Коллоидный журнал, 2023, T. 85, № 5, стр. 566-582
Стимул-чувствительные системы для доставки лекарств на основе бислойных липидных везикул: новые тенденции
А. А. Ефимова 1, *, А. В. Сыбачин 1
1 Московский государственный университет имени М.В. Ломоносова,
химический факультет
119991 Москва, Ленинские горы, 1, стр. 3, Россия
* E-mail: ephimova@genebee.msu.su
Поступила в редакцию 07.07.2023
После доработки 27.07.2023
Принята к публикации 31.07.2023
- EDN: ZAJLIH
- DOI: 10.31857/S0023291223600608
Аннотация
Разработка новых эффективных способов борьбы с тяжелыми заболеваниями, среди которых особое место занимают онкологические и инфекционные, остается актуальной задачей биомедицины и биотехнологии. В настоящее время усилия ученых сосредоточены на поисках лекарственных систем, обеспечивающих высокую эффективность лечения при минимальном воздействии на организм. Развитие этого направления привело к созданию стимул-чувствительных липосом, которые могут высвобождать инкапсулированное лекарство в ответ на определенный стимул: температуру, pH, электромагнитное поле, свет и др. Под воздействием стимула липидные бислойные везикулы изменяют структуру, размер, поверхностный заряд или фазовое состояние, что приводит к контролируемому высвобождению лекарственного препарата в определенном месте в организме, что позволяет добиться более точной и эффективной доставки. В данном обзоре обсуждаются современные тенденции в разработке стимул-чувствительных систем на основе липосом для контролируемой доставки биологически активных веществ.
ВВЕДЕНИЕ
Разработка новых эффективных способов борьбы с тяжелыми заболеваниями, среди которых особое место занимают онкологические и инфекционные, остается актуальной задачей биомедицины и биотехнологии. В настоящее время усилия ученых сосредоточены на поисках лекарственных систем, обеспечивающих высокую эффективность лечения при минимальном воздействии на организм. Среди проблем, ограничивающих применение многих препаратов, – малая биодоступность и низкая растворимость, риски развития побочных эффектов, а также резистентности и множественной лекарственной устойчивости, возникающих при длительной терапии [1]. Преодолеть эти трудности (хотя бы частично) можно, помещая лекарство в нано- или микроразмерный контейнер [2–4]. Такой прием позволяет заметно повысить биодоступность малорастворимых лекарств, увеличить время циркулирования в кровотоке и предотвратить их преждевременное разложение компонентами крови [5].
Благодаря впечатляющему прогрессу в материаловедении и фармацевтике за последние десятилетия был разработан широкий спектр наноносителей для лекарственных средств [6–8]. На сегодняшний день описаны десятки систем различного состава, архитектуры и морфологии для инкапсулирования и доставки биоактивных веществ: неорганические частицы [9, 10], мицеллы [11–13], дендримеры и полимерные микросферы [14–16], (био)коллоиды [17, 18], везикулы и т.д. Размер таких носителей обычно изменяется в интервале от нескольких десятков до нескольких сотен нанометров, что позволяет осуществлять системное (внутривенное) или местное (через слизистую оболочку) введение и способствует их диффузии внутри клетки. Среди перечисленных наноконтейнеров для доставки и контролируемого высвобождения биологически активных веществ наиболее широкое использование получили липосомы – сферические бислойные липидные везикулы [19, 20]. В настоящее время на фармацевтическом рынке представлено большое количество липосомальных препаратов для противоопухолевой, противовирусной и противомикробной терапии [21–24].
Развитие этого направления привело к созданию стимул-чувствительных липосом, которые могут высвобождать инкапсулированное водорастворимое лекарство в ответ на определенный (часто внешний) стимул, например температуру, ультразвук, электромагнитное поле, свет и др. [25–27]. Под воздействием стимула липидные бислойные везикулы изменяют структуру, размер, поверхностный заряд или фазовое состояние, что приводит к контролируемому высвобождению лекарственного препарата в определенном месте в организме, что позволяет добиться более точной и эффективной доставки лекарственных веществ/субстанций/агентов. Стимул-чувствительные системы для доставки лекарств на основе липидных бислойных везикул обладают рядом преимуществ над системами, использующими другие виды наночастиц. Это связано с тем, что липосомы сходны с клеточными природными мембранами по составу, биосовместимы и биодеградируемы, не токсичны и более устойчивы к разрушению в кровотоке. Кроме того, липосомы могут быть легко получены, а также функционализированы для улучшения специфичности и эффективности доставки биологически активных веществ [28].
В данном обзоре обсуждаются современные тенденции в разработке стимул-чувствительных систем на основе липосом для контролируемой доставки биологически активных веществ. Особое внимание уделено методам, приводящим к получению рН-чувствительных липосом, способных быстро высвобождать инкапсулированное вещество после попадания контейнера в области с пониженным значением рН, например, в опухоли, места воспалений или внутриклеточное пространство. Подробно рассмотрены представляющие большой интерес для исследователей магнито-чувствительные липосомы, содержащие магнитные наночастицы.
Одной из важнейших задач современной науки, возникающей при создании систем для доставки лекарств, является возможность контролируемого высвобождения препарата. Часто при попадании контейнера в целевые ткани не наблюдается высвобождения лекарственного вещества в достаточной концентрации. Поэтому в настоящее время активно разрабатываются системы, чувствительные к патологическим изменениям, например, в микроокружении опухоли или очагах воспалений. Среди подобных физиологических отличий чаще всего используют более низкие значения рН, повышенную температуру, измененный редокс-потенциал [29]. Стимул-чувствительные липосомы сохраняют свою структуру и свойства во время циркуляции в организме, но способны образовывать большое количество дефектов в бислое или полностью разрушаться при попадании в специфические условия раковой опухоли или места сильных/обширных/ существенных воспалений. При этом кинетика высвобождения заключенного в контейнер биоактивного препарата определяется многими факторами, в частности, концентрацией и природой инкапсулированного вещества, зарядом и составом липидного комплекса, размером и структурой липосом, а также внешними факторами, такими как температура, pH и др.
Термочувствительные липосомы
Термочувствительные липосомы способны удерживать лекарство в течение определенного периода времени, высвобождая содержимое в условиях локальной гипертермии: 41–45°С [30, 31]. Для получения подобных липосом используют липиды, температура фазового перехода которых лежит в пределах 38–42°С. При температуре фазового перехода липиды переходят из гелевого (твердого) в жидкокристаллическое (жидкое) состояние. Жидкое и твердое состояния мембран различаются набором параметров, одним из которых является подвижность липидов. В твердом состоянии подвижность липидных молекул как целого резко ограничена: углеводородные цепи имеют максимально вытянутую транс-конформацию и находятся в состоянии плотнейшей упаковки. При фазовом переходе плавление углеводородных цепей сопровождается резким увеличением их вращательной и колебательной подвижности за счет гош-транс-изомеризации, что приводит к разупорядочению упаковки липидов, что, как правило, сопровождается образованием дефектов в бислое и изменением проницаемости мембраны (рис. 1) [32–34].
Размер сквозных ионных каналов достаточен для прохождения большинства гидратированных ионов и гидрофильных органических веществ, составляющих основу множества лекарственных препаратов: радиус трансмембранных пор в липосомах достигает 1–3 нм [35].
В табл. 1. приведены значения температуры фазового перехода Тпл некоторых фосфолипидов, наиболее часто применяемых для получения термочувствительных липосом [36, 37].
Таблица 1.
Температуры фазового перехода (Тпл) некоторых фосфолипидов
| Фосфолипид | Тпл, °С |
|---|---|
| Дипальмитоилфосфатидилхолин | 41 |
| Дистеароилфосфатидилхолин | 53 |
| Димиристоилфосфатидилэтаноламин | 48 |
Использование смеси липидов с отличающимися температурами плавления в различных соотношениях позволяет создавать системы с необходимыми температурами фазовых переходов. Например, для липосом, содержащих дипальмитоилфосфатидилхолин (ДПФХ) и дистероилфосфатидилхолин (ДСФХ) в соотношении ДПФХ/ДСФХ = 3/1, температура плавления составляет 42–44°С [30]. Состав липидной мембраны обычно подбирают таким образом, чтобы температура фазового перехода не превышала 42°С, не вызывая значительного повреждения клеток тканей в результате гипертермии.
Одновременное использование термочувствительных липосом с локальным нагревом может увеличивать избирательность действия препарата [38].
Введение в мембрану на основе ДПФХ лизоформ липидов, имеющих отличающуюся от цилиндрической форму молекулы, увеличивает скорость высвобождения препарата, так как такие молекулы вызывают более эффективное дефектообразование в бислое при фазовом переходе. Одним из примеров подобных систем, прошедших все стадии клинических испытаний и рекомендованных для использования в медицинской практике, является препарат для лечения рака печени ThermoDox [39]. В состав ThermoDox входят лизолецитин и противоопухолевый агент доксорубицин. ThermoDox способен всего лишь за 20 с высвобождать до 50% активного вещества при температуре 39–42°С, что более чем на порядок превышает эффективность классического липосомального доксорубицина Doxil. Несмотря на активные исследования в данной области, термочувствительные липосомы до сих пор не нашли широкого применения, так как гипертермия может приводить к значительному повреждению тканей.
Создание термочувствительных липосомальных систем возможно также и с помощью формирования комплексов традиционных липосом полимером, обладающим термочувствительностью. Обычно в роли такого полимера используется поли-N-изопропилакриламид (ПНИПАМ) или его сополимеры [40, 41]. Гели на основе ПНИПАМ могут адсорбировать липосомы на своей “поверхности” за счeт гидрофобных или электростатических взаимодействий, формируя мультилипосомальные коллоидно стабильные системы [42, 43]. Увеличение температуры выше нижней критической температуры растворения полимера приводит к коллапсу микрогеля и, как результат, механическому разрушению контактирующих мембран липосом в адсобированном слое. В этом случае происходит высвобождение инкапсулированного в липосомы лекарства.
Фоточувствительные липосомы
Фоточувствительные липосомы высвобождают терапевтические средства при воздействии излучения разной длины волны: ультрафиолетового (УФ), видимого и ближнего инфракрасного (ИК). Среди других внешних стимулов воздействие светом является одним из наиболее привлекательных методов получения отклика. Это воздействие не инвазивно, может быть удаленным и точечным, что позволяет эффективно контролировать скорость высвобождения лекарственного средства путем изменения длины волны, времени и интенсивности воздействия. Другое преимущество фоточувствительных липосом заключается в том, что фотохимические процессы не требуют использования дополнительных реагентов или катализаторов, а большинство побочных продуктов, если таковые имеются, безвредны и не являются токсичными [44]. Фотодинамическая терапия является относительно новым терапевтическим методом, который хорошо зарекомендовал себя для лечения различных видов рака кожи, таких как базальноклеточная карцинома и др. [45]. При контроле проникновения света внутрь человеческого тела учитываются такие параметры как диаметр луча, длина волны, продолжительность воздействия, измеряемая интенсивность и т. д. Одним из примеров фоточувствительных липосомальных препаратов является Visudyne®, разработанный Novartis Ophthalmicis, для лечения влажной формы возрастной макулодистрофии, характеризующейся образованием хориоидальной неоваскуляризации [46].
Существует несколько подходов к созданию фоточувствительных липосом [47, 48]. Запускаемое светом высвобождение лекарственного средства вызывается обратимыми или необратимыми структурными модификациями фоточувствительных носителей. Одним из способов является встраивание в мембрану липосом синтетических фото-триггеров, например, производных азобензола. Другой метод основан на использовании фотохимически модифицированного липида в качестве одного из компонентов бислоя. Воздействие УФ-излучения на двойные связи таких липидов способствует изменению конформации углеводородных цепей, что приводит к нарушению в упаковке липидов в мембране и увеличивает ее проницаемость. Примером одного из фотоизомеризуемых липидов является (1,2-(4'-н-бутилфенил)азо-4'-(γ-фенилбутироил))-глицеро-3-фосфохолин, который встраивают в мембрану в низких концентрациях. В термодинамически выгодной транс-форме данный липид представляет собой компактную молекулу, легко встраивающуюся в липидный бислой. При УФ-воздействии фотохромный липид переходит в состояние, в котором доминирует цис-изомер, способствующий быстрому высвобождению инкапсулированного вещества [49].
Другим способом получения фоточувствительных систем на основе липосом является их нековалентное связывание с полиэлектролитами, модифицированными фоточувствительными группами [50]. Действие УФ-излучения приводит к фотоизомеризации таких групп, изменению сродства молекул к липидному бислою, а следовательно – нарушению его структуры и высвобождению инкапсулированного препарата. Облучение фоточувствительного источника протонов может вызывать значительное изменение рН среды, сопровождающееся резким изменением конформации макромолекул полиэлектролита, что также приводит к разрушению липосом.
Хотя фоточувствительные липосомы могут контролируемо высвобождать содержимое в соответствующих условиях, их применение в клинической практике ограничено низкой стабильностью в плазме, поэтому в настоящее время исследователи сфокусированы на разработке фотолипосом с длительной циркуляцией в кровотоке. Другим ограничивающим фактором является потенциальная токсичность и/или канцерогенность производных азобензола, используемых в качестве фототриггеров. Большинство фоточувствительных липосом откликается лишь на воздействие УФ-излучения, что также является серьезным ограничивающим фактором. Наиболее эффективно использование фоточувствительных липосом при лечении кожных и глазных заболеваний, когда ближнее УФ-излучение имеет достаточную прони-кающую способность. В тех случаях, когда требуется большая глубина проникновения, используют инициирование ближним ИК-светом, способным проникать глубоко в мягкие ткани. Следует учитывать, что длительное облучение УФ может быть опасным для человеческого организма. Другим недостатком является отсутствие возможности промышленного масштабирования фоточувствительных систем доставки, что также препятствует их клиническому применению.
Редокс-чувствительные липосомы
Принцип работы редокс-чувствительных липосом основан на разнице в окислительно-восстановительных потенциалах, которая существует между окислительной средой снаружи клетки и восстановительной внутри нее. Глутатион является восстановителем, в котором тиоловые группы нейтрализуют накопление активных форм кислорода в раковых тканях. Он защищает биологические системы, окисляясь до дисульфида глутатиона, который восстанавливается обратно с помощью глутатионредуктазы. Соотношение восстановленной (ВФГ) и окисленной форм (ОФГ) глутатиона в клетке является одним из важнейших параметров, который показывает уровень окислительного стресса. Было обнаружено, что концентрация ВФГ в раковой ткани примерно в 100 раз превышает таковую в здоровой ткани и в 100–1000 раз в крови [51]. Повышенные уровни ВФГ в раковых клетках по сравнению со здоровыми тканями представляют собой существенный стимул для стимул-чувствительной доставки лекарств. В липосомы внедряют редокс-чувствительные связи или линкеры, например, дисульфидные связи. При их восстановлении до тиольных групп целостность нарушается, и липосомы высвобождают заключенное в них вещество [52].
Нойхаузер и соавт. (Noyhouzer et al.) использовали модифицированные ферроценом однослойные фосфолипидные липосомы в качестве стимул-чувствительной системы для контролируемого высвобождения лекарства с помощью окислительно-восстановительного механизма внутри раковых клеток HeLa [53]. Поскольку ферроценовые группы на поверхности запускали окислительно-восстановительные реакции, оценка высвобождения лекарственного средства проводилась методом проточной цитометрии. При этом полученные результаты показали высокую специфичность метода.
Вэнг и соавт. (Wang et al.) разработали липосомы, характеризующиеся высокой емкостью, на основе дисульфидфосфатидилхолина, поли-этиленгликоля (ПЭГ-2000) и холестерина для доставки иринотекана [54], которые продемонстрировали превосходную фармакокинетику по сравнению с обычными липосомами, содержащими иринотекан, а также со свободным препаратом.
В работе [55] описаны редокс-чувствительные липосомы, построенные из диолеоилфосфатидилэтаноламина, к полярной голове которого ковалентно присоединен фрагмент, содержащий хинон. Больные клетки производят хиноновые редуктазы, которые восстанавливают хинон, что способствует разрыву ковалентных связей и индуцирует высвобождение лекарственного вещества из внутренней полости липосом.
Мирхади (Mirhadi et al.) с соавт. использовали чувствительное к окислительно-восстановительному потенциалу соединение (10,10'-диселендиилбисдекановую кислоту) для повышения терапевтической эффективности липосом, нагруженных доксорубицином [56]. Оптимальные составы показали быстрое высвобождение 30% препарата в присутствии 0.1% перекиси водорода при pH 6.5. Был также разработан многокомпонентный состав (с гиалуроновой кислотой) для усиленного синергетического противоопухолевого воздействия [57].
Использование окислительно-восстановительных стимулов высвобождения лекарственных препаратов является перспективным направлением нанофармакологии. Однако существует ряд сложностей, связанных с гетерогенной природой опухолевых клеток, что не позволяет установить специфические окислительно-восстановительные реакции для всех типов опухолевых тканей. Кроме того, поиск окислительно-восстановительных триггеров для доставки нескольких лекарственных веществ, которые высвобождались бы в разное время и с разной скоростью, для таких систем представляет собой сложную задачу.
рН-чувствительные липосомы
Особый интерес исследователей вызывает разработка pH-чувствительных липосом, которые для своего раскрытия не требуют внешнего воздействия: инкапсулированное вещество спонтанно высвобождается из липосом после попадания их в области с пониженным значением рН – опухоли и места воспалений [58]. Известно, что во многих опухолях и местах воспалений внеклеточные значения рН существенно отличаются от физиологических (7.4) и изменяются в интервале от 6 до 7.2, а в некоторых случаях демонстрируют значения менее 6 [59].
Существует несколько основных подходов для придания липосомам рН-чувствительности: изменение состава липидной мембраны, встраивание в бислой pH-чувствительных соединений, модификация поверхности липосом.
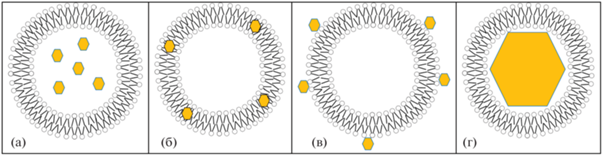
Некоторые липиды, у которых объeм полярной “головы” существенно меньше объeма, занимаемого “хвостами”, склонны образовывать обращенную гексагональную фазу вместо упаковки в бислойные структуры [60]. Устойчивый бислой не может образоваться только из одного такого липида, поэтому дополнительно вводят стабилизатор, содержащий кислотную группу (как правило, карбоксильную). При подкислении среды эта группа протонируется с погашением отрицательного заряда, наличие которого ранее стабилизировало липидный бислой (рис. 2). В результате молекулы липида стремятся образовать отдельную фазу, разрывая участки мембраны липосомы, и через образовавшиеся дефекты лекарство высвобождается [61].
Часто для синтеза pH-чувствительных липосом используют “вставки” – сайт-специфические лиганды, олигопептиды. В работе [62] описано использование для этой цели олигопептида из 30 звеньев с повторящимся фрагментом глутамилаланиллейцилаланина (ГАЛА). При физиологических значениях рН эта молекула располагается на поверхности липосомальной мембраны, а при более кислых значениях рН слои ГАЛА протонируются, принимают форму спиралей и внедряются в липосому с образованием пор, через которые содержимое липосом высвобождается во внешнюю среду.
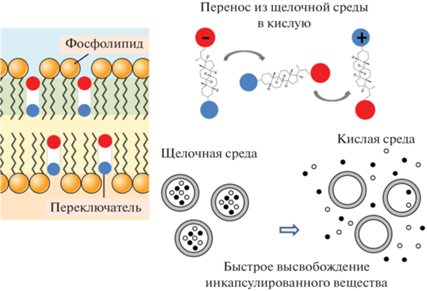
Рис. 3.
Схематичное представление работы рН-чувствительного переключателя- амфифильного производного литтохолевой кислоты.
Такие лиганды, как гиалуроновая и фолиевая кислоты, трансферрин и др., распознают и связываются со специфическим рецептором, сверхэкспрессированным на клетках-мишенях, запуская эндоцитоз и обеспечивая эндосомальную локализацию [63, 64]. Например, Миязаки и соавт. (Miyazaki et al.) разработали модифицированные гиалуроновой кислотой рН-чувствительные липосомы с целевыми свойствами в отношении клеток, экспрессирующих CD44 [65]. Было продемонстрировано более эффективное высвобождение доксорубицина, чем в случае немодифицированных липосом.
Оригинальный подход к созданию рН-чувствительных липосом был предложен авторами работы, в которой в мембрану липосом встраивали специально синтезированные липидоподобные соединения, способные изменять конформацию своих алкильных радикалов при понижении рН внешнего раствора [66–69]. Такие перестройки в молекуле “конформационного переключателя” вызывали нарушение упаковки гидрофобной части липидного бислоя и высвобождение инкапсулированных в липосомы водорастворимых веществ. Однако быстрое высвобождение было возможно только в том случае, если мембрана содержала значительные количества переключателя (30 вес. % и более), что приводило к нежелательному повышению (цито)токсичности липосом.
Еще одним типом конформационных переключателей, реагирующих на изменение pH, являются производные 3,7-диазобицикло[3.3.1]нонан-9-она (биспидинона). Эти вещества изменяют конформацию при добавлении солей двухвалентной меди. Эффект обусловлен комплексообразованием с ионами меди, что в итоге приводит к ослаблению упаковки алкильных цепей в мембране и повышению проницаемости липидного бислоя, сопровождающееся высвобождением инкапсулированного вещества [70].
Недавно был описан новый тип рН-чувствительного переключателя, представляющий собой производное литохолевой кислоты со стероидным ядром и двумя ионными группами, катионной и анионной [71]. При изменении кислотности внешнего раствора молекулы переключателя меняют свою ориентацию в липидном бислое, что приводит к нарушению упаковки липидов в бислое и формированию в нем дефектов, через которые происходит высвобождение водорастворимого инкапсулированного вещества (рис. 3). Вращение переключателя в мембране развивается очень быстро и сопровождается количественным вытеканием инкапсулированного в липосомах водорастворимого биологически активного вещества [72]. Принципиально, что для выхода лекарства достаточно встроить в мембрану 3 вес. % переключателя. Показано, что эффективность работы переключателя определяется природой ионных групп, присоединенных к стероидному ядру [73], а скорость высвобождения инкапсулированного вещества в первые минуты после подкисления максимальна для производного литохолевой кислоты, содержащего амино- и карбоксильные группы [72]. Для повышения эффективности доставки и увеличения терапевтического эффекта липосомы концентрируют на поверхности биодеградируемых носителей [74, 75]. В настоящее время усилия исследователей сосредоточены на поиске наиболее оптимального носителя для рН-чувствительных липосом.
Часто поверхность pH-чувствительных липосом модифицируют полимерами, содержащими чувствительные к рН среды группы, способные к диссоциации в ответ на изменение этого параметра. Диссоциация групп приводит к высвобождению терапевтического средства рядом с опухолью, при этом полимерная оболочка защищает здоровые клетки от токсического воздействия препарата. Некоторые полимеры могут дестабилизировать фосфолипидный бислой, другие – индуцировать слияние липосомы с мембраной клеток [76, 77].
Липосомальные контейнеры, как правило, модифицируют производными ПЭГ для увеличения продолжительности циркуляции в кровеносном русле. Таким образом получают так называемые липосомы-невидимки (“stealth” липосомы), время жизни которых в кровотоке составляет несколько дней [78, 79]. Введение функциональных сшивок между цепями ПЭГ и липидами, формирующими бислой, позволяет получить рН-чувствительные контейнеры. В работе [80] описано связывание ПЭГ и фосфатидилэтаноламина гидразоновым мостиком. При пониженных значениях рН происходит гидролиз гидразона, оболочка отщепляется от мембраны, что индуцирует высвобождение биоактивного вещества.
Еще одно направление связано с использованием новых материалов для модификации поверхности липосом. Например, модификация малеимидом позволяет избежать полной деградации после эндоцитоза при доставке в лизосомы раковых клеток. В работе [81] установлено, что использование рН-чувствительных липосом, покрытых полидофамином, существенно улучшает эффективность инкапсулированного 5-фторурацила по сравнению со свободным лекарством.
рН-чувствительные липосомы являются перспективными системами для контролируемой доставки лекарственных средств в целевые области организма. Кроме того, использование подобных липосом позволяет уменьшить эффективную дозу препарата, снизить токсичность и побочные эффекты от лечения. В настоящее время разработано большое количество pH-чувствительных липосом, поэтому требуется проведение клинических испытаний с наиболее оптимальными системами.
Магниточувствительные липосомы
Одним из наиболее широко используемых внешних воздействий на липосомальные наноконтейнеры является магнитное поле. Сами липосомы не обладают магниточувствительностью, поэтому для получения отклика на внешнее магнитное поле требуется дополнительная модификация липосом магнитными наночастицами. Наиболее подходящими объектами для такой модификации являются суперпарамагнитные частицы, для которых магнетизм проявляется только при воздействии магнитного поля. Обычно это наночастицы состоят из железа, кобальта, никеля и пр. металлов и их оксидов [82]. Размер наночастиц, при котором проявляется суперпарамагнетизм обычно не превышает 50 нм. Эта предельная величина превышает размер липидного бислоя и соизмерима со средним размером малых моноламеллярных липосом. Можно выделить три основных типа липосомальных контейнеров, модифицированных магнитными наночастицами: 1) гидрофильные наночастицы распределены внутри водной полости липосом; 2) гидрофобные наночастицы распределены в липидном бислое; 3) поверхность липосом модифицирована магнитными наночастицами (рис. 4а–4в). Отдельно можно выделить системы, в которых достаточно крупные суперпарамагнитные наночастицы с диаметром от 14–20 нм модифицированы липидным бислоем. В этом случае наночастица занимает практически весь внутренний объем липосом (рис. 4г). Обычно в таких частицах железо самостоятельно выполняет роль лекарственного препарата в дополнение к приданию системе магнитоуправляемости [83], например, для повышения доли железа в организме при анемии, вызванной его недостатком [84]. Таким образом, для модификации липосом магнитными наночастицами их желательный размер не должен превышать 14 нм.
Рис. 4.
Схема возможного распределения магнитных наночастиц в липосомах: гидрофильные наночастицы внутри водной полости (а); гидрофобные наночастицы внутри липидного бислоя (б); гидрофильные наночастицы на поверхности липосом (в); крупная наночастица, поверхность которой модифицирована липидным бислоем (г).
Магнитные наночастицы смешанного оксида Fe2+/Fe3+ (SPIONs – super paramagnetic iron oxide nanoparticles) наиболее часто применяют для модификации наноконтейнеров [85]. Данные наночастицы получают методами лазерного пиролиза, золь-гель синтеза, соосаждением солей железа в щелочи, термическим разложением карбонилов железа – последние два метода являются наиболее массовыми [86–90]. Другим распространенным оксидом железа, используемым для придания магниточувствительных свойств наноконтейнерам, является маггемит – гамма оксид железа. Структурно маггемит сходен с магнетитом, но обладает менее выраженными магнитными свойствами. При этом маггемит более термодинамически стабилен и не подвержен реакциям окисления и реакциям с участием радикалов, которые могут проходить в биологических средах при применении магнетита [91]. Маггемит может быть получен как путем окисления магнетита, так и прямым синтезом высокотемпературного разложения, так и синтезом в присутствии циклодекстринов в качестве стабилизаторов при комнатной температуре [92, 93].
Формирующиеся частицы в отсутствие стабилизатора склонны к агрегации. В качестве стабилизаторов обычно применяют декстран, хитозан, ПЭГ, поливиниловый спирт и низкомолекулярные поверхностно-активные вещества (ПАВ) [94–97]. Обычно синтезированные наночастицы измельчают воздействием ультразвука в присутствии стабилизатора [93, 98, 99].
С момента представления магнитных наночастиц как контрастирующих агентов в магнитно-резонансной томографии (МРТ) было зарегистрировано множество коммерческих продуктов на основе суперпарамагнитных оксидов железа – Venofer®, Ferrlecit®, INFeD®, Dexferrum®, Feraheme™, Feridex®, Resovist®, GastroMARK™, NanoTherm®, Combidex®, Clariscan®, Ferinject® и др., которые получили разрешение для клинического применения. Однако в течение небольшого промежутка времени были обнаружены побочные действия, а также низкая клиническая эффективность этих продуктов, что привело к запрету на их использование. На сегодняшний день только две формуляции присутствуют на рынке – Feraheme™ and NanoTherm® [100]. Причинами такого результата стали слишком маленькие размеры наночастиц, вследствие чего наблюдалось их накопление в различных (не целевых) органах, нарушение их нормального функционирования, нарушение работы нейронов и вызываемые действием этих наночастиц мутации. Применение же более крупных наночастиц приводило к их агломерации, выпадению в осадок и, как следствие, закупорке сосудов. Включение наночастиц в липосомы позволяет избавиться от таких негативных последствий внедрения в организм магнитных наночастиц.
Включение магнитных наночастиц в липосомы может осуществляться следующим путeм: добавление наночастиц на стадии приготовления липосом к раствору липидов в органическом растворителе в случае гидрофобных наночастиц и добавление водной дисперсии гидрофильных наночастиц к пленке липидов с последующим удалением несвязанных наночастиц с помощью хроматографии [91, 101, 102]. Прямая модификация поверхности липосом за счeт коньюгирования с магнитными наночастицам применяется довольно редко [101, 102], но бывает и эффективное решение модификации, например, за счeт электростатической адсорбции положительно заряженных наночастиц, стабилизированных маннозой, на отрицательно заряженную поверхность липосом путeм смешения водных дисперсий липосом и наночастиц с последующим удалением несвязанных наночастиц центрифугированием [98].
В случае использования гидрофобных наночастиц для создания магнитных липосом возникает проблема соответствия размеров наночастиц и бислоя. Уменьшение размера наночастиц ведeт к ухудшению магнитныx свойств, с другой стороны, размер включаемых в бислой наночастиц не должен превышать его толщину – 3–4 нм. Теоретические расчеты показывают, что максимальный размер наночастиц, которые можно включить в бислой составляет 5 нм [103]. При этом в ряде статей сообщается об успешном включении в липидный бислой магнитных наночастиц размером от 6 до 15 нм [104, 105]. Для этого использовали наночастицы оксида железа, стабилизированные олеиновой кислотой. Попытка смешать липиды и магнитные наночастицы в органическом растворителе с его последующим удалением и диспергированием пленки в водно-солевом буфере не привела к успешному включению крупных наночастиц. Поэтому авторами [104] было предложено использовать хлороформ в качестве растворителя липидов и наночастиц, при этом процедура приготовления липосом включала получение эмульсии в смеси водного буфера и хлороформа с соотношением органической фазы к водной 9 : 1. Далее методом ультразвуковой обработки была получена стабильная эмульсия по типу масло в воде. При нагревании этой эмульсии при 45°С в течение получаса хлороформ испарялся, а образующиеся структуры представляли собой магнитные липосомы с относительно невысокой полидисперсностью. Структурно в таких липосомах наночастицы представляют собой обернутые липидной мембраной включения, что вызывает значительное деформирование последней. Поэтому в таких системах наночастицы склонны не к симметричному распределению в мембране, а к агломерации внутри бислоя с целью понижения поверхностной энергии [105]. Таким образом, включенные в липосомы крупные магнитные наночастицы формируют янусоподобные структуры.
Основное применение магниточувствительные липосомы нашли в целевой доставке лекарств. Воздействие внешнего магнитного поля, вызываемого постоянным магнитом, источником переменного магнитного поля или микроволновым излучением, позволяет аккумулировать липосомальные наноконтейнеры в пораженных органах и тканях [106]. При этом использование магнитных липосом позволяет преодолевать гематоэнцефалический барьер (ГЭБ). ГЭБ образуется эндотелиальными клетками капилляров головного мозга, удерживаемыми близко друг к другу плотными соединениями, что ограничивает парацеллюлярный транспорт терапевтических средств. Таким образом, лекарственные препараты могут быть доставлены через ГЭБ только либо потому, что они обладают высокой липофильностью, либо потому, что они способны использовать естественные транспортеры организма, ведущие к внутриклеточному транспорту в мозге. При приложении внешней магнитной силы для доставки магнитных наночастиц в мозг частицы будут проникать через ГЭБ и далее в мозговую ткань. Исследования безопасности такого перемещения частиц через ГЭБ или нейроны не показали никаких цитотоксических эффектов, разрушения нервных волокон или влияния на функцию нейронных цепей [107].
Наличие пары Fe2+/Fe3+ в магнетите делает возможным создание липосомальных наноконтейнеров, способных помимо целевой доставки осуществлять дополнительное воздействие на опухолевые клетки за счет взаимодействия с перекисью водорода и генерации радикалов ОН·. Авторами [108] была предложена комплексная липосомальная система, содержавшая компоненты для химиотерапии, химиодинамической терапии, терапии голоданием и синергетической терапии магнитным наведением. В состав липосом были включены: а) партенолид, способный индуцировать апоптоз опухолевых клеток и потреблять избыточный глутатион, тем самым повышая эффективность химиодинамической терапии; б) глюкозооксидаза, которая может потреблять внутриопухолевую глюкозу, снижать рН и повышать уровень H2O2 в опухолевой ткани. Интегрированные в липосомы магнитные наночастицы выполняют две функции − эффективно катализируют превращение H2O2 в высокотоксичный гидроксильный радикал (·OH) и обеспечивают магнитное наведение.
Усиление эффективности целевой доставки магнитных липосомальных наноконтейнеров может быть достигнуто дополнительной векторизацией – модификацией поверхности липосом белками или антигенами, обеспечивающими специфическое связывание липосом с клетками-мишенями [109].
Также важным свойством модифицированных магнитными наночастицами липосом является возможность выступать в роли контрастирующих агентов в МРТ [86, 110]. Сами липосомы не обладают контрастом, который можно увидеть на МРТ-снимках. Использование данного метода позволяет неинвазивно оценить распределение липосомальных наноконтейнеров в различных органах. Также данный метод позволяет оценивать кинетику накопления липосомальных наноконтейнеров. С помощью модифицированных магнитными наночастицами липосом оценивают эффективность активного нацеливания наноконтейнеров путем модификации поверхности липидного бислоя специфическими лигандами, например, производными фолиевой кислоты, содержание рецепторов на которую у опухолевых клеток повышена. В комбинации с другими методами анализа, например, флуоресцентной микроскопией, можно оценить локализацию как наноконтейнера, так и доставляемого лекарства. Так оценивают локализацию противоопухолевого антибиотика доксору-бицина, обладающего флуоресценцией [111].
Отдельно стоит выделить способность магнитных наночастиц разогреваться при действии переменного магнитного поля, что позволяет создавать очаги локального нагрева в области нахождения подвергаемых воздействию наночастиц- гипертермии [112].
Магнитожидкостная гипертермия была впервые предложена Гилкристом и его коллегами. Суть процедуры заключается во введении магнитных наночастиц непосредственно в определенную ткань или орган с последующим воздействием на эту область переменного магнитного поля, что, в свою очередь, приводит к ее разогреванию до температуры 45–47°C и, как следствие, гибели клеток. Скорость и предельные температуры нагрева, как правило, зависят от состава наночастиц, их формы, размера и концентрации. При этом сам нагрев происходит за счет релаксационных процессов. А скорость этих процессов симбатно регулируется амплитудой и частотой переменного магнитного поля [101, 113–115]. При фиксированной частоте в 310 кГц липосомальные наноконтейнеры, содержащие во внутренней полости наночастицы маггемита со средним размером 12 нм, оказались способны к разогреванию окружающего раствора от 20 до 27°С при напряженности магнитного поля 200 Э; до 40°С при напряженности магнитного поля 400 Э и до 50°С при напряженности магнитного поля 800 Э. При этом использование дисперсии магнитных наноастиц, не включенных в липосомы, также приводило к повышению температуры, но в значительно меньшей степени [115]. По-видимому, это связано с локальной концентрацией наночастиц во внутренней полости липосом.
В то же время было показано, что сильное воздействие переменного магнитного поля может привести к неспецифическому нагреву областей без наночастиц вследствие вихревых токов. С целью исключить негативное воздействие на здоровые ткани были установлены ограничения параметров магнитного воздействия при терапии с помощью гипотермии, вызываемой магнитными наночастицами. Обычно рекомендуемые частоты магнитного поля составляют 100–200 кГц при магнитной индукции 20 мТл.
Способность магнитных наночастиц локально повышать температуру при действии внешнего магнитного поля используется для создания липосомальных наноконтейнеров с контролируемым высвобождением инкапсулированных препаратов. В основе данного подхода лежит формирование липосомальной мембраны из липидов, претерпевающих фазовый переход при температуре 38°С и выше. При этом включение наночастиц в мембрану приводит к снижению температуры фазового перехода липидов вследствие локального разупорядовачивания мембраны [116]. Авторами [117] было продемонстрировано, что анизотропия магниточувствительных наночастиц, включенных в липосомы, в значительной мере усиливает эффект нагревания липидного бислоя.
Авторами [113] было продемонстрировано, что использование липосом с магнитными наночастицами, сформированными двухфазной системой Fe3O4 La0.75Sr0.25MnO3, загруженными антибиотиком – паклитакселом, значительно усиливает терапевтический противоопухолевый эффект при гипертермии, индуцированной внешним переменным магнитным полем, по сравнению с индивидуальными магнитными липосомами и магнитными липосомами, заполненными паклитакселом, но без воздействия внешнего магнитного поля. При этом стоит отметить, что противоопухолевое действие гипертермии не такое эффективное по сравнению с действием паклитаксела – при равных концентрациях липосом выживаемость опухолевых клеток линии L929 in vitro составила 70% для магнитолипосом при действии переменного магнитного поля, 50% для магнитолипосом, заполненных паклитакселом, без действия поля. Кумулятивное применение магнитолипосом, заполненных паклитакселом, при воздействии магнитного поля привело к тому, что выжило лишь 30% клеток. Отдельно стоит отметить данные по экспериментам in vivo. Однократное введение дозы магнитолипосом с лекарством и использованием гипертермии привело к 2.5-кратному подавлению роста опухоли у мышей, а двукратное введение липосомального препарата привело к 3.6-кратному подавлению роста опухоли. При этом магнитные липосомальные наноконтейнеры успешно были локализованы в области опухоли и не были обнаружены в других органах мышей.
В работе [118] исследовали влияние воздействия переменного магнитного поля на липосомы из ДПФХ, заполненные доксорубицином, содержащие магнитные частицы оксида железа на внешней поверхности бислоя. Авторами отмечено, что наблюдалось частичное заглубление наночастиц в бислой, однако, целостность липидной мембраны не нарушалaсь. Воздействие внешнего магнитного поля (202 кГц и 30 мT) не привело к гибели клеток при использовании незагруженных антибиотиком липосом, в то время как для системы, содержащей доксорубицин, наблюдался значительный эффект гибели опухолевых клеток. Следует отметить, что цитотоксичность таких липосом (при воздействии магнитного поля) все равно оказалaсь меньше, чем цитотоксичность неинкапсулированного доксорубицина. Таким образом, главное преимущество данной системы – это магнитоуправляемая локализация антибиотика и его контролируемое высвобождение.
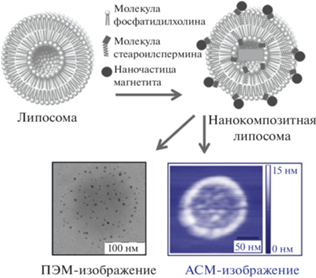
Контролируемое высвобождение лекарственного препарата из липосом, содержащих магнитные наночатицы, можно осуществлять без индуцируемых фазовых переходов в липидной мембране, а за счет механического нарушения целостности липидного бислоя или полного механического разрушения липосом. Хомутовым с коллегами было продемонстрировано, что модификация поверхности липосом, сформированных из стеароилспермина и фосфатидилхолина наночастицами магнетита, приводит к формированию коллоидно стабильных липосом. Комплексы формируются за счет взаимодействия фрагмента спермина с поверхностью наночастиц, не содержащих лигандов (рис. 5). Такие липосомы способны инкапсулировать доксорубицин и контролируемого высвобождать его при воздействии на суспензию короткочастотных импульсов [119, 120].
Рис. 5.
Липосома, модифицированная наночастицами магнетита. Схематическое изображение и микрофотографии атомно-силовой и просвечивающей электронной микроскопии.
Структурная организация липосомальной мембраны существенно сказывается на эффективности высвобождения инкапсулированных лекарств под действием магнитного поля на наночастицы, включенные в липидный бислой, и чем более упорядочена исходная липидная мембрана: жидкокристаллическая разупорядоченная, жидкокристаллическая упорядоченная и гелеобразная, тем больше высвобождается гидрофильного содержимого из внутренней полости во внешний раствор [121]. По-видимому, это связано с релаксационными процессами и подвижностью липидов в мембране, иными словами способностью липидов быстро залечивать формирующиеся в бислое дефекты.
Повышение эффективности действия магниточувстивтельных липосомальных наноконтейнеров может быть достигнуто за счет их дополнительного концентрирования на коллоидном носителе, чувствительнoм к воздействию ультразвука [122]. Таким образом, при двойном воздействии магнита и ультразвука повышается эффективность действия инкапсулированного противоопухолевого антибиотика – доксорубицина, однако такое повышение эффективности может быть связано не столько с двойным воздействием, сколько с аккумулированием липосом на носителе, что было ранее продемонстрировано авторами [123] – липoсомы, заполненные цисплатином, проявляли цитотоксичность при аккумулировании на полилактидных мицеллах, в то время как свободные липосомы при той же концентрации липидов цитотоксичности не проявляли.
Применение магниточувствительных липосом в тераностике
Уникальным свойством липосомальных наноконтейнеров, модифицированных магнитными наночастицами, является возможность их использования в тераностике – направлении медицины, использующем лекарственные препараты, обеспечивающие одновременную диагностику (обнаружение пораженных областей) и терапию (доставка лекарства) [124]. Так, включение в липосомы, несущие различные лекарственные молекулы и их комбинации, наночастиц оксида марганца позволило отследить с помощью МРТ распределение наноконтейнеров в различных органах во времени, эффективность накопления в опухолях, а также зарегистрировать времена, через которые наблюдались уменьшения опухолевых областей.
Применение комбинации лекарственного препарата и контрастирующего агента в липосомальном носителе может сказаться на эффективности действия индивидуальных компонентов. Так, применение фталоцианина цинка в качестве фотосенсибилизатора способно увеличить эффективность контрастирования в МРТ ионов гадолиния [125]. Однако природа этого эффекта авторами не исследована. Включение наночастиц маггемита в термочувствительные липосомы, в которых для высвобождения инкапсулированного доксорубицина использовали фототермическое возбуждение лазером при 808 нм, позволило снизить время, при котором наблюдалось взрывное высвобождение антибиотика по сравнению с классическим нагреванием термочувствительных липосом [111]. Для липосомальных тераностических систем на основе оксида марганца и паклитаксела как действующих веществ также было продемонстрировано кумулятивное повышение эффективности действия антибиотика [126]. Тераностическая система обеспечивала более пролонгированное действие и бóльшую степень высвобождения антибиотика, чем простая липосомальная форма паклитаксела. При этом накопление липосомального тераностического препарата в опухоли происходило дольше, чем для контрастирующего агента, стабилизированного ПАВ, однако времена удержания контрастирующего агента в опухоли отличались практически в три раза (12 ч для стабилизированного ПАВ MnO против 36 ч для липосомальной формы).
Для создания липосом для тераностики могут быть применены не только магнитные наночастицы, но и ионы Gd3+. Сами ионы гадолиния являются достаточно цитотоксичными, формирование хелатных комплексов с фосфолипидами, встраиваемыми в мембрану липосом, например, дистеароилглицерофосфоэтаноламин-N-диэтилентриаминпентауксусной кислотой, нивелирует эту токсичность [127].
Для тераностики возможна комбинация методов детектирования, например, сочетание МРТ и флуоресценции, для этого в магниточувствительные липосомы вводят флуоресцентный краситель, однако визуализация распределения липосомальных наноконтейнеров in vivo уже не может проводиться только неинвазивным методом и требует хирургического вмешательства [128].
Повышение эффективности терапевтического действия тераностических липосом может быть достигнуто при дополнительном воздействии на липосомы, напрямую не затрагивающим включенные магнитные наночастицы, например, сфокусированное ультразвуковое воздействие, также используемое для локальной гипертермии и обеспечивающее более эффективное накопление наноконтейнеров в опухолях за счет пассивного транспорта через сосуды, расширенные в результате воздействия ультразвука [129]. При этом особенно ярко эффект кумулятивного действия проявляется при наблюдении за действием лекарства в течение длительного времени после одновременного воздействия переменного магнитного поля и ультразвука. Также возможно придание тераностическим липосомам на основе магнитных наночастиц чувствительности к рН с целью увеличения эффективности высвобождения лекарств в опухолевых областях с закисленной окружающей средой [130]. Но для этого требуется еще одна стадия модификации липосом рН-чувствительными компонентами.
ЗАКЛЮЧЕНИЕ
Липосомы представляются одними из самых безопасных и наиболее эффективных биоразлагаемых и биосовместимых наноконтейнеров, которые могут быть легко метаболизированы в организме. Подобные носители лекарственных средств показали свою эффективность при лечении серьезных заболеваний, в том числе онкологических. Терапевтические преимущества противоопухолевых препаратов, инкапсулированных в липосомы, включают снижение токсичности и повышение эффективности лечения. Однако только липосомального лекарственного средства-носителя, нацеливающегося на опухолевые клетки с помощью специфических лигандов, не всегда достаточно для достижения успешного лечения. Липосомы способны реагировать на стимулы, что делает их незаменимыми в терапии, ориентированной на лечение конкретного заболевания. Использование стимул-чувствительных липосом позволяет достичь локализованного высвобождения лекарственного препарата с минимальным системным воздействием. Актуальной современной задачей представляется получение липосом, которые быстро реагируют на изменение стимула и высвобождают большую часть заключенного во внутреннюю полость соединения за короткое время. При разработке подобных систем следует учитывать ограничения, связанные с возможной цитотоксичностью модифицирующих липосому компонентов. Не менее важно, чтобы липосомы, обеспечив целевую доставку биоактивного вещества, безопасно метаболизировались и/или выводились из организма. При этом размер, поверхностный заряд и функциональные группы, модифицирующие липосому, должны поддерживать пролонгированную циркуляцию контейнера в кровотоке. Более того, для липосомальных носителей, содержащих несколько лекарственных носителей, крайне важно управлять соотношением препаратов.
В настоящее время активно разрабатываются комбинированные многофункциональные липосомы, одновременно реагирующие на несколько разных стимулов, такие как свет, pH, магнитное поле, температура, окислительно-восстановительный потенциал и др. Такие контейнеры могут быть в перспективе использованы в персонализированной медицине, что приведет к существенному улучшению качества жизни пациентов с серьезными заболеваниями.
Липосомы являются одними из лучших средств доставки биоактивных веществ, обладая рядом преимуществ перед другими наноконтейнерами. Несмотря на это, остается много вопросов, связанных с внедрением подобных конструкций в клиническую практику. Для перехода на крупномасштабное производство требуется проведение серьезных исследований, включающих выбор методики синтеза и оценки стоимости производства. Необходимо оценить специфичность и чувствительность предложенного метода, детально исследовать потенциальную безопасность и токсичность продуктов биодеградации контейнера.
Список литературы
Li Y.-J., Lei Y.-H., Yao N. et al. Autophagy and multidrug resistance in cancer // Chinese Journal of Cancer. 2017. V. 36. P. 1. https://doi.org/10.1186/s40880-017-0219-2
Migliore R., D’Antona N., Sgarlata C. et al. Co-loading of temozolomide and curcumin into a calix [4] arene-based nanocontainer for potential combined chemotherapy: Binding features, enhanced drug solubility and stability in aqueous medium // Nanomaterials. 2021. V. 11. № 11. P. 2930. https://doi.org/10.3390/nano11112930
Petrov R.A., Mefedova S.R., Yamansarov E.Y. et al. New small-molecule glycoconjugates of docetaxel and GalNAc for targeted delivery to hepatocellular carcinoma // Molecular Pharmaceutics. 2020. V. 18. № 1. P. 461–468. https://doi.org/10.1021/acs.molpharmaceut.0c00980
Vaneev A.N., Kost O.A., Eremeev N.L. et al. Superoxide dismutase 1 nanoparticles (nano-SOD1) as a potential drug for the treatment of inflammatory eye diseases // Biomedicines. 2021. V. 9. № 4. P. 396. https://doi.org/10.3390/biomedicines9040396
Pottanam Chali S., Ravoo B. J. Polymer Nanocontainers for Intracellular Delivery. Angewandte Chemie (International ed. in English) // 2020. V. 9 № 8. P. 2962–2972. https://doi.org/10.1002/anie.201907484
Zhang J., Lin Y., Lin Z. et al. Stimuli-responsive nanoparticles for controlled drug delivery in synergistic cancer immunotherapy // Advanced Science. 2022. V. 9. № 5. P. 2103444. https://doi.org/10.1002/advs.202103444
Barba A.A., Bochicchio S., Dalmoro A. et al. Engineering approaches for drug delivery systems production and characterization // Pharmaceutics. 2019. V. 581. P. 119267. https://doi.org/10.1016/j.ijpharm.2020.119267
Hou X., Zaks T., Langer R. et al. Lipid nanoparticles for mRNA delivery // Nat. Rev. Mater. 2021. V. 6. P. 1078–1094. https://doi.org/10.1038/s41578-021-00358-0
Wahlich J., Desai A., Greco F. et al. Nanomedicines for the delivery of biologics // Pharmaceutics. 2019. V. 11. № 5. P. 210. https://doi.org/10.3390/pharmaceutics11050210
Karim M.E., Shetty J., Islam R.A. et al. Strontium sulfite: A new pH-responsive inorganic nanocarrier to deliver therapeutic siRNAs to cancer cells. Pharmaceutics // 2019. V. 11. № 2. P. 89. https://doi.org/10.3390/pharmaceutics11020089
Cui Y., Yang Y., Ma M. et al. Reductive responsive micelle overcoming multidrug resistance of breast cancer by co-delivery of DOX and specific antibiotic // Journal of Materials Chemistry B. 2019. V. 7. № 40. P. 6075–6086. https://doi.org/10.1039/C9TB01093A
Zhang L., Wu C., Mu S. et al. A chemotherapeutic self-sensibilized drug carrier delivering paclitaxel for the enhanced chemotherapy to human breast MDA-MB-231 cells // Colloids Surf. B: Biointerfaces. 2019. V. 181. P. 902–909. https://doi.org/10.1016/j.colsurfb.2019.06.052
Madhumanchi S., Suedee R., Nakpheng T. et al. Binding interactions of bacterial lipopolysaccharides to polymyxin B in an amphiphilic carrier ‘sodium deoxycholate sulfate’ // Colloids Surf. B: Biointerfaces. 2019. V. 182. P. 110374. https://doi.org/10.1016/j.colsurfb.2019.110374
Wells C.M., Harris M., Choi L. et al. Stimuli-responsive drug release from smart polymers // J. Funct. Biomater. 2019. V. 10. № 3. P. 34. https://doi.org/10.3390/jfb10030034
Dhamecha D., Movsas R., Sano U. et al. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future // International Journal of Pharmaceutics. 2019. V. 569. P. 118627. https://doi.org/10.1016/j.ijpharm.2019.118627
Efimova A.A., Sorokina S.A., Trosheva, K.S. et al. Complexes of cationic pyridylphenylene dendrimers with anionic liposomes: The role of dendrimer composition in membrane structural changes // Int. J. Mol. Sci. 2023. V. 24. № 3. P. 2225. https://doi.org/10.3390/ijms24032225
Angelova A., Garamus V.M., Angelov B. et al. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and antitumor agents // Advances in Colloid and Interface Science. 2017. V. 249. P. 331–345. https://doi.org/10.1016/j.cis.2017.04.006
Carmona-Ribeiro A.M., de Melo Carrasco L.D. Novel Formulations for antimicrobial peptides // International Journal of Molecular Sciences. 2014. V. 15. № 10. P. 18040–18083. https://doi.org/10.3390/ijms151018040
Li M., Du C., Guo N. et al. Composition design and medical application of liposomes // European Journal of Medicinal Chemistry. 2019. V. 164. P. 640–653. https://doi.org/10.1016/j.ejmech.2019.01.007
Tretiakova D., Le-Deigen I., Onishchenko N. Phosphatidylinositol stabilizes fluid-phase liposomes loaded with a melphalan lipophilic prodrug // Pharmaceutics. 2021. V. 13. № 4. P. 473. https://doi.org/10.3390/pharmaceutics13040473
Sheoran R., Khokra S.L., Chawla V. et al. Recent patents, formulation techniques, classification and characterization of liposomes // Recent patents on nanotechnology. 2019. V. 13. № 1. P. 17–27. https://doi.org/10.2174/1872210513666181127110413
Amarandi R.-M., Ibanescu A., Carasevici E. et al. Liposomal-based formulations: A path from basic research to temozolomide delivery inside glioblastoma tissue // Pharmaceutics. 2022. V. 14. P. 308. https://doi.org/10.3390/pharmaceutics14020308
Park H., Otte A., Park K. Evolution of drug delivery systems: From 1950 to 2020 and beyond // Journal of Controlled Release. 2022. V. 342. P. 53–65. https://doi.org/10.1016/j.jconrel.2021.12.030
Barba A.A., Bochicchio S., Dalmoro A. et al. Lipid delivery systems for nucleic-acid-based-drugs: From production to clinical applications // Pharmaceutics. 2019. V. 11. № 8. P. 360. https://doi.org/10.3390/pharmaceutics11080360
Monteiro L.F., Malachias Â., Poundlana G. et al. Paclitaxel-loaded pH-sensitive liposome: New insights on structural and physicochemical characterization // Langmuir. 2018. V. 34. P. 5728–5737. https://doi.org/10.1021/acs.langmuir.8b00411
Tokudome Y., Nakamura K., Itaya Y. et al. Enhancement of skin penetration of hydrophilic and lipophilic compounds by pH-sensitive liposomes // Journal of Pharmacy and Pharmaceutical Sciences. 2015. V. 18. P. 249–257. https://doi.org/10.18433/J3H89S
Awad N.S., Paul V., AlSawaftah N.M. et al. Ultrasound-responsive nanocarriers in cancer treatment: A review // ACS Pharmacology & Translational Science. 2021. V. 4. № 2. P. 589–612. https://doi.org/10.1021/acsptsci.0c00212
Yan W., Leung S.S., To K.K.W. Updates on the use of liposomes for active tumor targeting in cancer therapy // Nanomedicine. 2020. V. 15. P. 303–318. https://doi.org/10.2217/nnm-2019-0308
Nikolova M.P., Kumar E.M., Chavali M.S. Updates on responsive drug delivery based on liposome vehicles for cancer treatment // Pharmaceutics. 2020. V. 14. P. 2195. https://doi.org/10.3390/pharmaceutics14102195
Yatvin M.B., Weinstein J.N., Dennis W.H. Design of liposomes for enhanced local release of drugs by hyperthermia // Science, New Series. 1978. V. 202. № 4374. P. 1290–1293. https://doi.org/10.1126/science.364652
Kong G., Dewhirst M.W. Review hyperthermia and liposomes // International Journal of Hyperthermia. 1999. V. 15. № 5. P. 345–370. https://doi.org/10.1080/026567399285558
Evans E., Needham D. Physical properties of surfactant bilayer membranes: Thermal transitions, elasticity, rigidity, cohesion, and colloidal interactions // J. Phys. Chem. 1987. V. 91. P. 4219–4228.
Trosheva K.S., Sorokina S.A., Efimova A.A. et al. Interaction of multicomponent anionic liposomes with cationic pyridylphenylene dendrimer: Does the complex behavior depend on the liposome composition? // Biochimica et Biophysica Acta (BBA) – Biomembranes. 2021. V. 1863. № 12. P. 183761. https://doi.org/10.1016/j.bbamem.2021.183761
Efimova A.A., Abramova T.A., Popov A.S., Grokhovskaya T.E. Interaction of chitosan with anionic liquid liposomes: Reversibility of structural rearrangements in lipid bilayer // Russian Journal of General Chemistry. 2022. V. 92. № 11. P. 2429–2435. https://doi.org/10.1134/S1070363222110275
Антонов В.Ф. Эволюция липидных пор в бислое при фазовом переходе мембранных липидов // Регулярная и хаотическая динамика / Под ред. А.Б. Рубина. М., 2006.
Dluhy R.A., Chowdhry B.Z., Cameron D.G. Infrared characterization of conformational differences in the lamellar phases of 1,3-dipalmitoyl-sn-glycero-2-phosphocholine // Biochimica et Biophysica Acta (BBA) – Biomembranes. 1985. V. 821. № 3. P. 437–444. https://doi.org/10.1016/0005-2736(85)90048-3
Watts A., Spooner P.J.R. Phospholipid phase transitions as revealed by NMR // Chem. Phys. Lipids. 1991. V. 57. № 2–3. P. 195–211. https://doi.org/10.1016/0009-3084(91)90076-n
Bozzuto G., Molinari A. Liposomes as nanomedical devices // International Journal of Nanomedicine. 2015. V. 10. P. 975–999. https://doi.org/10.2147/IJN.S68861
Needham D., Anyarambhatla G., Kong G., Dewhirst M.W. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model // Cancer Res. 2000. V. 60. № 5. P. 1197–1201.
Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery // Adv. Drug. Deliv. Rev. 2006. V. 58. № 15. P.1655–1670. https://doi.org/10.1016/j.addr.2006.09.020
Chernikova E.V., Plutalova A.V., Mineeva K.O. et al. Ternary copolymers of acrylic acid, n-isopropylacrylamide, and butyl acrylate: Synthesis and aggregative behavior in dilute solutions // Polymer Science, Series B. 2016. V. 58. № 5. P. 564–573. https://doi.org/10.1134/S1560090416050031
MacKinnon N., Guérin G., Liu B., Gradinaru C.C., Rubinstein L., Macdonald P.M. Triggered instability of liposomes bound to hydrophobically modified core-shell PNIPAM hydrogel beads // Langmuir. 2010. V. 26. № 2. P. 1081–1089. https://doi.org/10.1021/la902423v
Yaroslavov A., Panova I., Sybachin A. et al. Payload release by liposome burst: Thermal collapse of microgels induces satellite destruction // Nanomedicine. 2017. V. 13. № 4. P. 1491–1494. https://doi.org/10.1016/j.nano.2017.02.001
Alvarez-Lorenzo C., Bromberg L., Concheiro A. Light-sensitive intelligent drug delivery systems // Photochemistry and Photobiology. 2009. V. 85. № 4. P. 848–860. https://doi.org/10.1111/j.1751-1097.2008.00530.x
Ericson M.B., Wennberg A.M., Larko O. Review of photodynamic therapy in actinic keratosis and basal cell carcinoma // Ther. Clin. Risk Manag. 2008. V. 4. P. 1–9. https://doi.org/10.2147/TCRM.S1769
Konan Y.N., Gurny R., Allemann E. State of the art in the delivery of photosensitizers for photodynamic therapy // J. Photochem. Photobiol. B. 2002. V. 66. P. 89–106. https://doi.org/10.1016/s1011-1344(01)00267-6
Wang J.-Y., Wu Q.-F., Li J.-P. et al. Photo-sensitive liposomes: Chemistry and application in drug delivery // Mini-Reviews in Medicinal Chemistry. 2010. V. 10. № 2. P. 172–181. https://doi.org/10.2174/138955710791185091
Pan P., Svirskis D., Rees S. W.P. et al. Photosensitive drug delivery systems for cancer therapy: Mechanisms and applications // Journal of Controlled Release. 2021. V. 338. P. 446–461. https://doi.org/10.1016/j.jconrel.2021.08.053
Bisby R.H., Mead C., Morgan C.G. Active uptake of drugs into photosensitive liposomes and rapid release on UV photolysis. Photochemistry and Photobiology. 2000. V. 72. № 1. P. 57–61. https://doi.org/10.1562/0031-8655(2000)0720049mscpob2.0.co2
Ghosh S., Carter K.A., Lovell J.F. Liposomal formulations of photosensitizers // Biomaterials. 2019. V. 218. P. 119341. https://doi.org/10.1016/j.biomaterials.2019.119341
Torchilin V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery // Nat. Rev. Drug Discov. 2014. V. 13. P. 813–827. https://doi.org/10.1038/nrd4333
Liu M., Du H., Zhang W., Zhai G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications // Mater. Sci. Eng. C. 2017. V. 71. P. 1267–1280. https://doi.org/10.1016/j.msec.2016.11.030
Noyhouzer T., L’Homme C., Beaulieu I. et al. Ferrocene-modified phospholipid: An innovative precursor for redox-triggered drug delivery vesicles selective to cancer cells // Langmuir. 2016. V. 32. P. 4169–4178. https://doi.org/10.1021/acs.langmuir.6b00511
Wang T., He W., Du Y., Wang J., Li X. Redox-sensitive irinotecan liposomes with active ultra-high loading and enhanced intracellular drug release // Colloids Surf. B. Biointerfaces. 2021. V. 206. P. 111967. https://doi.org/10.1016/j.colsurfb.2021.111967
Ong W., Yang Y., Cruciano A.C., McCarley R.L. Redox-triggered contents release from liposomes // J. Am. Chem. Soc. 2008. V. 130. P. 14739–14744. https://doi.org/10.1021/ja8050469
Mirhadi E., Mashreghi M., Askarizadeh A. et al. Redox-sensitive doxorubicin liposome: A formulation approach for targeted tumor therapy // Sci. Rep. 2022. V. 12. P. 11310. https://doi.org/10.1038/s41598-022-15239-x
Yin T., Liu Y., Yang M. et al. Novel chitosan derivatives with reversible cationization and hydrophobicization for tumor cytoplasm-specific burst co-delivery of siRNA and chemotherapeutics // ACS Appl. Mater. Interfaces. 2020. V. 12. P. 14770–14783. https://doi.org/10.1021/acsami.9b19373
Mahmoudzadeh M., Magarkar A., Koivuniemi A., Róg T., Bunker A. Mechanistic insight into how PEGylation reduces the efficacy of pH-sensitive liposomes from molecular dynamics simulations // Molecular pharmaceutics. 2021. V. 18. № 7. P. 2612–2621. https://doi.org/10.1021/acs.molpharmaceut.1c00122
Lee E.S., Oh K.T., Kim D., Youn Y.S., Bae Y.H. Tumor pH-responsive flower-like micelles of poly(L-lactic acid)-b-poly(ethylene glycol)-b-poly(L-histidine) // J. Control. Release 2007. V. 123. P. 19–26. https://doi.org/10.1016/j.jconrel.2007.08.006
Efimova A.A., Sybachin A.V., Yaroslavov A.A. Effect of anionic-lipid-molecule geometry on the structure and properties of liposome-polycation complexes // Polymer Science Series C. 2011. V. 53. № 1. P. 18. https://doi.org/10.1134/S1811238211040011
Ferreira D.S., Lopes S.C. de A., Franco M.S., Oliveira M.C. pH-sensitive liposomes for drug delivery in cancer treatment // Therapeutic Delivery. 2013. V. 4. № 9. P. 1099–1123. https://doi.org/10.4155/tde.13.80
Li W., Nicol F., Szoka F.C. A designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery // Adv. Drug Deliv. Rev. 2004. V. 56. № 7. P. 967–985. https://doi.org/10.1016/j.addr.2003.10.041
Zhao Y., Ren W., Zhong T. et al., Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity // J. Control. Release. 2016. V. 222. P. 56. https://doi.org/10.1016/j.jconrel.2015.12.006
Miyazaki M., Yuba E., Hayashi H. et al. Hyaluronic acid-based pH-sensitive polymer-modified liposomes for cell-specific intracellular drug delivery systems // Bioconjug. Chem. 2018. V. 29. P. 44. https://doi.org/10.1021/acs.bioconjchem.7b00551
Samoshina N.M., Liu X., Brazdova B. et al. Fliposomes: pH-sensitive liposomes containing a trans-2-morpholinocyclohexanol-based lipid that performs a conformational flip and triggers an instant cargo release in acidic medium // Pharmaceutics. 2011. V. 3. № 3. P. 379–405. https://doi.org/10.3390/pharmaceutics3030379
Liu X., Zheng Y., Samoshina N.M. et al. Fliposomes: pH-triggered conformational flip of new trans-2-aminocyclohexanol-based amphiphiles causes instant cargo release in liposomes // J. Liposome Res. 2012. V. 22. № 4. P. 319–328. https://doi.org/10.3109/08982104.2012.698420
Zheng Y., Liu X., Samoshina N.M. et al. Fliposomes: trans-2-aminocyclohexanol-based amphiphiles as pH-sensitive conformational switches of liposome membrane – a structure-activity relationship study // Chem. Phys. Lipids. 2018. V. 210. P. 129–141. https://doi.org/10.1016/j.chemphyslip.2017.10.004
Zaborova O.V., Timoshenko V.A., Nardin C. et al. New insights on the release and self-healing model of stimuli-sensitive liposomes // J. Colloid Interface Sci. 2023. V. 640. P. 558–567. https://doi.org/10.1016/j.jcis.2023.02.099
Veremeeva P.N., Grishina I.V., Lapteva V.L. et al. pH-Sensitive liposomes with embedded 3,7-diazabicyclo[3.3.1]nonane derivative // Mendel. Commun. 2014. V. 3. № 24. P. 152–153. https://doi.org/10.1016/j.mencom.2014.04.008
Veremeeva P.N., Lapteva V.L., Palyulin V.A. et al. Bispidinone-based molecular switches for construction of stimulus-sensitive liposomal containers // Tetrahedron. 2014. V. 70. № 7. P. 1408–1411. https://doi.org/10.1016/j.tet.2014.01.012
Yaroslavov A., Efimova A., Smirnova N. et al. A novel approach to a controlled opening of liposomes // Colloids Surf. B: Biointerfaces. 2020. V. 190. P. 110906. https://doi.org/10.1016/j.colsurfb.2020.110906
Efimova A.A., Popov A.S., Kazantsev A.V. et al pH-Sensitive liposomes with embedded 3-(isobutylamino)cholan-24-oic acid: What is the possible mechanism of fast cargo release? // Membranes. 2023. V. 13. № 4. P. 407. https://doi.org/10.3390/membranes13040407
Popov A.S., Efimova A.A., Kazantsev A.V. et al. pH-Sensitive liposomes with embedded ampholytic derivatives of cholan-24-oic acid // Mendel. Commun. 2021. V. 31. № 6. P. 827–829. https://doi.org/10.1016/j.mencom.2021.11.019
Yaroslavov A.A., Efimova A.A., Abramova T.A. et al. Multi-compartment containers from a mixture of natural and synthetic lipids // Mend. Commun. 2023. V. 33. № 2. P. 221–224. https://doi.org/10.1016/j.mencom.2023.02.023
Grozdova I., Melik-Nubarov N., Efimova A. et al. Intracellular delivery of drugs by chitosan-based multi-liposomal complexes // Colloids Surf. B: Biointerfaces. 2020. V. 193. P. 11062. https://doi.org/10.1016/j.colsurfb.2020.111062
Abri Aghdam M., Bagheri R., Mosafer J. et al. Recent advances on thermosensitive and pH-sensitive liposomes employed in controlled release // J. Control Release. 2019. V. 315. P. 1–22. https://doi.org/10.1016/j.jconrel.2019.09.018
Paliwal S.R., Paliwal R., Vyas S.P. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery // Drug Deliv. 2015. V. 22. № 3. P. 231–242. https://doi.org/10.3109/10717544.2014.882469
Nandi U., Onyesom I., Douroumis D. An in vitro evaluation of antitumor activity of sirolimus-encapsulated liposomes in breast cancer cells // J. Pharm. Pharmacol. 2021. V. 73. № 3. P. 300–309. https://doi.org/10.1093/jpp/rgaa061
El Knidri H., Dahmani J., Addaou A. et al. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight // Int. J. Biol. Macromol. 2019 V. 139. P. 1092–1102. https://doi.org/10.1016/j.ijbiomac.2019.08.079
Sawant R.M., Hurley J.P., Salmaso S., et al. “SMART” drug delivery systems: Double-targeted pH-responsive pharmaceutical nanocarriers // Bioconjug. Chem. 2006. V. 17. № 4. P. 943–949. https://doi.org/10.1021/bc060080h
Zong W., Hu Y., Su Y. et al. Polydopamine-coated liposomes as pH-sensitive anticancer drug carriers // J. Microencapsul. 2016. V. 33. № 3. P. 257–262. https://doi.org/10.3109/02652048.2016.1156176
Sandler S.E., Fellows B., Mefford O.T. Best practices for characterization of magnetic nanoparticles for biomedical applications // Anal. Chem. 2019. V. 91. № 22. P. 14159–14169. https://doi.org/10.1021/acs.analchem.9b03518
Hadinoto K., Sundaresan, Cheow W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review // Eur. J. Pharm. Biopharm. 2013. V. 85. № 23. P. 427–443. https://doi.org/10.1016/j.ejpb.2013.07.002
Fathy M.M., Fahmy H.M., Balah A.M.M. et al. Magnetic nanoparticles-loaded liposomes as a novel treatment agent for iron deficiency anemia: In vivo study // Life Sci. 2019. V. 234. P. 116787. https://doi.org/10.1016/j.lfs.2019.116787
Dormer K., Seeney C., Lewelling K. et al. Epithelial internalization of superparamagnetic nanoparticles and response to external magnetic field // Biomaterials. 2005. V. 26. № 14. P. 2061–2072. https://doi.org/10.1016/j.biomaterials.2004.06.040
Li X., Li W., Wang M., Liao Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects // J. Control. Release. 2021. V. 335. P. 437–448. https://doi.org/10.1016/j.jconrel.2021.05.042
Ansari M.J., Kadhim M.M., Hussein B.A. et al. Synthesis and stability of magnetic nanoparticles // BioNa-noSci. 2022. V. 12. № 2. P. 627–638. https://doi.org/10.1007/s12668-022-00947-5
Lyer S., Singh R., Tietze R. et al. Magnetic nanoparticles for magnetic drug targeting // Biomed. Tech. 2015. V. 60. № 5. P. 465–475. https://doi.org/10.1515/bmt-2015-0049
Mireles L.K., Sacher E., Yahia L. et al. A comparative physicochemical, morphological and magnetic study of silane-functionalized superparamagnetic iron oxide nanoparticles prepared by alkaline coprecipitation // Int. J. Biochem. Cell. Biol. 2016. V. 75. P. 203–211. https://doi.org/10.1016/j.biocel.2015.12.002
Lassenberger A., Grünewald T.A., van Oostrum P.D.J., et al. Monodisperse iron oxide nanoparticles by thermal decomposition: elucidating particle formation by second-resolved in situ small-angle X-ray scattering // Chem. Mater. 2017. V. 29. № 10. P. 4511–4522. https://doi.org/10.1021/acs.chemmater.7b01207
Israel L.L., Galstyan A., Holler E., Ljubimova J.Y. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain // J. Control. Release. 2020. V. 320. P. 45–62. https://doi.org/10.1016/j.jconrel.2020.01.009
Vernaya O.I., Shumilkin A.S., Shabatin V.P. et al. The synthesis of maghemite nanoparticles by thermal decomposition of cryochemically modified iron (III) acetylacetonate // Mos. Univ. Chem. Bull. 2020. V. 75. P. 265–268. https://doi.org/10.3103/S0027131420050089
Pigareva V.A., Alekhina Y.A. Grozdova I.D. et al. Magneto-sensitive and enzymatic hydrolysis-resistant systems for the targeted delivery of paclitaxel based on polylactide micelles with an external polyethylene oxide corona // Polym. Int. 2021. V. 71. № 4. P. 456–463. https://doi.org/10.1002/pi.6306
Abdollah M.R., Kalber T., Tolner B. et al. Prolonging the circulatory retention of SPIONs using dextran sulfate: In vivo tracking achieved by functionalisation with near-infrared dyes // Faraday Discuss. V. 2014. V. 175. P. 41–58. https://doi.org/10.1039/c4fd00114a
Saravanakumar K., Sathiyaseelan A., Manivasagan P. et al. Photothermally responsive chitosan-coated iron oxide nanoparticles for enhanced eradication of bacterial biofilms // Biomater. Adv. 2022. V. 141. P. 213129. https://doi.org/10.1016/j.bioadv.2022.213129
Ramnandan D., Mokhosi S., Daniels A. et al. Chitosan, polyethylene glycol and polyvinyl alcohol modified MgFe2O4 ferrite magnetic nanoparticles in doxorubicin delivery: A comparative study in vitro // Molecules. 2021. V. 26. № 13. P. 3893. https://doi.org/10.3390/molecules26133893
Rajan A., Sharma M., Sahu N.K. Assessing magnetic and inductive thermal properties of various surfactants functionalised Fe3O4 nanoparticles for hyperthermia // Sci. Rep. 2020. V. 10. № 1. P. 15045. https://doi.org/10.1038/s41598-020-71703-6
Wang X., Wang Y., Xue Z. et al. Magnetic liposome as a dual-targeting delivery system for idiopathic pulmonary fibrosis treatment // J. Colloid Interface Sci. 2023. V. 636. P. 388–400. https://doi.org/10.1016/j.jcis.2023.01.007
Halevas E., Mavroidi B., Swanson C.H. et al. Magnetic cationic liposomal nanocarriers for the efficient drug delivery of a curcumin-based vanadium complex with anticancer potential // J. Inorg. Biochem. 2019. V. 199. P. 110778. https://doi.org/10.1016/j.jinorgbio.2019.110778
Soares F.A., Costa P., Sousa C.T. et al. Rational design of magnetoliposomes for enhanced interaction with bacterial membrane models // Biochim. Biophys. Acta Biomembr. 2023. V. 1865. P. 184115. https://doi.org/10.1016/j.bbamem.2022.184115
Monnier C.A., Burnand D., Rothen-Rutishauser B. et al. Magnetoliposomes: Opportunities and challenges // Eur. J. Nanomed. 2014. V. 6. № 4. P. 201–215. https://doi.org/10.1515/ejnm-2014-0042
Floris A., Ardu A., Musinu A., et al. SPION@ liposomes hybrid nanoarchitectures with high density SPION association // Soft Matter. 2011. V. 7. № 13. P. 6239–6247. https://doi.org/10.1039/C1SM05059A
Amstad E., Kohlbrecher J., Muller E. et al. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes // Nano Lett. 2011. V. 11. № 4. P. 1664–1670. https://doi.org/10.1021/nl2001499
Choi W.I., Sahu A., Wurm F.R. et al. Magnetoliposomes with size controllable insertion of magnetic nanoparticles for efficient targeting of cancer cells // RSC Adv. 2019. V. 9. № 26. P. 15053–15060. https://doi.org/10.1039/c9ra02529d
Hermann C.A., Hofmann C., Duerkop A. et al. Magnetosomes for bioassays by merging fluorescent liposomes and magnetic nanoparticles: Encapsulation and bilayer insertion strategies // Anal. Bioanal. Chem. 2020. V. 412. P. 6295–6305. https://doi.org/10.1007/s00216-020-02503-0
Pradhan P., Banerjee R., Bahadur D., Koch C., Mykhaylyk, O., Plank C. Targeted magnetic liposomes loaded with doxorubicin. In: D’Souza, G. (eds) Liposomes. Methods in Molecular Biology. V. 1522. Humana Press. New York. NY. 2017.
Thomsen L.B., Linemann T., Birkelund S. et al. Evaluation of targeted delivery to the brain using magnetic immunoliposomes and magnetic force // Materials. 2019. V. 31. № 21. P. 3576. https://doi.org/10.3390/ma12213576
Gao W., Wei S., Li Z. et al. Nano magnetic liposomes-encapsulated parthenolide and glucose oxidase for ultra-efficient synergistic antitumor therapy // Nanotechnology. 2020. V. 31. P. 355104. https://doi.org/10.1088/1361-6528/ab92c8
Yang R., An L.Y., Miao Q.F. et al. Effective elimination of liver cancer stem-like cells by CD90 antibody targeted thermosensitive magnetoliposomes // Oncotarget. 2016. V. 7. № 24. P. 35894. https://doi.org/10.18632/oncotarget.9116
Thébault C.J., Ramniceanu G., Michel A. et al. In vivo evaluation of magnetic targeting in mice colon tumors with ultra-magnetic liposomes monitored by MRI // Mol. Imaging. Biol. 2019. V. 21. P. 269–278. https://doi.org/10.1007/s11307-018-1238-3
Ma G., Kostevšek N., Monaco I. et al. PD1 blockade potentiates the therapeutic efficacy of photothermally-activated and MRI-guided low temperature-sensitive magnetoliposomes // J. Control Release. 2021. V. 332. P. 419–433. https://doi.org/10.1016/j.jconrel.2021.03.002
Luiz M.T., Dutra J.A.P., Viegas J.S.R. et al. Hybrid magnetic lipid-based nanoparticles for cancer therapy // Pharmaceutics. 2023. V. 15. № 23. P. 751. https://doi.org/10.3390/pharmaceutics15030751
Gogoi M., Jaiswal M.K., Sarma H.D. et al. Biocompatibility and therapeutic evaluation of magnetic liposomes designed for self-controlled cancer hyperthermia and chemotherapy // Integr. Biol. 2017 V. 9. № 6. P. 555–565. https://doi.org/10.1039/c6ib00234j
Farzin A., Etesami S.A., Quint J. et al. Magnetic nanoparticles in cancer therapy and diagnosis // Adv. Healthc. Mater. 2020. V. 9. № 9. P. 1901058. https://doi.org/10.1002/adhm.201901058
Alonso J., Khurshid H., Devkota J. et al. Superparamagnetic nanoparticles encapsulated in lipid vesicles for advanced magnetic hyperthermia and biodetection // J. Appl. Phys. 2016. V. 119. P. 083904. https://doi.org/10.1063/1.4942618
Oliveira R.R., Carrião M.S., Pacheco M.T. et al. Triggered release of paclitaxel from magnetic solid lipid nanoparticles by magnetic hyperthermia // Mater. Sci. Eng. C. 2018. V. 92. P. 547–553. https://doi.org/10.1016/j.msec.2018.07.011
Cardoso B.D., Rodrigues A.R.O., Bañobre-López M. et al. Magnetoliposomes based on shape anisotropic calcium/magnesium ferrite nanoparticles as nanocarriers for doxorubicin // Pharmaceutics. 2021. V. 13. № 8. P. 1248. https://doi.org/10.3390/pharmaceutics13081248
Fortes Brollo M.E., Domínguez-Bajo A., Tabero A. et al. Combined magnetoliposome formation and drug loading in one step for efficient alternating current-magnetic field remote-controlled drug release // ACS Appl. Mater. Interfaces. 2020. V. 12. № 4. P. 4295–4307. https://doi.org/10.1021/acsami.9b20603
Khomutov G.B., Kim V.P., Koksharov Y.A. et al. Nanocomposite biomimetic vesicles based on interfacial complexes of polyelectrolytes and colloid magnetic nanoparticles // Colloid. Surf. A. 2017. V. 532. P. 26–35. https://doi.org/10.1016/j.colsurfa.2017.07.035
Gulyaev Y.V., Cherepenin V.A., Taranov I.V. et al. Activation of nanocomposite liposomal capsules in a conductive water medium by ultra-short electric exposure // J. Commun. Technol. Electr. 2021. V. 66. P. 88–95. https://doi.org/10.1134/S1064226921010022
Trilli J., Caramazza L., Paolicelli P. et al. The impact of bilayer rigidity on the release from magnetoliposomes vesicles controlled by PEMFs // Pharmaceutics. 2021. V. 13. № 10. P. 1712. https://doi.org/10.3390/pharmaceutics13101712
Dwivedi P., kiran S., Han S. et al. Magnetic targeting and ultrasound activation of liposome-microbubble conjugate for enhanced delivery of anticancer therapies // ACS Appl. Mater. Interfaces 2020. V. 12. № 21. P. 23737–23751. https://doi.org/10.1021/acsami.0c05308
Sybachin A.V., Khlynina P.O., Spiridonov V.V. et al. Amino-terminated polylactide micelles with an external poly(ethylene oxide) corona as carriers of drug-loaded anionic liposomes // Polym. Int. 2018. V. 67. № 10. P. 1352–1358. https://doi.org/10.1002/pi.5629
Shete M.B., Patil T.S., Deshpande A. et al. Current trends in theranostic nanomedicines // J. Drug Delivery Sci. Tech. 2022. V. 71. P. 103280. https://doi.org/10.1016/j.jddst.2022.103280
Skupin-Mrugalska P., Sobotta L., Warowicka A. et al. Theranostic liposomes as a bimodal carrier for magnetic resonance imaging contrast agent and photosensitizer // J. Inorg. Biochem. 2018. V. 180. P. 1–14. https://doi.org/10.1016/j.jinorgbio.2017.11.025
Li J., Li Q., He M. et al. AS1411 aptamer-modified theranostic liposomes co-encapsulating manganese oxide nano-contrast agent and paclitaxel for MRI and therapy of cancer // RSC Adv. 2019. V. 9. № 60. P. 34837–34846. https://doi.org/10.1039/c9ra06878c
Šimečková P., Hubatka F., Kotouček J. et al. Gadolinium labelled nanoliposomes as the platform for MRI theranostics: In vitro safety study in liver cells and macrophages // Sci. Rep. 2020. V. 10. № 1. P. 4780. https://doi.org/10.1038/s41598-020-60284-z
Chen Q., Shang W., Zeng C., et al. Theranostic imaging of liver cancer using targeted optical/MRI dual-modal probes // Oncotarget. 2017. V. 8. № 20. P. 32741. https://doi.org/10.18632/oncotarget.15642
Thébault C.J., Ramniceanu G., Boumati S. et al. Theranostic MRI liposomes for magnetic targeting and ultrasound triggered release of the antivascular CA4P // J. Control. Release. 2020. V. 322. P. 137–148. https://doi.org/10.1016/j.jconrel.2020.03.003
Guo H., Chen W., Sun X. et al. Theranostic magnetoliposomes coated by carboxymethyl dextran with controlled release by low-frequency alternating magnetic field // Carbohydr. Polym. 2015. V. 118. P. 209–217. https://doi.org/10.1016/j.carbpol.2014.10.076
Дополнительные материалы отсутствуют.
Инструменты
Коллоидный журнал




