 1. P. 43–56. https://doi.org/10.1080/08927022.2021.1939330
Vaitheeswaran S., Reddy G., Thirumalai D. Water-mediated interactions between hydrophobic and ionic species in cylindrical nanopores // Journal of Chemical Physics. 2009. V. 130. № 9. P. 094502. https://doi.org/10.1063/1.3080720
Prieve D.C., Yezer B.A., Khair A.S. et al. Formation of charge carriers in liquids // Advances in Colloid and Interface Science. 2017. V. 244. P. 21–35. https://doi.org/10.1016/j.cis.2016.11.004
Smith G.N., Eastoe J. Controlling colloid charge in nonpolar liquids with surfactants // Physical Chemistry Chemical Physics. 2013. V. 15. № 2. P. 424–439. https://doi.org/10.1039/c2cp42625k
Varela L., Andraus S., Trizac E. et al. Relaxation dynamics of two interacting electrical double-layers in a 1D Coulomb system // Journal of Physics Condensed Matter. 2021. V. 33. № 39. https://doi.org/10.1088/1361-648X/ac1237
Eyvazi N., Biagooi M., Nedaaee Oskoee S.E. Molecular dynamics investigation of charging process in polyelectrolyte-based supercapacitors // Scientific Reports. 2022. V. 12. № 1. P. 1098. https://doi.org/10.1038/s41598-022-04837-4
Dukhin A.S., Goetz P.J. How non-ionic “electrically neutral” surfactants enhance electrical conductivity and ion stability in non-polar liquids // Journal of Electroanalytical Chemistry. 2006. V. 588. № 1. P. 44–50. https://doi.org/10.1016/j.jelechem.2005.12.001
Peri J.B. The state of solution of aerosol OT in nonaqueous solvents // Journal of Colloid and Interface Science. 1969. V. 29. № 1. P. 6–15. https://doi.org/10.1016/0021-9797(69)90340-3
Kotlarchyk M., Chen S.H., Huang J.S. et al. Structure of three-component microemulsions in the critical region determined by small-angle neutron scattering // Physical Review A. 1984. V. 29. № 4. P. 2054–2069. https://doi.org/10.1103/PhysRevA.29.2054
Kotlarchyk M., Huang J.S., Chen S.H. Structure of AOT reversed micelles determined by small-angle neutron scattering // Journal of Physical Chemistry. 1985. V. 89. № 20. P. 4382–4386. https://doi.org/10.1021/j100266a046
Zhang J., Bright F.V. Nanosecond reorganization of water within the interior of reversed micelles revealed by frequency-domain fluorescence spectroscopy // Journal of Physical Chemistry. 1991. V. 95. № 20. P. 7900–7907. https://doi.org/10.1021/j100173a064
Eicke H.-F., Christen H. Is water critical to the formation of micelles in apolar media?? // Helvetica Chimica Acta. 1978. V. 61. № 6. P. 2258–2263. https://doi.org/10.1002/hlca.19780610631
Fowkes F.M., Lloyd T.B., Chen W.-J. et al. Zeta-potentials and heats of adsorption of charge-control agents on liquid toners // Proc. SPIE 1253. Hard Copy and Printing Materials, Media, and Processes. 1990. V. 1253. P. 52−62.https://doi.org/10.1117/12.19840
Birkett K.L., Gregory P. Metal complex dyes as charge control agents // Dyes and Pigments. 1986. V. 7. № 5. P. 341–350. https://doi.org/10.1016/0143-7208(86)80002-X
Strubbe F., Beunis F., Neyts K. Detection of elementary charges on colloidal particles // Physical Review Letters. 2008. V. 100. № 21. P. 218301. https://doi.org/10.1103/PhysRevLett.100.218301
Hogg R., Healy T.W., Fuerstenau D.W. Mutual coagulation of colloidal dispersions // Transactions of the Faraday Society. 1966. V. 62. № 615. P. 1638–1651. https://doi.org/10.1039/tf9666201638
Haoping W., Jun J., Blum L. Improvement on the Derjaguin’s method for the interaction of spherical particles // Colloid and Polymer Science. 1995. V. 273. № 4. P. 359–363. https://doi.org/10.1007/BF00652350
Bowen R.W., Filippov A.N., Sharif A.O. et al. Model of the interaction between a charged particle and a pore in a charged membrane surface // Advances in Colloid and Interface Science. 1999. V. 81. № 1. P. 35–72. https://doi.org/10.1016/S0001-8686(99)00004-4
Sun J., Velamakanni B.V., Gerberich W.W. et al. Aqueous latex/ceramic nanoparticle dispersions: Colloidal stability and coating properties // Journal of Colloid and Interface Science. 2004. V. 280. № 2. P. 387–399. https://doi.org/10.1016/j.jcis.2004.08.014
Bulavchenko A.I., Popovetskiy P.S. Structure of adsorption layer of silver nanoparticles in sodium bis(2-ethylhexyl) sulfosuccinate solutions in n-decane as observed by photon-correlation spectroscopy and nonaqueous electrophoresis // Langmuir. 2014. V. 30. № 43. P. 12729–12735. https://doi.org/10.1021/la5004935
Brown M.A., Abbas Z., Kleibert A. et al. Determination of surface potential and electrical double-layer structure at the aqueous electrolyte-nanoparticle interface // Physical Review X. 2016. V. 6. № 1. P. 011007. https://doi.org/10.1103/PhysRevX.6.011007
Dukhin A.S., van de Ven T.G.M. Electrokinetic characterization of polydisperse colloidal particles // Journal of Colloid and Interface Science. 1994. V. 165. № 1. P. 9–18. https://doi.org/10.1006/jcis.1994.1200
Bulavchenko A.I., Pletnev D.N. Electrophoretic concentration of nanoparticles of gold in reversed micellar solutions of AOT // Journal of Physical Chemistry C. 2008. V. 112. № 42. P. 16365–16369. https://doi.org/10.1021/jp805268w
Strubbe F., Beunis F., Neyts K. Determination of the effective charge of individual colloidal particles // Journal of Colloid and Interface Science. 2006. V. 301. № 1. P. 302–309. https://doi.org/10.1016/j.jcis.2006.04.034
Bulavchenko A.I., Popovetsky P.S. Electrokinetic potential of nanoparticles in reverse AOT micelles: Photometric determination and role in the processes of heterocoagulation, separation, and concentration // Langmuir. 2010. V. 26. № 2. P. 736–742. https://doi.org/10.1021/la903583r
Poovarodom S., Poovarodom S., Berg J.C. Effect of alkyl functionalization on charging of colloidal silica in apolar media // Journal of Colloid and Interface Science. 2010. V. 351. № 2. P. 415–420. https://doi.org/10.1016/j.jcis.2010.07.058
Poovarodom S., Berg J.C. Effect of particle and surfactant acid-base properties on charging of colloids in apolar media // Journal of Colloid and Interface Science. 2010. V. 346. № 2. P. 370–377. https://doi.org/10.1016/j.jcis.2010.03.012
Tscharnuter W.W., McNeil-Watson F., Fairhurst D. A new instrument for the measurement of very small electrophoretic mobilities using phase analysis light scattering // ACS Symposium Series. 1998. V. 693. P. 327–340. https://doi.org/10.1021/bk-1998-0693.ch023
Thomas J.C., Crosby B.J., Keir R.I. et al. Observation of field-dependent electrophoretic mobility with phase analysis light scattering (PALS) // Langmuir. 2002. V. 18. № 11. P. 4243–4247. https://doi.org/10.1021/la011758e
Vinogradova O.I., Silkina E.F., Asmolov E.S. Transport of ions in hydrophobic nanotubes // Physics of Fluids. 2022. V. 34. № 12. https://doi.org/10.1063/5.0131440
Sainis S.K., Merrill J.W., Dufresne E.R. Electrostatic interactions of colloidal particles at vanishing ionic strength // Langmuir. 2008. V. 24. № 23. P. 13334–13337. https://doi.org/10.1021/la8024606
Park J.K., Ryu J.C., Kim W.K. et al. Effect of electric field on electrical conductivity of dielectric liquids mixed with polar additives: DC conductivity // Journal of Physical Chemistry B. 2009. V. 113. № 36. P. 12271–12276. https://doi.org/10.1021/jp9015189
Hsu M.F., Dufresne E.R., Weitz D.A. Charge stabilization in nonpolar solvents // Langmuir. 2005. V. 21. № 11. P. 4881–4887. https://doi.org/10.1021/la046751m
Nave S., Eastoe J., Penfold J. What is so special about aerosol-OT? 1. Aqueous systems // Langmuir. 2000. V. 16. № 23. P. 8733–8740. https://doi.org/10.1021/la000341q
Nave S., Eastoe J., Heenan R.K. et al. What is so special about aerosol-OT? 2. Microemulsion systems // Langmuir. 2000. V. 16. № 23. P. 8741–8748. https://doi.org/10.1021/la000342i
Nave S., Eastoe J., Heenan R.K. et al. What is so special about aerosol-OT? Part III – Glutaconate versus sulfosuccinate headgroups and oil−water interfacial tensions // Langmuir. 2002. V. 18. № 5. P. 1505–1510. https://doi.org/10.1021/la015564a
Nave S., Paul A., Eastoe J. et al. What is so special about aerosol-OT? Part IV. Phenyl-tipped surfactants // Langmuir. 2005. V. 21. № 22. P. 10021–10027. https://doi.org/10.1021/la050767a
Bulavchenko A.I., Podlipskaya T.Y., Demidova M.G. et al. The formation of Me(AOT)n micelles as nanoreactors, crystallizers, and charging agents: Cation-exchange solvent extraction versus direct injection solubilization // Solvent Extraction and Ion Exchange. 2020. V. 38. № 4. P. 455–471. https://doi.org/10.1080/07366299.2020.1733747
Fioretto D., Freda M., Mannaioli S. et al. Infrared and dielectric study of Ca(AOT)2 reverse micelles // Journal of Physical Chemistry B. 1999. V. 103. № 14. P. 2631–2635. https://doi.org/10.1021/jp9837028
Petit C., Lixon P., Pileni M.P. In situ synthesis of silver nanocluster in AOT reverse micelles // Journal of Physical Chemistry. 1993. V. 97. № 49. P. 12974–12983. https://doi.org/10.1021/j100151a054
Lisiecki I., André P., Filankembo A. et al. Mesostructured fluids. 1. Cu(AOT)2−H2O−isooctane in oil rich regions // Journal of Physical Chemistry B. 1999. V. 103. № 43. P. 9168–9175. https://doi.org/10.1021/jp991242s
Tanori J., Gulik-Krzywicki T., Pileni M.P. Phase diagram of copper(II) bis(2-ethylhexyl) sulfosuccinate, Cu(AOT)2–isooctane–water // Langmuir. 1997. V. 13. № 4. P. 632–638. https://doi.org/10.1021/la960427c
Smith G.N., Brown P., James C. et al. The effects of counterion exchange on charge stabilization for anionic surfactants in nonpolar solvents // Journal of Colloid and Interface Science. 2016. V. 465. P. 316–322. https://doi.org/10.1016/j.jcis.2015.11.062
Eastoe J., Fragneto G., Robinson B.H. et al. Variation of surfactant counterion and its effect on the structure and properties of aerosol-OT-based water-in-oil microemulsions // Journal of the Chemical Society, Faraday Transactions. 1992. V. 88. № 3. P. 461–471. https://doi.org/10.1039/FT9928800461
Bulavchenko A.I., Shaparenko N.O., Kompan’kov N.B. et al. The formation of free ions and electrophoretic mobility of Ag and Au nanoparticles in n-hexadecane-chloroform mixtures at low concentrations of AOT // Physical Chemistry Chemical Physics. 2020. V. 22. № 26. P. 14671–14681. https://doi.org/10.1039/d0cp02153a
Strubbe F., Neyts K. Charge transport by inverse micelles in non-polar media // Journal of Physics Condensed Matter. 2017. V. 29. № 45. P. 453003.https://doi.org/10.1088/1361-648X/aa8bf6
Park E., Lee S., Lee H. et al. Full-color electrophoretic display using charged colloidal arrays of core–shell microspheres with enhanced color tunability in non-polar medium // Advanced Optical Materials. 2021. V. 9. № 21. P. 2100833. https://doi.org/10.1002/adom.202100833
Shaparenko N.O., Beketova D.I., Demidova M.G. et al. Regulation of the charge and hydrodynamic diameter of silica nanoparticles in AOT microemulsions // Colloid Journal. 2019. V. 81. № 1. P. 43–49. https://doi.org/10.1134/S1061933X19010101
Lee J. Charge carriers created by interaction of a nonionic surfactant with water in a nonpolar medium // Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2018. V. 554. P. 211–217. https://doi.org/10.1016/j.colsurfa.2018.06.050
Parent M.E., Yang J., Jeon Y. et al. Influence of surfactant structure on reverse micelle size and charge for nonpolar electrophoretic inks // Langmuir. 2011. V. 27. № 19. P. 11845–11851. https://doi.org/10.1021/la202660d
Pugh R.J., Matsunaga T., Fowkes F.M. The dispersibility and stability of carbon black in media of low dielectric constant. 1. Electrostatic and steric contribution to colloidal stability // Colloids and Surfaces. 1983. V. 7. № 3. P. 183–207. https://doi.org/10.1016/0166-6622(83)80046-8
Gacek M.M., Berg J.C. Effect of synergists on organic pigment particle charging in apolar media // Electrophoresis. 2014. V. 35. № 12–13. P. 1766–1772. https://doi.org/10.1002/elps.201300593
Pugh R.J., Fowkes F.M. The dispersibility and stability of carbon black in media of low dielectric constant. 2. Sedimentation volume of concentrated dispersions, adsorption and surface calorimetry studies // Colloids and Surfaces. 1984. V. 9. № 1. P. 33–46. https://doi.org/10.1016/0166-6622(84)80140-7
Pugh R.J., Fowkes F.M. The dispersibility and stability of coal particles in hydrocarbon media with a polyisobutene succinamide dispersing agent // Colloids and Surfaces. 1984. V. 11. № 3–4. P. 423–427. https://doi.org/10.1016/0166-6622(84)80295-4
Gacek M.M., Berg J.C. Investigation of surfactant mediated acid-base charging of mineral oxide particles dispersed in apolar systems // Langmuir. 2012. V. 28. № 51. P. 17841–17845. https://doi.org/10.1021/la303943k
Singh H., Ray D., Kumar S. et al. Probing the adsorption of nonionic micelles on different-sized nanoparticles by scattering techniques // Physical Review E. 2020. V. 102. № 6. P. 062601. https://doi.org/10.1103/PhysRevE.102.062601
Beunis F., Strubbe F., Marescaux M. et al. Micellization and adsorption of surfactant in a nonpolar liquid in micrometer scale geometries // Applied Physics Letters. 2010. V. 97. № 18. P. 2008–2011. https://doi.org/10.1063/1.3503968
Popovetskiy P.S. Synthesis and characterization of silver nanoparticles in reverse micelles of nonionic surfactants and in their mixed micelles with AOT // Colloid Journal. 2020. V. 82. № 2. P. 144–151. https://doi.org/10.1134/S1061933X2002009X
Popovetskiy P.S., Kolodin A.N., Maximovskiy E.A. et al. Electrophoretic concentration and production of conductive coatings from silver nanoparticles stabilized with non-ionic surfactant Span 80 // Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2021. V. 625. P. 126961. https://doi.org/10.1016/j.colsurfa.2021.126961
Varshosaz J., Pardakhty A., Hajhashemi V.I. et al. Development and physical characterization of sorbitan monoester niosomes for insulin oral delivery // Drug Delivery: Journal of Delivery and Targeting of Therapeutic Agents. 2003. V. 10. № 4. P. 251–262. https://doi.org/10.1080/drd_10_4_251
Gacek M.M., Berg J.C. Effect of surfactant hydrophile-lipophile balance (HLB) value on mineral oxide charging in apolar media // Journal of Colloid and Interface Science. 2015. V. 449. P. 192–197. https://doi.org/10.1016/j.jcis.2014.11.075
Ponto B.S., Berg J.C. Nanoparticle charging with mixed reverse micelles in apolar media // Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2020. V. 586. P. 124275. https://doi.org/10.1016/j.colsurfa.2019.124275
Yezer B.A., Khair A.S., Sides P.J. et al. Use of electrochemical impedance spectroscopy to determine double-layer capacitance in doped nonpolar liquids // Journal of Colloid and Interface Science. 2015. V. 449. P. 2–12. https://doi.org/10.1016/j.jcis.2014.08.052
Popovetskiy P., Kasyanov A., Maximovskiy E. et al. Electrophoretic mobility of silver nanoparticles stabilized with nonionic surfactant Ecosurf SA4: Origin of charged particles, concentration by electrophoresis and production of conductive coatings // Journal of Molecular Liquids. 2023. V. 374. P. 121273. https://doi.org/10.1016/j.molliq.2023.121273
Дополнительные материалы отсутствуют.
Инструменты
Коллоидный журнал



